Abstract
Tea protein has received much attention due to its potential health functions. This article studied the molecular weight, foaming capacity, foam stability, emulsifying capacity, emulsion stability, and oil absorption of tea protein prepared from tea residue by alkali extraction. The results showed that tea protein contained two components with different molecular weights (1.26 × 106 ± 1.3 × 104 and 2.4 × 104 ± 1.1 × 103). The content of the lower molecular component in tea protein was higher than the higher molecular one. When the concentration of tea protein was 4 mg/mL, its sparkling ability was satisfied, while the foaming capacity and foam stability was the best, respectively, at the conditions of pH 9 and 7. The emulsifying capacity was 62 ± 2% as well as 99 ± 1% of emulsion stability, and oil absorption was 170 ± 7%.
INTRODUCTION
Tea is one of the most consumed beverages in the world. Numerous experimental and epidemiological studies suggest that tea may contribute to reducing the risk of cardiovascular diseases and cancers, and has other advantageous effects on health, such as antioxidant and antibacterial activity, body weight control, oral health promotion, and solar ultraviolet protection.[Citation1CitationCitation−Citation4] Previous research has mainly focused on water-soluble components (especially polyphenols, polysaccharides, etc.) that were regarded as the most significant constituents for health beneficial effects in tea,[Citation3,Citation5Citation−Citation7] while little attention has been paid to water-insoluble ones.
Moreover, in the tea industry, a certain quantity of tea is used for the extraction of tea polyphenols and for the production of instant tea powder or tea drinks. With the recent increase in tea consumption, a large amount of tea residue was produced. Like other biomass residues, tea residue wastes represent an unused resource and pose environmental pollution problems. Although the usages of tea residues as forage,[Citation8] fertilizer,[Citation9] and adsorbent for heavy metals[Citation10,Citation11] have been reported, most of them were still disposed of without further utilization.
Researches indicated that tea residues contained around 21–28% of crude protein in dry basis.[Citation12] Tea protein, which mainly contains 82% glutelin and 13% prolamin, is water-insoluble. So far, proteins from non-animal sources have attracted more and more attention in the food industry. Soy protein is probably the most common ingredient due to its highly nutritious and desirable functional properties.[Citation13] Other proteins, like corn protein[Citation14] and seaweed protein[Citation15] have also been studied for their potential food applications. Interestingly, tea protein has recently been found to be effective in decreasing blood lipid of hyperlipidemia[Citation16] and eliminating peroxide free radicals;[Citation17] therefore, it is not only a nutrient component but also a potential functional additive for the food industry.[Citation13] In view of its good functional activities, at present, some researchers have focused on the extraction and purification of tea protein.[Citation12,Citation18] However, few research reports have been done on the components or functional properties of tea protein. As a consequence, the applications of tea protein are still limited. Thus, the aim of this study was to investigate the molecular weight and functional properties of tea protein extracted from tea residue.
MATERIALS AND METHODS
Materials and Equipment
Tea residue (25.14% protein, w/w, dry weight), the remainder after the production of instant tea, was obtained from Zhejiang Tata Tea Technology Co., Ltd., Zhejiang, China. Other chemicals used in this study were of analytical grade and were purchased from the East China Pharmaceutical Co., Ltd., Hangzhou, China. All equipment, including a grinder, water-bath oscillator, centrifuge, Kjeldahl nitrogen device, and spray drier, were conventional ones.
Preparation of Tea Protein
After drying and grinding, the raw material was extracted using an alkali extraction method, with conditions as follows: alkali liquor concentration of 0.05 mol/L, solid-liquid ratio of 1:30, and extracting at 80°C for 60 min. The extracts were centrifuged to remove insolubles. The collected supernatant, after acid precipitation and desalination, was dissolved in distilled water. Finally, a dry powder of tea protein was obtained from the solution by spray drying.[Citation18]
Molecular Weight Determination of Tea Protein
Gel filtration chromatography was used for separation of components in tea protein and for analysis of molecular weight. Superdex 200 10/300 GL was packed into a column, and then equilibrated with glycine-NaOH buffer (50 mmol/L, pH 10.0, containing 0.15 mol/L NaCl). After concentration by ultrafiltration, 0.5 mL of protein solution was added onto the top of the gel. The column was run with the same buffer of equilibration at a flow rate of 0.5 mL/min. Eluent was divided and fractions were collected separately. The molecular mass standards used were transferrin (8.0 × 104 Da), bovine serum albumin (BSA, 6.7 × 104 Da), albumin egg (4.5 × 104 Da) and ribonuclease A (RNase A, 1.37 × 104 Da), which were dissolved with glycine-NaOH buffer (50 mmol/L, pH 10.0, containing 0.15 mol/L NaCl). The concentrations of BSA and the other three solutions were 8 and 5 mg/mL, respectively. Void volume (Vo) was determined using Dextran Blue 2000. The distribution coefficient (Kav) for a given protein was calculated according to its retention time. Calibration curve was generated by linear regression of Kav values (Y-axis) against their respective log Mr values (X-axis). Mr is the relative molecular weight.
A sample solution of tea protein in 3 mg/mL was dissolved with glycine-NaOH buffer (pH 10.0, containing 0.15 mol/L NaCl). The solution was centrifuged at the speed of 8900× g for 30 min at 4°C and then the supernatant was collected for gel filtration chromatography analysis. The gel filtration chromatography procedure of samples was similar to that of the standards. With elution volume value (Ve) as an independent variable, relative average molecular weight of the routine sample was calculated according to the calibration curve.
Foaming Capacity (FC) and Foam Stability (FS)
FC and FS were determined according to the method described by Nath and Rao[Citation19] with slight modifications. First, 2 mg/mL solutions of tea protein at a certain pH (ranging from 1 to 9) were homogenized at 17,400× g for 2 min using a homogenizer. Both the initial solution volumes and the foam volumes after homogenization were recorded. The FC was expressed as the ratio of the foam volume to the initial solution volume. The calculation was described as follows:
A similar procedure was used to determine the FS, but the samples were allowed to stand for 10 min at room temperature and the residue foam volume was measured. The FS was measured as:
Emulsifying Capacity (EC) and Emulsion Stability (ES)
According to the method by Chau and Cheung,[Citation20] aqueous dispersions of 1, 2, 3, 4, 5, and 6 mg/mL of protein samples were separately prepared in centrifuge tubes with 8 mL of mixture (4 mL of distilled water and 4 mL of vegetable oil). The dispersions were homogenized at 17,400× g for 5 min using a homogenizer, and then were centrifuged at 200× g for 5 min in a centrifuge. EC was the ratio of height of the emulsified layer to the total height of liquid in the centrifuge tube, which can be expressed as:
To determine the ES, emulsions prepared by the above procedure were heated at 80°C for 30 min, cooled to room temperature, and centrifuged at 130× g for 5 min. Both the height of the initial emulsified layer before heating and the height of the remaining emulsified layer were recorded. ES was calculated as follows:
Oil Absorption (OA)
The method of Bencini[Citation21] was used with slight modifications. To start, 0.500 g of tea protein sample and 6 mL of vegetable oil (soybean oil) were added into a centrifuge tube. After stirring for 2 min and standing for 30 min, the mixture was centrifuged at 800× g for 20 min to remove free oil. The amount of oil absorbed by 1 g of tea protein represents the OA:
Statistical Analysis
All of the experiments were carried out in triplicate. Treatment effect was analyzed using analysis of variance and Duncan multiple range test to determine the differences between treatments. Each point was the mean of replicate experiments. All statistical methods were performed by the statistical software—Statistical Package for Social Sciences 13.0 (SPSS, Chicago, IL, USA). Values of P < 0.05 and P < 0.01 were considered to be significant and very significant, respectively.
RESULTS AND DISCUSSION
Molecular Weight of Tea Protein
Molecular weight is a very important parameter characterizing the physical properties of a biomacromolecule.[Citation3] The calibration curve for proteins by gel filtration chromatography on Sephadex G-200 10/300 GL was shown in . The calibration curve equation of proteins was: Y = -0.29861x + 1.80542 (R2 = 0.99992). The calibration curve showed good linearity between Kav values against log Mr values over the calibration ranges. A routine sample calculation was made by the comparison of its Ve value with Kav value of the standard curve, and relative molecular weight was calculated according to the above equation.
Figure 1 Molecular weight determination of tea protein by gel filtration chromatography: (a) calibration curve; (b) chromatogram.
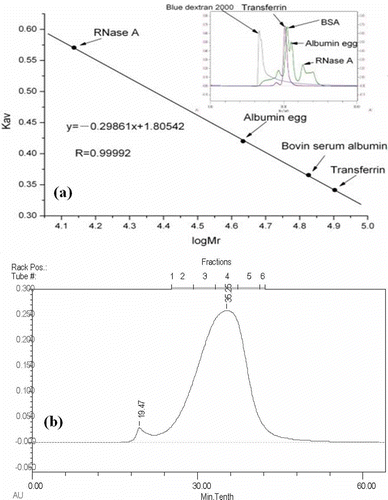
Through gel filtration chromatography, molecules can be separated according to their size. showed a chromatogram of gel filtration chromatography of tea protein sample. As can be seen from this figure, there were two peaks and they were separated well under chromatogram conditions. The current chromatogram implied that there were two components with different molecular weights: 1.26 × 106 ± 1.3 × 104 and 2.4 × 104 ± 1.1 × 103, respectively, in tea protein. The molecule weights were calculated by using a calibration curve. Meanwhile, the content of the component (2.4 × 104 ± 1.1 × 103) was higher than that of the other one (1.26 × 106 ± 1.3 × 104).
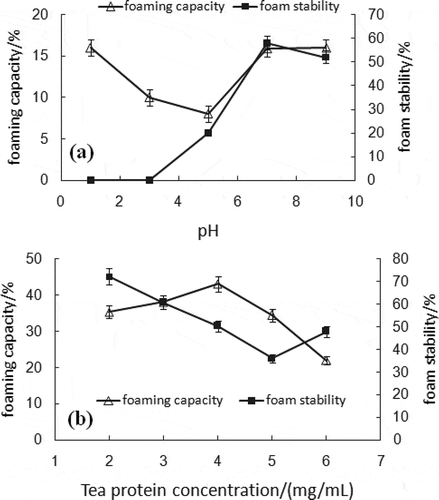
Effects of pH on FC and FS
Two different properties of foaming, effectiveness of gas encapsulation (FC) and lifetime of the foam (FS), are two crucial parameters in the characterization of the functional properties of protein. The pH, ranging from 1 to 9, was designed to investigate the effects on these foaming properties. Results presented in showed that the pH had a significant influence on FC and FS of tea protein. At pH 9, the FC of tea protein was best, while at a pH of 3–5, the values were low. Meanwhile, the pH of the protein system also affected the FS significantly. The FS of tea protein was poor when below a pH of 3. It indicated that foam disappeared rapidly without stirring under this condition. With the increase in pH value, the FS of tea protein tended to improved rapidly and reached the maximum at pH 7. Further increase in pH led to a drop of the FS. Both FC and FS were low near the isoelectric point that contributes to its lower solubility at this point. It may be attributed that net charge influences the adsorption of the protein at the air-water interface, as net charge increases, foaming property enhances.[Citation22]
Effects of Tea Protein Concentration on FC and FS
Under the above developed pH condition, the effect of tea protein concentration on the FC and FS was examined. As shown in , the FC increased when concentration was below 4 mg/mL. Excess tea protein caused a decrease in FC value. The possible reason was mainly that the new stretched foam interface would be covered rapidly by mass protein with high diffusion speed, and then both the foam coarsening and the FS were decreased. Furthermore, it has been reported that the FC of protein, on a great degree, depends on its solubility. The current study showed that the tea protein solubility was best when pH was 7, consistent with the FC results.
Additionally, the change trend of FS was opposite to that of FC. Results indicated that when the tea protein concentration changed from 2 to 6 mg/mL, the FS value decreased quickly and increased slightly above 5 mg/mL. The maximum FS value was observed at 2 mg/mL. Thus, the FC of tea protein was relatively low. When the concentration of tea protein was 4 mg/mL, the sparkling ability was satisfied.
EC and ES of Tea Protein
Another two critical functional characteristics of proteins are EC and ES, which affect the practical application of proteins in industrial production. The protein is exposed to the mixing of the oil and aqueous phase, and last but not least, to the creation of the oil-water interface. Proteins are amphiphilic molecules, possessing both hydrophilic and lipophilic groups, and they have been used for their surfactant properties in many fields to stabilize oil-in-water emulsions. This also makes them susceptible to structural changes when exposed to the interfaces. Proteins can reduce tension at the water-oil interface and help prevent coalescence.[Citation23] Generally, emulsifying properties of proteins are related to the aqueous solubility. Compared to soybean protein, the aqueous solubility of tea protein was lower and the amount of soluble protein was less at the same protein concentration. Besides, molecular structures of proteins determine their emulsifying properties. Proteins with more compact structure possess poorer emulsifying properties. Furthermore, the ratios of hydrophilic and lipophilic groups in protein also affect the interaction between products in emulsions.
The concentrations of tea protein ranging from 1 to 6 mg/mL were designed to investigate the emulsifying properties (EC and ES) of tea protein. showed the results of EC and ES parameters at 1 mg/mL (data under other concentrations not shown). The values of EC and ES were 62.33 ± 1.85% and 98.85 ± 1.09%, respectively. As presented, the emulsifying properties of tea protein were satisfied, especially the ES. However, it was worth noting that concentration of 1 mg/mL was still too high for tea protein emulsification. A more suitable concentration value needs to be further investigated. Many factors affected emulsifying properties of protein. Emulsifiers used in the food industry require not only good EC but also satisfied ES value. Thus, the emulsifying properties of tea protein were not inferior to other kinds of proteins, considering both EC and ES parameters. It indicated that tea protein has potential for applications in various food products, like meat, ice cream, cake, bread, and other baked goods.
Table 1 EC, ES, and OA of tea protein
OA of Tea Protein
The OA mainly reflects the combined ability between protein and free fat. As for flavor foods, OA of the protein is an important functional feature. It could improve fat absorption and retention in food and reduce fat loss in process, thus improving the palatability and flavor of the food. The OA of tea protein was examined by using soybean oil (). As presented in the table, the OA of tea protein reached 169.86 ± 6.86%. Compared to soybean protein, tea protein possesses stronger OA ability. It was likely due to combined ability between lipophilic groups of protein and oxhydryl of lipids. It indicated that there were more hydrophobic groups present on the surface of tea protein. Strong OA makes tea protein more suitable for applications in meat, fish, and other high-fat products.
CONCLUSION
In this study, tea protein was extracted from tea residue effectively by alkali extraction method. This one-time alkali extraction rate of tea protein reached 77.4%, which was higher than normal methods. Present results indicated that tea protein contained two components with different molecular weights, 1.26 × 106 ± 1.3 × 104 and 2.4 × 104 ± 1.1 × 103, respectively. The content of the latter one in tea protein is higher than the former. The tea protein has good FC, FS, EC, ES, and OA properties. When the concentration of tea protein was 4 and 2 mg/mL, its FC and FS were the best, respectively, while its sparkling ability was satisfied at the concentration of 4 mg/mL. Moreover, the FC and FS were the best, respectively, at the condition of pH 9 and 7. The EC was 62.33 ± 1.85%, while ES was 98.85 ± 1.09%. The OA was 169.86 ± 6.86%. The physicochemical properties of tea protein indicate that tea protein has a good application prospect. The structure of tea protein can be modified to yield relative derivatives. Molecular biology methods, like changing the sequence of amino acid by specific enzyme, changing the space structure of protein by extrusion texturization modification, and changing the covalent structure of protein by introducing or removing chemical groups, can be used to modify tea protein to improve its functional properties. Modified tea protein can be used to develop blood substitutes, plasma expanders, and anti-tumor agents. Just as tea polysaccharides, tea protein will be another important macromolecule in tea.
REFERENCES
- McKay, D.L.; Blumberg, J.B. The role of tea in human health: An update. Journal of the American College of Nutrition 2002, 21, 1–13.
- Cabrera, C.; Artacho, R.; Gimenez, R. Beneficial effects of green tea—A review. Journal of the American College of Nutrition 2006, 25, 79–99.
- Chen, X.Q.; Lin, Z.; Ye, Y.; Zhang, R.; Yin, J.F.; Jiang, Y.W.; Wan, H.T. Suppression of diabetes in non-obese diabetic (NOD) mice by oral administration of water-soluble and alkali-soluble polysaccharide conjugates prepared from green tea. Carbohydrate Polymers 2010, 82, 28–33.
- Pekal, A.; Drozdz, P.; Pyrznska, K. Comparison of the antioxidant properties of commonly consumed commercial teas. International Journal of Food Properties 2012, 15, 1101–1109.
- Jiao, H.L.; Ye, P.; Zhao, B.L. Protective effects of green tea polyphenols on human HepG2 cells against oxidative damage of fenofibrate. Free Radical Biology and Medicine 2003, 35, 1121–1128.
- Chen, X.Q.; Ye, Y.; Cheng, H.; Jiang, Y.W.; Wu, Y.L. Thermal effects on the stability and antioxidant activity of an acid polysaccharide conjugate derived from green tea. Journal of Agricultural and Food Chemistry 2009, 57, 5795–5798.
- Chen, X.Q.; Wang, Y.F.; Wu, Y.L.; Han, B.Y.; Zhu, Y.J.; Tang, X.L.; Sun, Q.L. Green tea polysaccharide-conjugates protect human umbilical vein endothelial cells against impairments triggered by high glucose. International Journal of Biological Macromolecules 2011, 49, 50–54.
- Yoshimi, S.; Yasuhito, F.; Hidenori, K.; Nobuyoshi, M.; Takashi, S.; Takanori, S. Tea-residue silage and method for preparing and storing the same. Japan Patent No. 2002272385; 2001.
- Chen, L.Y. Study on the process of “organic-inorganic compound fertilizer” made from tea residue and effect of the fertilizer on tea quality and soil environment. Masters Thesis, Zhejiang University, Hangzhou, China, 2003.
- Mahvi, A.H.; Naghipour, D.; Vaezi, F.; Nazmara, S. Teawaste as an adsorbent for heavy metal removal from industrial wastewaters. American Journal of Applied Sciences 2005, 2, 372–375.
- Amarasinghe, B.M.W.P.K.; Williams, R.A. Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chemical Engineering Journal 2007, 132, 299–309.
- Shen, L.Q.; Wang, X.Y.; Wang, Z.Y.; Wu, Y.F.; Chen, J.S. Studies on tea protein extraction using alkaline and enzyme methods. Food Chemistry 2008, 107, 929–938.
- Wang, X.S.; Tang, C.H.; Li, B.S.; Yang, X.Q.; Li, L.; Ma, C.Y. Effects of high-pressure treatment on some physicochemical and functional properties of soy protein isolates. Food Hydrocolloids 2008, 22, 560–567.
- Li, X.X.; Han, L.J.; Chen, L.J. In vitro antioxidant activity of protein hydrolysates prepared from corn gluten meal. Journal of the Science of Food and Agriculture 2008, 88, 1660–1666.
- Fleurence, J. Seaweed proteins: Biochemical, nutritional aspects and potential uses. Trends in Food Science & Technology 1999, 10, 25–28.
- Huo, P.; Huang, G.R.; Zhang, X.H.; Xia, Y.; Jiang, J.X.; Fu, J.Y.; Zhang, K.C. Studies on lipid-depressing function of water insoluble tea protein. Journal of Tea Science 2005, 25, 95–99.
- Wang, H.X.; Hu, C.Y. Study on modification and functional properties of tea dregs protein. Food Science 2005, 26, 135–140.
- Zhang, S.K.; Zhu, K.X.; Lu, C.; Zhou, H.M.; Wang, B.; Peng, W.; Jiang, Y.L. Producing tea protein product. China Patent No. 102302083; 2012.
- Nath, J.P.; Rao, M.S.N. Functional-properties of guar proteins. Journal of Food Science 1981, 46, 1255–1259.
- Chau, C.F.; Cheung, P.C.K. Functional properties of flours prepared from three Chinese indigenous legume seeds. Food Chemistry 1998, 61, 429–433.
- Bencini, M.C. Functional-properties of drum-dried chickpea (Cicer-Arietinum L.) flours. Journal of Food Science 1986, 51, 1518–1521.
- Naqash, S.Y.; Nazeer, R.A. In vitro antioxidant and antiproliferative activities of bioactive peptide isolated from Nemipterus Japonicus backbone. International Journal of Food Properties 2012, 15, 1200–1211.
- Cano-Medina, A.; Jimenez-Islas, H.; Dendooven, L.; Herrera, R.P.; Gonzalez-Alatorre, G.; Escamilla-Silva, E.M. Emulsifying and foaming capacity and emulsion and foam stability of sesame protein concentrates. Food Research International 2011, 44, 684–692.