Abstract
The characterization of thirty-one honey samples using melisopalynological and physicochemical characteristics as diastase activity, invertase activity, pH, moisture, electrical conductivity, hydroxymethylfurfural content, and color, as well as phenolic and flavonoid content, mineral composition, and sugar content was performed. All the samples were produced in the Eurosiberian area of the Iberian Peninsula. Relationships between certain pollen types and the selected physicochemical parameters have been found using a Spearman rank correlation analysis. The principal component analysis of data set was an important tool for the determination of the variables used to characterize this honey type.
INTRODUCTION
Eucalyptus honeys are an important unifloral honey commercialized worldwide. In Europe the main areas for this honey type production are in Italy, Spain, and Portugal. Outside Europe, large amounts of Eucalyptus honey are produced in the countries where the genera is native (Australia and New Zealand) or, as in Europe, were widely introduced (North and South Africa, Israel, Central and Southern America).[Citation1]
The bees use about 800 Eucalyptus species to obtain this unifloral honey type that is labelled as eucalyptus honey in the European market, without any specific mention regarding the plant species from which the samples were produced. However, Australian honeys from different Eucalyptus species can be found in the market.[Citation2] This is due honeys from different species of Eucalyptus trees displaying wide variations in the sensorial characteristics.[Citation3]
The aroma of the eucalyptus honey has long been investigated,[Citation4] including compounds as hydroxycetones, sulfur compounds, diketones, norisoprenoids, alkanes, aliphatic compounds, and monoterpenes, as characteristic compounds in their composition.[Citation5,Citation6] Flavonoids are other important group found in different eucalyptus honey types and has been used for their differentiation.[Citation2,Citation7] Hydrogen peroxide-dependent antibacterial activity was found in honey derived from Eucalyptus marginata from Western Australia; although the level of antibacterial activity varied widely among samples during storage, the floral source and region were clearly important in the production of active honey.[Citation8] Other studies applied 2-DZ technique to discriminate between different honey types.[Citation9] The influence of geographical origin appears to be relevant in the case of eucalyptus honeys.
The EU Directive for honey, introduce that the product name of honey may be supplemented by information referring to the floral or vegetable origin, if the product comes wholly or mainly from the indicated source and possesses the organoleptic, physicochemical, and microscopic characteristics of the source and the regional, territorial or topographical origin, if the product comes entirely from the indicated source.[Citation10] European eucalyptus honeys are produced mainly from Eucalyptus camaldulensis and Eucalyptus globulus. It must be emphasised that the UE Directive provides some exceptional criteria for Eucalyptus camaldulensis honey. These were about the sucrose content (maximum of 10 g 100 g−1) and the electrical conductivity (EC)(no more than 0.8 mS cm−1 for blossom honeys excepting, among others, eucalyptus honey).[Citation10] Although there are several studies in which the characteristics of Eucalyptus camaldulensis honeys were studied,[Citation1,Citation11−Citation14] there is still little information on the specific characteristics of Eucalyptus globulus honeys.
Eucalyptus globulus Labill. (Myrtaceae) has been introduced in the coast of Eurosiberian area of Europe during the late 19th and 20th century. Fifty-three percent of the worldwide area of Eucalyptus globulus is located in this territory of the Iberian Peninsula (22% in Spain and 31% in Portugal).[Citation15] This plant became first known as an important resource used by the pulp and paper industry due to its high quality cellulosic fibers. Recently it was demonstrated that the plant has been also used for medicinally essential oil[Citation16] and honey production. In this territory, Eucalyptus globulus blooms in winter, between November and March, achieving a high yield in nectar secretion, so an important quantity of unifloral honey could be produced. The honey was described with amber-colored, floral aroma, and waxy smell of medium intensity and low persistence with sweet and slightly acidic flavor.[Citation17]
The Eurosiberian phytosociological region of the Iberian Peninsula provides optimal weather conditions for Eucalyptus globulus crops. However, Eucalyptus camaldulensis is widely spread in the Mediterranean region in which blooming occurs in summer. Both regions have clearly different flora and climatic production conditions therefore a strong influence on the particularities of the final product was expected, since the composition and properties depend on the floral and ecological origin of honey.[Citation18] The main objective of this study was to describe the palynological and physicochemical characteristics of the Eucalyptus globulus honey produced in Europe.
MATERIALS AND METHODS
Honey Samples and Geographical Production Area
Thirty-one honey samples produced in the Northwest Iberian Peninsula were provided directly by beekeepers (). All the samples were fresh honeys collected from the Eurosiberian phytosociological area near the coast. In this area, Eucalyptus globulus was intensively planted during the twentieth century. The species occupies an important extension of the Atlantic territory as monospecific forest or mixed forest associate with Pinus pinaster. Other important bee resources in the studied area are represented by forests with Castanea sativa and Quercus robur; shrubs of Leguminosae (some Ulex, Cytisus and Genista species), Ericaceae (Erica species and Calluna vulgaris, mainly) and an important extension of natural and cultivated prairies. In any case, Eucalyptus globulus is the main bee resource in the area, so the blooming of this species completely determines the honey production for beekeepers.
Figure 1 Geographical origin of the samples. Map extracted from Rivas-Martínez et al.[Citation19]
![Figure 1 Geographical origin of the samples. Map extracted from Rivas-Martínez et al.[Citation19]](/cms/asset/3f2da0d8-cbed-4fcf-9556-5a935f2edddf/ljfp_a_790050_f0001_b.gif)
Palynological Analysis
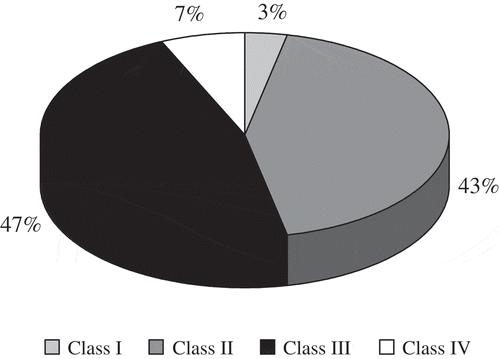
The pollen analysis was based on the method established by Louveaux et al.[Citation20] Ten grams of honey were dissolved in bi-distilled water and centrifuged at 3373 g for 10 min. The obtained sediment was used for the quantitative and qualitative analyses. Quantitative analysis was accomplished by a volumetric method. The quantity of pollen grains was counted under light microscopy at 400X. The results were expressed as the number of pollen grains per gram of honey (PG g−1) and the Maurizio’s classification was used,[Citation21] distributing the honey samples in five classes: Class I (less than 2000 PG g−1), Class II (between 2000 and 10,000 PG g−1), Class III (between 10,000 and 50,000 PG g−1), Class IV (between 50,000 and 100,000 PG g−1), and Class V (more than 100,000 PG g−1). In addition, fungi, yeasts, and algae were quantified. These elements were indicative of honeydew (HDE).
Qualitative analysis let to perform the pollen spectra. For this a minimum of 800 pollen grains were counted in two aliquots using light microscopy (400X·or 1000X, when necessary). The results were expressed in percentages and divided into the following frequency classes: P, present pollen (lower than 1% of the pollen spectrum); R, minor pollen (between 1 and 3%); I, important pollen (between 3 and 15%); A, accompanying pollen (between 15 and 45%); and D, dominant pollen (equal to or more than 45%).
Physicochemical Analyses
In order to establish the quality of honey, physicochemical analyses (hydroxymethylfurfural content [HMF], diastase activity, EC, and moisture) were conducted using AOAC methods.[Citation22] HMF content was determined using the White spectrophotometric method. The absorbance of the solutions was determined at 284 and 336 nm with a UV-VIS spectrophotometer (Jenway 6505; Jenway LTD, Dunmow, Essex, England). Diastase activity was measured as the speed at which the diastase in buffered honey solution hydrolyses a 1% starch solution. The endpoint for this reaction was established by measuring the absorbance at 660 nm with a UV-VIS spectrophotometer until it achieved an absorbance of less than 0.235. EC was determined with a portable conductivity meter (Knick Portamess® 913 (X) Conductivity, Beuckestr, Berlin) and the results were expressed as mS cm−1. Moisture content was evaluated using a refractometer (ABBE URA-2WAJ-325; Auxilab S.L., Navarra, Spain). A pH meter (Crison micropH 2001; Crison Instruments S.A., Barcelona, Spain) was used to directly measure the pH of 5 g of honey dissolved in 25 ml of bi-distilled water. Color determination was performed by using a digital instrument from Hanna (HANNA C 221 Honey Color Analyzer, Rhode Island, USA). The color intensity of the analyzed samples was measured directly in a cuvette of 1 cm side. The results were expressed using the Pfund scale (mm Pfund). Invertase activity was determined by spectrophotometric measurement of the decomposition of 4-nitrophenyl-α-D-glucopyranoside at 400 nm, with the samples incubated at 40°C. Invertase activity was expressed as invertase number (IN).
Phenol and Flavonoid Analyses
Total phenol concentration was measured by spectrophotometry, based on the Folin-Ciocâlteu method,[Citation23] with gallic acid as phenol reference. The content was expressed as equivalent mg of gallic acid. To plot the calibration curve, a stock solution of gallic acid (0.5 mg ml−1) was prepared for dilutions. The linearity was 0.995 (R2). Each honey sample was diluted with bi-distilled water (0.1 g ml−1). Ten milliliters of bi-distilled water and 1 ml of Folin-Ciocâlteu reagent were added to 1 ml of each diluted honey sample. The mixture was gently agitated and left to rest for 2 min, and then 4 ml of Na2CO3 was added. This solution was incubated at room temperature in the dark for 1 h, and the absorbance was then read at 765 nm against a blank solution. The flavonoid content was determined using an adapted Dowd’s method,[Citation24] with quercetin as reference and the results were expressed as equivalent mg of quercetin. Different concentrations of quercetin (0.002 – 0.01 mg ml−1) were used for calibration, and the linearity was 0.998 (R2). Each honey sample was diluted in bi-distilled water (0.3 g ml−1). Two milliliters of the honey solution was mixed with 0.5 ml of AlCl3, and bi-distilled water was added for a final volume of 25 ml. The solution was left for 30 min in the dark, and the absorbance was measured at 425 nm against a blank solution.
Sugar Profile Analysis
The quantification of the sugars in the studied honey samples were performed using an ion chromatography system (Dionex ICS-3000 SP) incorporating an analytical column (Carbopac PA1; 3 250 mm), guard column, and a pulse amperometric detector.[Citation25] The samples were dissolved in water until a concentration of 10 mg l−1 was attained and finally the sugars were separated using a gradient of two mobile phases (A and B). Phase A involved ultrapure water and phase B involved 200 mM NaOH. Ten microliters of the prepared sample was injected into the loop of the chromatograph. The identified sugars by this method were fructose, glucose, sucrose, trehalose, melezitose, and maltose. The performance method was evaluated by the determination of linearity, precision, and quantification limits. The calibration curves obtained by triplicate injection of standard solutions of each sugar showed good linearity with a regression coefficients (>0.99).
Mineral Content Analysis
The mineral content of honey was determined using an atomic absorption spectrophotometer (Varian Spectra A-220 Fast Squencial; Agilent Technologies, Santa Clara, CA, USA). The identified minerals were K, Ca, Fe, Mg, Na, P, Zn, and Cu. Samples were heated and sonicated to facilitate honey homogenisation. Aliquots of 0.5 g honey were transferred into teflon-coated tubes and digested in a microwave oven (CEM MARSX press model) after adding 5 ml of 9:2 HNO3-H2O2 mixtures.[Citation26]
Statistical Analysis
For the interpretation of the data, the SPSS 17.0 Statistics software and STATGRAPHICS® Centurion XVI software were used. A descriptive analysis of the variables was carried out and the normality of data was also verified by the Kolmogorov test. The relationships among the measured variables were checked by Spearman’s rank correlation analysis. To carry out the above mentioned, the most representative pollen types of honey samples were taken into consideration, together with other microscopic variables (PG g−1, HDE g−1, HDE P−1) and physicochemical variables (HMF, diastase activity, activity invertase, pH, moisture, EC, color, phenols and flavonoids content, mineral content, and sugar content). Principal component analysis (PCA) was conducted for a data matrix reduction in order to establish which variables are the most important. A total of 13 variables were selected for this purpose: Eucalyptus, Castanea sativa, Rubus, Cytisus t., Erica, phenols, flavonoids, fructose, glucose, sucrose, diastase activity, invertase activity, and color.
RESULTS AND DISCUSSION
Pollen Analysis
The pollen richness of honey and the content of HDE elements provide valuable information about honey processing and its botanical origin. Thus, honeys such as Robinia, are characterized by low pollen content, while others, such as Myosotis honeys, have high pollen content.[Citation20] In general, the evaluated honey samples had medium-low pollen content with an average value of 18108 PG g−1 (). Ninety percent of honey samples belonged to Maurizio’s classes II and III, with a maximum value of 40751 PG g−1 (). Furthermore, the analyzed samples did not exceed 100,000 PG g−1. Similar pollen richness can be observed in some studies that describes the eucalyptus honey from the same floral origin.[Citation27,Citation28] Evaluated pollen richness in this study was clearly lower than the values reported for Eucalyptus camaldulensis honey.[Citation1] Regarding the presence of HDE elements, all samples showed a null HDE index (), corroborating the blossom origin of the honeys. The microscopical analysis of the samples showed the frequency of Metschnikowia cells as representative nectariferous yeast for this type of honey.[29]
Table 1 Descriptive statistics of quantitative variables of the palynological analysis
Concerning the pollen spectrum of honeys, a total of 70 pollen types belonging to 37 botanical families were identified (). Eucalyptus pollen was the dominant pollen, with an average value of 75% of the pollen spectra in the analyzed samples. According to the frequency classes, the pollen types Castanea sativa, Rubus, Cytisus, and Crataegus monogyna were accompanying pollens, while the important pollens were Quercus, Salix, Prunus t., Lithodora, Conium maculatum t., Trifolium t., Acacia, and various Ericaceae (Erica umbellata, Erica arborea, Erica australis, and Erica cinerea). It is worth mentioning the presence of Brassica, Echium, Plantago, and Scrophularia as rare pollens with low values in the pollen spectra but as very frequent pollen in more than 50% of the samples. In these honeys, it should also be noted, the presence of the pollen combination Castanea sativa, Rubus, Cytisus t., Quercus, Salix, and Erica in more than 90% of samples. This combination was representative for the main flora of apicultural interest in the studied area.
Table 2 Percentage of presence of pollen types and their frequency classes in the honey samples. P: minor pollen (≤1%); R: frequent pollen (1–3%); I: important pollen (3–15%); A: accompanying pollen (15–45%), and D: dominant pollen (≥45%)
Table 2 (Continued)
Table 3 Descriptive statistics of the physicochemical parameters assessed in honey
Pollen analysis is a useful tool used to differentiate the geographical origin of the product and, at this moment, it is the unique procedure for this purpose. So, the presence of the mentioned pollen combination could be useful to differentiate this honey type from other eucalyptus honeys of different geographical origins and different Eucalyptus species. In this way, the pollen spectra of eucalyptus honeys from the Mediterranean region (mainly Eucalyptus camaldulensis), frequently includes the presence of Olea europaea, Cistus ladanifer, Hedysarum coronarium, Lavandula, or Citrus. Furthermore, the presence of Echium as accompanying pollen is very common.[Citation11–Citation13,Citation30–Citation32] In eucalyptus honeys from other regions such as Australia, pollen types can also be found belonging to Angophora and Melaleuca, Euphorbiaceae, Proteaceae, along with Raphanus, Echium, and Citrus.[Citation28]
Physicochemical Analyses
Descriptive statistics for the results of the physicochemical analyses and color are shown in . The study and interpretation of enzymatic activity (diastase and invertase), together with the HMF content can be used to evaluate the quality of honey.[Citation13,Citation33,Citation34] The studied honeys had a low HMF content and complied with the limitation of the EU Directive. These were fresh honeys, provided by beekeepers that use no heating treatment for the extraction and processing the honey. Generally, diastase and invertase content had a low mean value (11.7° Gothe and 13.2 IN, respectively). Other studies showed for Eucalyptus camaldulensis honey, values higher than 13.2° Gothe and 19.1 IN.[Citation1,Citation33–Citation37] The low enzymatic content of this honey type could be related with the time of honey production in early spring. In this period, the population of the bee colony increases, with the queen bee laying a high number of eggs, therefore honeybees have to feed the brood. At this time honeybee glands produce fewer enzymes.[Citation33] In addition, the nectar secretion is very abundant and occurs fast. Honeybees forage the nectar and deposit it in the combs in a very quick process. As a consequence the enzymatic content is low due to the honeybees’ activity.
The studied honeys had average values of pH and EC in accordance with the values attributed to nectar honeys. Both physicochemical parameters were in agreement with the results reported in other studies concerning this honey type, produced from Eucalyptus globulus and Eucalyptus camaldulensis.[Citation1,Citation14,Citation31,Citation35,Citation37,Citation38] However, other authors[Citation13,Citation30] indicated higher values of EC in Eucalyptus camaldulensis honeys, this variation being recorded by the European Directive. This Directive included the Eucalyptus honey in a group whose EC may go beyond the 0.8 mS cm−1 limit.[Citation1] Nevertheless Eucalyptus globulus honey always had EC below this value.
Regarding moisture content, the studied honeys showed a mean value of 17.8%. No differences were found between the moisture of the studied samples of Eucalyptus globulus and Eucalyptus camaldulensis samples studied by other authors[Citation1,Citation12,Citation14,Citation30,Citation31,Citation35−Citation39] due to the wide moisture range (14.8–19.2%). Moisture content of the honey largely depends on the processing of the product and on its preservation. Also, the environmental conditions influence the moisture of this product, thus in warmer and drier regions the moisture of the honey is usually lower.
Regarding the color, studied honey samples presented light amber color (with an average value of 73 mm Pfund). But it is worth mentioning a variation in color from the 53 mm Pfund value (amber light), similar to some Italian eucalyptus honey[Citation1,Citation37] to 96 mm Pfund value (amber) similar to the values reported in some Moroccan honeys.[Citation36]
Recently a large number of papers about the phenol and flavonoid content in the honey were published. These are components which have a great importance due to their health benefits and their influence in the antioxidant capacity of the honey.[Citation7,Citation40] This content largely depends on the botanical origin. In the studied samples, the phenol and flavonoid content was lower than in HDE, heather and chestnut honeys produced in Northern Portugal.[Citation41] K, Ca, and Na were the best represented mineral elements in these honeys (). The other elements had a considerably lower content. The mineral fraction of the honey depends on the nutrients which have been absorbed by plants, their availability in the soil, the own soil, and the pollution of the environment.[Citation42] In relation to Na content, it should be mentioned that samples were taken from coastal areas, so the proximity of the sea could influence the Na content in the soil-plant complex and therefore, in the honey. Mineral elements seemed to be good candidates for the classification of honeys because they are stable over a long period of time and can be good indicators of the geographic origin of honey.[Citation43] Average values of the studied honey samples were similar to the eucalyptus honeys described by Fernández-Torres et al.,[Citation44] although they were slightly higher than eucalyptus honeys described by González-Miret et al.[Citation42] Other honeys from Northwest Spain, such as Rubus honeys, had a similar content.[Citation45]
Table 4 Descriptive statistics of phenol and flavonoid content, minerals, and sugars identified
Honey is composed essentially of sugars. The most abundant sugars are fructose and glucose, followed by maltose and sucrose, while trehalose was only quantified in one sample and melezitose was not detected in any of the studied honeys (). This study highlighted the low sucrose content compared with works that reported studies of Eucalyptus camaldulensis honeys.[Citation12,Citation14,Citation30,Citation35] Concerning the glucose/water and the fructose/glucose ratio, the studied eucalyptus honeys showed a slow tendency to crystallization.[Citation46]
Statistical Analysis
Spearman rank correlation analysis showed good correlation coefficients between some of the evaluated variables (). The pollen types Rubus and Castanea sativa presented a strong positive correlation, so both appeared as accompanying pollen in these honeys. These plants bloom at the same time (between May and June) at the end of the honey season and have great honeybee attractiveness, therefore, are involved at the last stage of honey harvest. These pollen types are characteristic taxa of honeys from the Northwest Iberian Peninsula. On the contrary, between Eucalyptus and Echium there was a negative correlation. Echium was poorly represented in the pollen spectrum of the studied honeys however; it was best represented in eucalyptus honeys from the Mediterranean area.[Citation11−Citation13,Citation30−Citation32] The analysis showed that darker honeys contained higher percentages of Erica and higher phenolic content. As other studies also emphasized,[Citation47] these honeys proved a clear relationship between color, phenolic content, and EC. In addition, reduced sugars showed a strong correlation between them, because both are formed by the enzymatic action up on the sucrose contained in the nectar. Furthermore, samples with higher fructose content showed higher phenolic content, higher EC, and darker color. Finally, the invertase and diastase enzymes had positive correlation as other authors have also shown.[Citation33,Citation34] As a result of the mentioned correlation, the presence of Erica in honeys could be related with some minor variations in these samples such as, slightly darker samples with higher fructose, phenol, and flavonoid content. This occurred in the honeys collected from the mountain areas of the Cantabric coast from the Iberian Peninsula in which shrubs are very common.
Table 5 Results of Spearman’s rank correlation analysis among physicochemical and microscopic variables
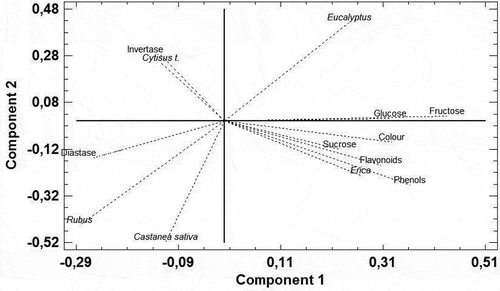
The mentioned influence of Erica in the honey features was emphasized with the multivariate analysis using PCA. The performed PCA extracted a total of five components that explain 81.6% of the variability of the data. shows the most important variables for each component. The variables of the first two components were highlighted; these explained the 52.4% of the variability of the data. In the first component, the significant variables were the physicochemical parameters, fructose, phenols, color, glucose, and flavonoids along with Erica pollen type. While in the second component, the relevant variables were only pollen types such as Rubus, Castanea sativa, and Eucalyptus ().
Table 6 Component weights of the PCA
CONCLUSIONS
Eucalyptus globulus honey is the main unifloral honey in the coastal lands of the Eurosiberian area of the Iberian Peninsula. Different pollen spectra were observed in the analyzed honey samples in comparison with other eucalyptus honeys from other areas. The typical pollen combination of the studied honeys was Eucalyptus, Castanea sativa, Rubus, Cytisus t., Quercus, Salix, and Erica being a useful characteristic for the differentiation of the Eucalyptus globulus honeys. The studied honeys were mainly characterised by light amber color, low phenolic content, low sucrose content, low enzymatic content, and low EC.
ACKNOWLEDGMENTS
The authors wish to thank the collaboration of the Spanish and Portuguese beekeepers who have generously provided the samples, and to the technician of the ‘Centro de Apoio Científico e Tecnolóxico á Investigación’ (CACTI) from Vigo University for their help.
REFERENCES
- Persano-Oddo, L.; Piro, R. Main European unifloral honeys: Descriptive sheets. Apidologie 2004, 35 (1), 38–81.
- Martos, I.; Ferreres, F.; Yao, L.; D’Arcy, B.; Caffin, N.; Tomás-Barberán, F.A. Flavonoids in monospecific eucalyptus honeys from Australia. Journal of Agricultural and Food Chemistry 2000, 48, 4744–4748.
- Crane, E. Honey. A Comprehensive Survey. IBRA, International Bee Research Association: Heinemann, London, UK, 1975; 608.
- Alissandrakis, E.; Tarantilis, P.A.; Pappas, C.; Harizanis, P.C. Investigation of organic extractives from unifloral chestnut (Castanea sativa L.) and eucalyptus (Eucalyptus globulus Labill.) honeys and flowers to identification of botanical marker compounds. Food Science and Technology Lebensmittel Wissenschaft and Technologie 2011, 44, 1042–1051.
- Serra-Bonvehí, J.; Ventura-Coll, F. Flavour index and aroma profiles of fresh and processed honeys. Journal of Science of Food and Agriculture 2003, 83, 275–282.
- De la Fuente, E.; Valencia-Barrera, R.M.; Martinez-Castro, I.; Sanz, J. Occurrence of 2-hydroxy-5-methyl-3-hexanone and 3-hydroxy-5-methyl-2-hexanone as indicators of botanic origin in eucalyptus honeys. Food Chemistry 2007, 103, 1176–1180.
- Yao, L.; Jiang, Y.; Singanusong, R.; Datta, N.; Raymont, K. Phenolic acid and abscisic acid in Australian Eucalyptus honeys and their potential for floral authentication. Food Chemistry 2004, 86, 169–177.
- Irish, J; Blair, S.; Carter, D.A. The antibacterial activity of honey derived from Australian flora. PLoS ONE 2011, 6 (3), e.18229. DOI:10.1371/journal.pone.0018229.
- Rossano, R.; Larocca, M.; Polito, T.; Perna, A.M.; Padula, M.C.; Martelli, G.; Riccio, P. What are the proteolytic enzymes of honey and what they do tell us? A fingerprint analysis by 2-D Zymography of unifloral honeys. PLoS ONE 2012, 7 (11), e.49164. DOI:10.1371/journal.pone.0049164.
- Council directive 2001/110/EC of 20 December 2001 relating to honey. Official Journal of the European Communities 2002, 47–52.
- Serra-Bonvehí, J.; Cañas-Lloria, S. Caratteristiche fisico chimiche, composizione e spettro pollinico dei miele di Eucalipto (Eucalyptus) prodotto in Spagna. Apicoltura 1988, 4, 59–81.
- Sorkun, K.; Doğan, C.; Başoğlu. N. Physicochemical characteristics and composition of Eucalyptus camaldulensis Dehnh. Honey produced in Turkey. Apiacta 2001, 4, 182–189.
- Terrab, A.; Diez, M.J.; Heredia, F.J. Palynological, physico-chemical, and color characterization of Moroccan honeys: I. River red gum (Eucalyptus camaldulensis Dehnh) honey. International Journal of Food Science and Technology 2003, 38, 379–386.
- Serrano, S.; Villarejo, M.; Espejo, R.; Jodral, M. Chemical and physical parameters of andalusian honey: Classification of Citrus and Eucalyptus honeys by discriminant analysis. Food Chemistry 2004, 87, 619–625.
- Potts, B.M.; Vaillancourt, R.E.; Jordan, G.J.; Dutkowski, G.W.; Costa e Silva, J.; McKinnon, G.E.; Steane, D.A.; Volker, P.W. Exploration of the Eucalyptus globulus gene pool. In: Eucalyptus in a Changing World; Tomé, M.; Eds.; RAIZ, Instituto Investigação de Floresta e Papel: Aveiro, Portugal, 2004; 46–61.
- Ghalem, B.R.; Mohamed, B. Antibacterial activity of leaf essential oils of Eucalyptus globulus and Eucalyptus camaldulensis. African Journal of Pharmacy and Pharmacology 2008, 2 (10), 211–215.
- Commission Regulation (EC) No 868/2007 of 23 July 2007 entering a designation in the Register of protected designations of origin and protected geographical indications (Miel de Galicia or Mel de Galicia (PGI)) Official Journal of the European Union 2007, 11–18.
- Gómez-Díaz, D.; Navaza, J.M.; Quintáns-Riveiro, L.C. Physicochemical characterization of Galician Honeys. International Journal of Food Properties 2012, 15 (2), 292–300.
- Rivas-Martínez, S.; Díaz-González, T.E.; Fernández-González, F.; Izco, J.; Loidi, J.; Lousâ, M.; Penas, A. Vascular plant communities of Spain and Portugal. Addenda to the syntaxonomical checklist of 2001. Itínera Geobotánica 2002, 15 (1––2), 5–-922.
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 59 (4), 139–157.
- Maurizio, A. Untersuchungen zur quantitativen pollenanalyse des honigs. Mitteilungen aus dem Gebiete der Lebensmittel Untersuchung und Hygiene 1939, 30, 27–69.
- Association of Official Analytical Chemists, Inc. (AOAC). Official Methods of Analysis. 15th Ed; Helrich, K.; Ed.; AOAC: Arlington, VA, USA, 1990.
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture 1965, 16, 144–158.
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardisation d´un extraait de propolis et identification des principaux constituants. Journal de Pharmacie de Belgique 1994, 49, 462–468.
- Devillers, J.; Morlot, M.; Pham-Delégue, M.H.; Dore, J.C. Classification of monofloral honeys based on their quality control data. Food Chemistry 2004, 86, 305–312.
- Caroli, S.; Forte, G.; Lamiceli, A.L.; Galoppi, B. Determination of essential and potentially toxic trace elements in honey by inductively Coupled Plasma-Based Techniques. Talanta 1999, 50 (2), 327–336.
- De Luis, P.; Gómez-Ferreras, C. Contribución al análisis polínico de mieles de Asturias Occidental (España). Botanica Complutensis 1989, 15, 163–173.
- Seijo, M.C.; Aira, M.J.; Jato, M.V. Distribución y características palinológicas de las mieles de eucalyptus gallegas. Botanica Complutensis 1998, 22,133–143.
- Escuredo, O.; Fernández-González, M.; Seijo, M.C. Differentiation of blossom honey and honeydew honey from Northwest Spain. Agriculture 2012, 2, 25–37.
- Makhloufi, C.; Kerkvliet, J.D.; Ricciardelli-D’Albore, G.; Choukri, A.; Samar, R. Characterization of Algerian honeys by palynological and physico-chemical methods. Apidologie 2010, 41 (5), 509–521.
- Mateo, R.; Bosch-Reig, F. Classification of Spanish unifloral honeys by discriminant analysis of electrical conductivity, color, water content, sugars, and pH. Journal of Agricultural and Food Chemistry 1998, 46, 393–400.
- De La Fuente, E.; Ruiz-Matute, A.I.; Valencia-Barrera, R.M.; Sanz, J.; Martínez-Castro, I. Carbohydrate composition of Spanish unifloral honeys. Food Chemistry 2011, 129 (4), 1483–1489.
- Persano-Oddo, L.; Piazza, M.G.; Pulcini, P. Invertase activity in honey. Apidologie 1999, 30, 57–65.
- Serrano, S.; Espejo, R.; Villarejo, M.; Jodral, M.L. Diastase and invertase activities in Andalusian honeys. International Journal of Food Science and Technology 2007, 42, 76–79.
- Benaziza-Bouchema, D.; Schweitzer, P. Characterization of the main honeys from the northern regions of Algeria. Cahiers Agricultures 2010, 19 (6), 432–438.
- Chakir, A.; Romanea, A.; Marcazzan, G.L.; Ferrazzi, P. Physicochemical properties of some honeys produced from different plants in Morocco. Arabian Journal of Chemistry 2011, DOI:10.1016/j.arabjc.2011.10.013.
- Marini, F.; Magri, A.L.; Balestrieri, F.; Fabretti, F.; Marinia, D. Supervised pattern recognition applied to the discrimination of the floral origin of six types of Italian honey samples. Analytica Chimica Acta 2004, 515, 117–125.
- Feás, X.; Pires, J.; Estevinho, M.L.; Iglesias, A.; Pinto, J.P. Palynological and physicochemical data characterisation of honeys produced in the Entre-Douro e Minho region of Portugal. International Journal of Food Science and Technology 2010, 45, 1255–1262.
- Malika, N.; Mohamed, F.; Chakib, E. Microbiological and physic-chemical properties of Moroccan honey. International Journal of Agriculture and Biology 2005, 7 (5), 773–776.
- Herken, E.N.; Erel, O.; Guzel, S.; Celik, H.; Ibanoglu, S. Total antioxidant, phenolic compounds, and total oxidant status of certified and uncertified Turkey’s honeys. International Journal of Food Properties 2010, 5 (13), 599–607.
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food and Chemical Toxicology 2008, 46, 3774–3779.
- González-Miret, M.L.; Terrab, A.; Hernanz, D.; Fernandez-Recamales, M.A.; Heredia, F.J. Multivariate correlation between color and mineral composition of honeys and by their botanical origin. Food Chemistry 2005, 53 (7), 2574–2580.
- Latorre, M.J.; Peña, R.; Pita, C.; Botana, A.; García, S.; Herrero, C. Chemometric classification of honeys according to their type II. Metal content data. Food Chemistry 1999, 66, 263–268.
- Fernández-Torres, R.; Pérez-Bernal, J.L.; Bello-López, M.A.; Callejón-Mochón, M.; Jiménez-Sánchez, J.C.; Guiraúm-Pérez, A. Mineral content and botanical origin of Spanish honeys. Talanta 2005, 65 (3), 686–691.
- Escuredo, O.; Seijo, M.C.; Fernández-González, M. Descriptive analysis of Rubus honey from the north-west of Spain. International Journal of Food Science and Technology 2011, 46, 2329–2336.
- Manikis, I.; Thrasivoulou, A. La relación entre las características fisicoquímicas de la miel y los parámetros de sensibilidad a la cristalización. Apiacta 2001, 36 (2), 106–112.
- Escuredo, O.; Míguez, M.; Fernández-González, M.; Seijo, M.C. Nutritional value and antioxidant activity of honeys produced in a European Atlantic area. Food Chemistry 2013, 138, 851–856.