Abstract
Effect of peroxidase on arabinoxylans (pentosans) isolated from whole wheat flour and instrumental textural parameters of whole wheat flour dough were studied. Significant increase in dough hardness and decrease in adhesiveness was observed on treatment of dough with peroxidase. Arabinoxylans isolated from peroxidase treated dough had higher molecular weight, viscosity, arabinose to xylose ratio, ferulic acid, and protein contents as compared to that of untreated dough. Arabinoxylans isolated from treated dough had higher absorption at 280 and 320 nm indicating the association of proteins and ferulic acid with arabinoxylans. Thus, the treatment of dough with peroxidase may catalyze the formation of cross-linking between arabinoxylans as well as protein-arabinoxylan that could be responsible for the alteration of the whole wheat flour dough characteristics.
INTRODUCTION
A variety of food products are prepared using wheat due to the unique visco-elastic properties of its dough. Chapati is the main traditional staple food product prepared in the Indian subcontinent and also consumed in UK and other countries, particularly by the Asian ethnic community.[Citation1] Due to change in the socioeconomic conditions, demand for ready-to-eat chapatis is increasing and therefore, mechanization for various processing steps is happening. However, one of the limitations in mechanization processing steps is to overcome the adhesiveness (stickiness) of chapati dough.
Whole wheat flours are used for the preparation of chapatis, while refined wheat flour is used for bread preparation. The composition of these two different flours varies with respect to their protein and arabinoxylan contents, which control water holding capacities of dough. Arabinoxylans constitutes 2–3% in refined wheat flour, while its content is higher in whole wheat flour (5–8%).[Citation2,Citation3] Arabinoxylans are the major non-starch polysaccharides in wheat. They are one of the important components of dough because of their high water holding capacity. The water soluble arabinoxylans hold up to 10 times of their weight in water and therefore, the content of water soluble arabinoxylans has been positively related to bread quality[Citation4] as well as tandoori roti-making quality.[Citation5] They are important in determining the dough handling properties.[Citation6]
Chemical or biological additives, like enzymes are added to improve the dough handling properties.[Citation7−Citation9] The positive effect of peroxidases in bread making has been attributed to cross-linking of feruloyated arabinoxylans into larger aggregates,[Citation10] cross-linking of gluten proteins or attachment of arabinoxylans to gluten proteins.[Citation11−Citation14] Thus, these reactions may alter the structural properties of the arabinoxylans or decrease the water soluble arabinoxylans, which decrease water holding capacity and alter dough rheology and texture.[Citation15] In vitro studies indicate that combination of peroxidase and hydrogen peroxide catalyse the gelation of arabinoxylans via the formation of diferulic acid linkages.[Citation16] In literature, most of the work on cross-linking of arabinoxylans using oxidative agents was on in vitro studies i.e., isolated pentosans were directly treated with oxidizing agents.[Citation17] However, no studies are available on the characteristics of the arabinoxylans isolated from the dough after its treatment with peroxidase.
Therefore, in the present study, different amounts of peroxidase were added to the whole-wheat flour to study (a) their effects on instrumental textural profile of whole wheat flour dough and (b) their changes in the physico-chemical properties of arabinoxylans.
MATERIALS AND METHODS
Materials
Wheat variety, DWR-162 was obtained from College of Agriculture, Dharwad, India. Wheat was milled into whole flour in a commercial disc mill. Freshly milled whole-wheat flour was cooled and stored at 4°C. Glucoamylase (EC 3. 2. 1. 3) and horseradish peroxidase (EC 1. 1. 11. 7) were obtained from Sigma Chemical Company, St Louis, USA. Termamyl (EC 3. 2. 1. 1) was from Novo, Denmark. Sepharose CL-2B and T-series dextran standards (T-10, T-20, T-40, T-70, T-500, and T-2000) were procured from Pharmacia Fine Chemicals, Uppsala, Sweden. All other chemicals were of analytical grade. Solvents used in this study were either analytical or HPLC grade. Bovine serum albumin (BSA) and hydrogen peroxide were from Sisco-Research Laboratory. Mumbai, India.
Instrumental Texture Profile Analysis of Wheat Dough
Wheat dough (control) was prepared by adding optimum amount of distilled water (based on their farinograph water absorption value) to the whole wheat flour taken in Hobart mixer and mixed for 2 min. Peroxidase (different activities) treated doughs with fixed concentration of hydrogen peroxide (0.05%) were prepared same as control dough. After 30 min of resting period, they were subjected to textural profile analysis using a Universal Textural Measuring system (Lloyds, LR5K, Fareham, Hampshire, UK). Dough was cut into cylindrical pieces of 3 cm diameter and 1.5 cm height and measured for dough hardness (peak force during the first compression cycle or first bite), cohesiveness (ratio of the positive force area during the second compression to that of the first compression), and adhesiveness (negative peak area for the first bite). Triplicate measurements were done for each treatment. Texture profile analysis was measured with crosshead speed of 60 mm/min, load cell of 49 N, compression of 50% of sample height and probe diameter of 3.5 cm.
Isolation and Purification of Arabinoxylans
Arabinoxylans from control dough and peroxidase treated doughs were isolated using barium hydroxide and purified according to the method reported earlier.[Citation18] Whole wheat flour doughs were subjected to Termamyl and glucoamylase digestion and centrifuged. The residue obtained was extracted twice with saturated barium hydroxide solution containing 250 mM sodium borohydride and centrifuged. The supernatant obtained was precipitated with alcohol under acidic conditions and precipitate was digested with glucoamylase at 60°C for 4 h. After the digestion, the pentosans were precipitated by adding two volumes of alcohol and centrifuged. The residue obtained was uniformly dispersed in water and lyophilized.
Gel Permeation Chromatography (GPC)
Purified pentosans (10 mg) were dissolved in 1 mL of water and were analyzed by gel filtration chromatography on a precalibrated (with T-series dextran standards) Sepharose CL-2B column (1.6 × 92 cm) using water as eluent. Fractions (3 mL) were collected using LKB Bromma 2211 fraction collector at 18 mL/h flow rate and aliquots were analyzed by the phenol-sulphuric acid method.[Citation19] Fractions containing carbohydrates were pooled, dialyzed, and freeze-dried.
Relative Viscosity
Different amounts of purified pentosans (0.1 to 0.5%) were dissolved in water and the relative viscosity of purified pentosans with respect to water was determined in an Ostwald viscometer.[Citation20]
Sugar Content
Purified pentosans (10 mg) were dissolved in 1 ml of water and estimated for protein, sugar, and uronic acid contents. Estimation of total sugars was done by phenol-sulphuric acid method,[Citation19] uronic acids by carbazole method,[Citation21] and protein content by the method described by Lowry et al.[Citation22] For determination of sugar composition of the purified pentosans, the samples were hydrolysed with 2 N trifluroacetic acid in sealed tubes at 100°C for 5–6 h and the sugars were analyzed by gas liquid chromatography as alditol acetates[Citation23] on an OV-225 column at column temperature of 200°C using a Shimadzu GLC.
Ferulic Acid Content
The purified pentosans (10 mg) were treated with 1 N NaOH solution (5 ml) at room temperature for 4 h under nitrogen.[Citation24] The mixture was acidified to pH 3 with HCl (2 N) and extracted three times with equal volume of ethyl acetate. The extracts were evaporated to dryness in a rotary evaporator and the residue dissolved into a known amount of methanol. Ferulic acid content was analyzed by using HPLC method as reported earlier.[Citation25]
UV-Spectroscopy
UV absorption spectra of purified pentosan solutions taken in quartz cuvette were recorded between 200–400 nm using a UV-visible spectrophotometer (Shimadzu, Kyoto, Japan).[Citation26]
Statistical Analysis
Data obtained from three experiments were treated statistically by Duncan’s new multiple range test to determine the significance of results.[Citation27] The results were expressed as mean ± standard deviation (SD). A value of P < 0.05 was considered to be statistically significant.
RESULTS AND DISCUSSION
Effect of Peroxidase on Textural Properties
Textural characteristics of dough influences baking properties and play an essential role in determining the global acceptability of the food by consumers. Good quality dough will have certain degree of hardness, cohesiveness, and low adhesiveness.[Citation28]
Table 1 Effect of peroxidase on textural profile analysis of wheat dough
Textural profile analysis of whole wheat dough is given in . Significant increase in dough hardness was observed with the incorporation of peroxidase in the dough and it was maximum at 1 mg peroxidase/100 g flour, with a hardness value of about 7.16 N. Incorporation of 2 mg peroxidase had showed significant increase in dough hardness compared to control, however, it was significantly lower than 0.5 and 1 mg peroxidase incorporated doughs. Earlier, Selinheimo et al.[Citation29] had made similar observations when the dough was treated with higher levels of laccase. They speculated that softening phenomenon of dough might be due to radical catalyzed break down of the cross-linked arabinolxylan network at higher dosages. Incorporation of peroxidase or hydrogen peroxide did not bring much changes in cohesiveness, while addition of peroxidase along with hydrogen peroxide decreased the adhesiveness of the dough significantly compared to control. For dough, this property (adhesiveness) is of vital importance, because it is related to handling properties and machinability and low value of adhesiveness is preferred. These results indicated that dough became less sticky after the addition of peroxidase. Decrease in stickiness of dough by the addition of peroxidases has also been reported by Hilhorst et al.[Citation7] These changes in rheological properties of peroxidase treated dough may be due to the changes in the structural characteristics of arabinoxylans and proteins. Peroxidase catalyzed cross-linking of ferulylated arabinoxylans[Citation10] or cross-linking of glutenin subunits[Citation14] has been observed in wheat dough.
The addition of peroxidase along with hydrogen peroxide may catalyse oxidative gelation of arabinoxylan molecules via the formation of diferulic acid linkages.[Citation12] In dough, such cross-linking was suggested to be responsible for the improvement of dough physico-chemical properties.[Citation6] Further, peroxidases also cross-link arabinoxylans to side chains of amino acids.[Citation30] Evidence for the occurrence of thiol-ferulic acid linkages has also been observed in wheat dough.[Citation31] Accordingly peroxidase action in dough has been attributed to the formation of inter-chain bonds between arabinoxylans or by the coupling of arabinoxylans to gluten proteins. Ultimately peroxidase in dough may modify arabinoxylan and gluten chemical properties which in turn lead to changes in the textural properties.[Citation32]
Effect of Peroxidase on Molecular Weight of Arabinoxylans (Pentosans)
Pentosans isolated from the whole wheat dough of varying levels of peroxidase along with fixed concentration of hydrogen peroxide (0.05%) were subjected to GPC analysis. Results indicated that all the pentosans eluted as a single broad peak (). Out of all the chromatograms, T4, which represents pentosans isolated from dough incorporated with 1mg of peroxidase, had the least elution volume indicating that the molecular weight of arabinoxylan was the highest as compared to the pentosans isolated from control and other treated doughs. Increase in size of arabinoxylans would be attributed to the cross-linking between the arabinoxylan molecules in presence of peroxidase.[Citation9] Pentosans isolated from dough incorporated with 2 mg peroxidase showed slight increase in size but it was much lesser than that of pentosans isolated from 1 mg peroxidase incorporated dough. As explained earlier, it may be due to radical catalyzed break down of the cross-linked arabinoxylan network at high dosage of peroxidase.[Citation29,Citation33]
Figure 1 Properties of arabinoxylans isolated from control and peroxidase treated doughs. (a) Elution profile of the purified pentosans; (b) relative viscosity of purified pentosans; (c) UV spectra of purified arabinopxylans. T1- Control; T2- 0.05% H2O2; T3- 1 mg peroxidase; T4- 1 mg peroxidase + 0.05 H2O2; T5- 2 mg peroxidase + 0.05 H2O2.
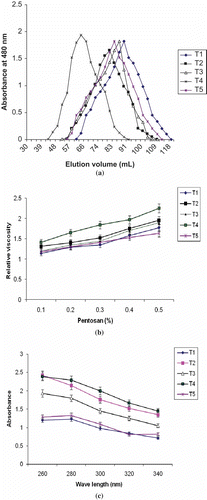
Effect of Peroxidase on Relative Viscosity of Arabinoxylans
The viscosity of the purified arabinoxylans isolated from doughs treated with peroxidase and hydrogen peroxide are shown in . The pentosans from native samples showed low relative viscosity compared to arabinoxylans isolated from peroxidase treated doughs. Among the peroxidase treated doughs, the pentosans isolated from dough incorporated with peroxidase (1 mg) and hydrogen peroxide showed relatively more viscosity compared to control. The viscosity of pentosans depends on the size of arabinoxylans, degree of substitution of arabinosyl residues along with the xylan back bone, and content of ferulic acid molecules in the arabinoxylan fractions.[Citation34] Similar results were also obtained earlier in wheat arabinoxylans treated with peroxidase.[Citation12,Citation13] Increase in relative viscosity could be attributed to cross-linking of pentosans in the peroxidase treated dough and results in increase in molecular weight of arabinoxylans which in turn leads to increase in relative viscosity of the pentosans. However, further increase in peroxidase (2 mg) concentration resulted decrease in relative viscosity compared to pentosans isolated from dough incorporated with 1 mg of peroxidase. The decrease in viscosity might result from the oxidative degradation of carbohydrate chains taking place competitively with the cross-linking reaction. Such degradation of polysaccharides is known to be caused by hydroxyl radicals formed from hydrogen peroxide and other reducing agents.[Citation8]
Effect of Peroxidase on Amount and Chemical Composition of Arabinoxylans
Comparison of the amount and composition of purified arabinoxylans from doughs treated with varying levels of peroxidase gives information on the mechanism of action of peroxidase. Notably, changes in ferulic acid, proteins, and arabinose/xylose ratio in the isolated pentosans are of interest, since peroxidase could affect all these components. Amount of pentosans increased in treated doughs compared to control dough (). Increase in pentosans could be due to cross-linking of water-soluble arabinoxylans, which leads to formation of more amounts of water-insoluble arabinoxylans[Citation7] in treated dough as it was extracted by barium hydroxide. Uronic acid content was highest in pentosans extracted from peroxidase (1 mg) treated dough and lowest in control dough (). Significant changes are observed in protein and ferulic acid contents among the pentosan fractions extracted from dough samples. Pentosans contain higher levels of proteins and ferulic acids in doughs incubated with peroxidase (1 mg) and hydrogen peroxide.
Table 2 Chemical composition of purified arabinoxylans from control and enzyme treated doughs
Peroxidase has been shown to cross-link arabinoxylans and the formation of ferulic acid dimers.[Citation7,Citation30] Proteins have also been suggested as targets for cross-linking to arabinoxylans by peroxidase.[Citation6] Increase in ferulic acid and proteins in pentosans from dough treated with peroxidase could be due to cross-linking between arabinoxylans or between arabinoxylan and proteins and leads changes in chemical properties of pentosans.
Analysis of sugar composition by GLC revealed that changes in the contents of arabinose and xylose between the pentosan fractions isolated from doughs of varying levels of peroxidase along with hydrogen peroxide (). The changes in the arabinose to xylose ratio were prominent among the pentosan fractions. Higher arabinose to xylose (A/X) ratio was observed in the pentosan fractions isolated from dough having peroxidase (1 mg). Increased A/X ratio indicated their higher degree of substitution in arabinoxylan fractions due to the action of peroxidase. Degree of substitution of arabinoxylans could influence solubility of arabinoxylans, viscosity, and distribution of water in dough and leads to changes in textural and chemical properties of dough.[Citation6,Citation13]
Hilhorst et al.[Citation7] have shown that peroxidase had no significant effect on the composition of water extractable pentosans, while it had significant effect on the water unextractable pentosans with respect to arabinose to xylose ratio and yield levels are higher from wheat dough treated with peroxidase. These results also indicated that by the appropriate combination of hydrogen peroxide and peroxidase in dough leads to isolation of arabinoxylans with higher degree of substitution with higher amount of ferulic acid and protein contents.
Effect of Peroxidase on UV-Absorption Spectra of Purified Arabinoxylans
The UV absorption spectra of the pentosans from different dough samples are shown in . Pentosans extracted from peroxidase treated doughs had higher absorption intensity at 280 nm than from control dough. The increased absorption could be due to increased amount of protein associated with pentosans resulted due to cross-linking of pentosan components with proteins. Patil et al.[Citation26] also found increased absorption intensity at 280 nm when dough is treated with oxidative agents and the increase was attributed to protein component. The absorption peak at 320 nm is due to the presence of ferulic acid and it was more pronounced in pentosans isolated from peroxidase (1 mg) treated dough compared to control dough (results not shown). Earlier, Yeh et al.[Citation35] have shown that increase in absorption at 280 and 320 nm was due to increased content of associated proteins and ferulic acid with pentosans fraction.
Thus, the results indicate that pentosans isolated from peroxidase treated dough had more amounts of protein and ferulic acid, and also showed high absorption at 280 and 320 nm. Increase in contents of protein and ferulic acid in pentosan fraction give a possible indication of formation of cross-linking of protein and pentosans through ferulic acid.
CONCLUSIONS
Treatment of dough with peroxidase had improved the whole wheat flour dough handling properties by decreasing the adhesiveness of dough. The improvements in dough properties are mainly responsible due to the changes in the properties of the constituents of the treated dough. Increase in the molecular weight of purified arabinoxylans may be one of the factors responsible for the improved dough handling properties. UV spectral studies and increase in protein and ferulic acid contents in purified arabinoxylans indicate that the molecular weight increase in arabinoxylans may be due to ferulic acid mediated cross-linking between arabinoxylans as well as protein and arabinoxylans.
ACKNOWLEDGMENTS
The authors would like to thank R. Ravi, Department of Sensory Science, CFTRI, Mysore, for helping in the analysis of TPA and statistical analysis. RBS acknowledges Council of Scientific and Industrial Research, New Delhi, India, for the award of research fellowship.
FUNDING
The authors would also like to thank the Department of Atomic Energy (No. 2007/37/30/BRNS) Mumbai, for financial assistance.
REFERENCES
- Hemalatha, M.S.; Bhagwat, S.G.; Salimath, P.V.; Prasada Rao, U.J.S. Enhancement of soluble dietary fibre, polyphenols, and antioxidant properties of chapatis prepared from whole wheat flour dough treated with amylases and xylanase. Journal of the Science of Food and Agriculture 2012, 92, 764–771.
- Pomeranz, Y. Composition and functionality of wheat-flour components. In: Wheat Chemistry and Technology; Pomeranz, Y. Ed.; American Association of Cereal Chemists Inc.: St.Paul, MN, 1978; 585–674.
- Pritchard, J.R.; Lawrence, G.J.; Larroque, O.; Li, Z.; Laidlaw, H.K.C.; Morell, M.K.; Rahman, S. A survey of β-glucan and arabinoxylan content in wheat. Journal of the Science of Food and Agriculture 2011, 91, 1298–1303.
- Kim, S.K.; D‘Appolonia, B.L. Bread staling studies III. Effect of pentosans on dough, bread and bread staling rate. Cereal Chemistry 1977, 54, 225–229.
- Saxena, D.C.; Salimath, P.V.; Haridas Rao, P. Indian wheat cultivars: Their carbohydrate profile and its relation to tandoori-roti quality. Food Chemistry 2000, 68, 185–190.
- Labat, E.; Morel, M.H.; Rouau, X. Effects of laccase and manganese peroxidase on wheat gluten and pentosans during mixing. Food Hydrocolloids 2000, 15, 47–52.
- Ponzio, N.R.; Ferrero, C.; Puppo, M.C. Wheat varietal flours: Influence of pectin and DATEM on dough and bread quality. International Journal of Food Properties 2013, 16, 33–44.
- Bahal, G.; Sudha, M.L.; Ramasarma, P.R. Wheat germ lipoxygenase: Its effect on dough rheology, microstructure, and bread making quality. International Journal of Food Properties 2013, 16 (8), 1730–1739.
- Pescador-Piedra, J.C.; Garrido-Castro, A.; Chanona-Pérez, J.; Farrera-Rebollo, R.; Gutiérrez-López, G.; Calderón-Domínguez, G. Effect of the addition of mixtures of glucose oxidase, peroxidase and xylanase on rheological and breadmaking properties of wheat flour. International Journal of Food Properties 2009, 12, 748–765.
- Van Oort, M. Oxidases in bread making. International Food Ingredients 1996, 4, 42–45.
- Hilhorst, R.; Gruppen, H.; Orsel, R.; Laane, C.; Schols, H.A.; Voragen, A.G.J. Effects of xylanase and peroxidase on soluble and insoluble arabinoxylans in wheat bread dough. Journal of Food Science 2002, 67, 497–506.
- Ciacco, C.F.; D’Appolonia, B.L. Characterization of pentosans from different wheat flour classes and of their gelling capacity. Cereal Chemistry 1982, 59, 96–99.
- Izydorczyk, M.S.; Biliaderis, C.G. Cereal arabinoxylans: Advances in structure and physico-chemical properties. Carbohydrate Polymers 1995, 28, 33–48.
- Manu, B.T.; Prasada Rao, U.J.S. Role of peroxidase and H2O2 in cross-linking ofgluten proteins. Journal of Food Biochemistry 2011, 35, 1695–1702.
- Rouau, X.; Moreau, D. Modification of some physico-chemical properties of wheat flour pentosans by an enzyme complex recommended for baking. Cereal Chemistry 1993, 70, 626–632.
- Schooneveld-Bergmans, M.E.F.; Dignum, M.J.W.; Grabber, J.H.; Beldman, G.; Voragen, A.G.J. Studies on the oxidative cross-linking of feruloylated arabinoxylans from wheat flour and wheat bran. Carbohydrate Polymers 1999, 38, 309–317.
- Izydorczyk, M.S.; Biliaderis, C.G.; Bushuk, W. Oxidative gelation studies of water-soluble pentosans from wheat. Journal of Cereal Science 1990, 11, 153–169.
- Nandini, C.D.; Salimath, P.V. Structural features of arabinoxyalns from sonalika variety of wheat comparison between whole wheat flour and wheat bran. Journal of the Science of Food and Agriculture 2003, 83, 1297–1302.
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Analytical Chemistry 1956, 28, 350–356.
- Muralikrishna, G.; Ramdas Bhat, U.; Tharanathan, R.N. Functional characteristics of the mucilaginous polysaccharides derived from cow pea (Vignasinensis), Blackgram (Phaseolus mungo), and linseed (Linumusitatissimum). Starke 1987, 39, 107–109.
- Dische, Z. A new specific colour reaction of hexuronic acids. Journal of Biological Chemistry 1947, 167, 189–198.
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry 1951, 193, 265–271.
- Sawardekar, J.S.; Slonekar, LS.; Jeanes, A. Quantitative determination of monosaccharides as their alditol acetates by gas liquid chromatography. Analytical Chemistry 1967, 59, 430–435.
- Fausch, H.; Kuendig, W.; Neukom, H. Ferulic acid as a component of glycoprotein from wheat flour. Nature 1963, 199, 287–288.
- Shyama Prasad Rao, R.; Muralikrishna, G. Non-starch polysaccharide-phenolic acid complexes from native and germinated cereals and millet. Food Chemistry 2004, 84, 527–531.
- Patil, S.K.; Tsen, C.C.; Lineback, D.R. Water soluble pentosans of wheat flour. I. Characterization of pentosans and glycoproteins from wheat flour and dough mixed under various conditions. Cereal Chemistry 1975, 52, 57–69.
- Steel, G.D.; Torrie, J.H. Principles and Procedures of Statistics, McGraw Hill: New York, USA, 1980.
- Daniela, F.O.; Viviana, O.S. Textural characterization of lasagna made from organic whole wheat. International Journal of Food Science and Technology 2006, 41, 63–69.
- Selinheimo, E.; Kruus, K.; Buchert, J.; Hopia, A.; Autio, K. Effects of laccase, xylanase, and their combination on the rheological properties of wheat dough. Journal of Cereal Science 2006, 43, 152–159.
- Neukom, H.; Markwalder, H.U. Oxidative gelation of wheat flour pentosans: A new way of cross-linking polymers. Cereal Foods World 1978, 23, 374–375.
- Jackson, G.M.; Hoseney, R.C. Fate of ferulic acid in overmixed wheat flour doughs: Partial characterization of a cysteine-ferulic acid adducts. Journal of Cereal Science 1986, 4, 87–95.
- Piber, M.; Koehler, P. Identification of dehydro-ferulic acid-tyrosine in rye and wheat: Evidence for a covalent cross-link between arabinoxylans and proteins. Journal of Agricultural and Food Chemistry 2005, 53, 5276–5284.
- Hilhorst, R.; Dunnewind, B.; Orsel, R.; Stegeman, P.; Van Vliet, T.; Gruppen, H.; Schols, H.A. Baking performance, rheology, and chemical composition of wheat dough and gluten affected by xylanase and oxidative enzymes. Journal of Food Science 1999, 64, 808–813.
- Courtin, C.M.; Delcour, J.A. Arabinoxylans and endoxylanases in wheat flour bread making. Journal of Cereal Science 2002, 35, 225–243.
- Yeh, Y.F.; Hoseney, R.C.; Lineback, D.R. Changes in wheat flour pentosans as a result of dough mixing and oxidation. Cereal Chemistry 1980, 57, 144–148.
