Abstract
The amount of phenolics, flavonoids, and anthocyanins in ethanol extracts and antioxidant activity of both ethanol and lipophilic extracts of common fruity vegetables in Bangladesh were studied. Among the ethanol extracts of 15 fruity vegetables, M. oleifera had the highest total polyphenol content (85.05 mg gallic acid equivalent/g extract) followed by L. acutangula (61.74 mg gallic acid equivalent/g extract) and A. esculentus (48.92 mg gallic acid equivalent/g extract). L. acutangula had high a content of flavonoids (14.46 mg (+)-catechin equivalent/g extract), which was almost similar to L. siceraria (13.67 mg catechin equivalent/g extract) followed by A. esculentus (11.95 mg catechin equivalent/g extract) and S. melongena (11.42 mg catechin equivalent/g extract). Highest anthocyanins content was in F. hispida (2.22 μmol/g extract) followed by S. melongena (1.04 μmol/g extract). Ethanol extracts of A. esculentus, F. hispida, L. acutangula, L. siceraria, and S. melongena exhibited high DPPH free radical scavenging activity with IC50 of 70.4, 64.9, 70.4, 64.9, and 94.3 μg/mL respectively, whereas for the same lipophilic extracts of F. hispida and S. melongena showed lowest (37 μg/mL) IC50 followed by M. oleifera (47.6 μg/mL), L. siceraria (57.5 μg/mL), and A. esculentus (63.3 μg/mL). These vegetables also showed high reducing powers, NO scavenging and total antioxidant capacity. Therefore, the top five potential fruity vegetables consist of both hydrophilic and lipophilic antioxidant(s), the order being F. hispida > M. oleifera > A. esculentus, L. acutangula > L. siceraria > and S. melongena.
INTRODUCTION
Fruits and vegetables are major source of dietary functional components. Of these components, antioxidants are most important because they inhibit pathogenesis of various diseases such as cardiovascular disorders, diabetes, cancer, inflammation, aging, and brain dysfunction. Reportedly, reactive oxygen species (ROS) have been linked to over 100 disorders.[Citation1] Therefore, for maintaining a healthy biological system, it is critical to have the balance between oxidation and antioxidation. Excess generation of ROS causes oxidative stress that spreads over all the cell targets (DNA, lipids, and proteins). A variety of polyphenols, flavonoids, anthocyanins, and vitamins have been reported as showing antioxidants.[Citation2–Citation4] Various epidemiological studies have suggested that consumption of fruits and vegetables is associated with reduced risk of cardiovascular diseases and cancer,[Citation5] neurodegenerative diseases such as Parkinson’s and Alzheimer’s diseases,[Citation6] as well as with inflammation and aging.[Citation7] The majority of antioxidants are hydrophilic and they can easily contribute to keep up physiological health of hydrophilic organs whether lipophilic antioxidants are essential for lipophilic organs such as the brain. Besides, antioxidants are used in the food industry and pharmaceuticals as additives. Widely used synthetic antioxidants are now under question due to their side effects like carcinogenicity.[Citation8] Therefore in response to the growing consumer concern, search for antioxidants and/or antioxidant principles from natural sources have gained interest and many plants reportedly have potential antioxidant activity.[Citation9−Citation13]
People all over Bangladesh consume fruity vegetables cooked and sometimes in various preparations though no scientific data is available comparing their content of functional components such as polyphenols, flavonoids, anthocyanins, and antioxidants. Since fruits and vegetables have various health-promoting components, they might be playing a vital role in maintaining public health. It is mainly due to their antioxidants as improvement of body’s antioxidant defenses ameliorates various diseases. Since both hydrophilic and lipophilic antioxidants are essential for sound health, it will be noteworthy to categorize common vegetables depending on their content of both types of antioxidants. In this article, ethanol was used as solvent because it extracts a part of both hydrophilic and lipophilic components, whereas pentane was used only to extract lipophilic components.
MATERIALS AND METHODS
Materials
Chemicals
Ascorbic acid, Folin-Ciocalteu’s phenol reagent, (+)-catechin and gallic acid (GA) were purchased from Sigma-Aldrich Co (St. Louis, MO). DPPH was purchased from Wako Pure Chemical Industry, Ltd., Osaka, Japan. Aluminium chloride, ammonium molybdate, ethanol, methanol, potassium ferricyanide, sodium carbonate, sodium nitroprusside, sulfanilamide, and trichloroacetic acid were purchased from Merck, Germany.
Sample collection
The fruity vegetables namely Abelmoschus esculentus (L.) Moench, Benincasa hispida (Thunb.) Cogn., Cucumis dipsaceus (Ehrenb.), Cucurbita mixta (Thumb.), Ficus hispida (L.), Luffa acutangula (L.) Roxb., Luffa aegyptiaca (M.), Lablab purpureus (L.), Lagenaria siceraria (Mol.) Stan., Momordica charantia (L.), Moringa oleifera (Lamk.), Solanum melongena (L.), Trichosanthes cucumerina (L.), Trichosanthes dioica (Roxb.), and Vigna unguiculata (L.) Walp. were collected from local markets of Khulna city, Bangladesh. After washing with distilled water the collected fruity vegetables were sliced into small pieces and shed dried. Then, each dried sample was ground by use of a grinding machine into powdered form and stored in an air tight container.
Methods
Extraction
Twenty five grams of powder of each sample was taken in 100 mL of ethanol and kept in an air tight bottle. After seven days, the ethanol was filtered by Whatman No. 1 filter paper. The filtrate was air dried and the solid extracts were kept in the refrigerator. For the preparation of lipophilic extract, 10 g powder was shaken vigorously by hand with 200 mL pentane and after filtration, the filtrate was air dried to obtain pentane extract. Finally, 10 mg of the solid extract was dissolved in 1 mL ethanol or DMSO (10 mg/mL) and used to conduct the experiments.
Determination of total phenolics (TPH)
The TPH in ethanol extracts was determined according to the Folin-Ciocalteu method[Citation14] with GA as the standard and expressed (mg) as gallic acid equivalents (GAE)/g of extract. One milliliter of diluted extract was mixed with 1 mL of Folin-Ciocalteu’s reagent and vortexed for 5 s. Then, 1 mL of a 10% (w/v) sodium carbonate aqueous solution was added to the mixture. The mixture was incubated at room temperature for 1 h and thereafter colorimetric measurement was made at 700 nm. Each experiment was conducted three times.
Determination of total flavonoids (TF)
TF content was determined by using a colorimetric method according to the Zhishen et al.[Citation15] Briefly, a test tube containing 50 μg ethanol extract or different concentrations of (+)-catechin standard solution (20–80 μg/mL) mixed with 75 μL of 5% (w/v) NaNO2 solution. After 6 min, 150 μl of a 10% (w/v) AlCl3.6H2O solution was added and the mixture was allowed to stand for a further 5 min before 0.5 mL of 1 M NaOH was added. The mixture was brought to 2.5 mL with distilled water and mixed well. The absorbance was measured immediately at 510 nm using a spectrophotometer. The results were expressed as the mean ± SD mg of (+)-catechin equivalents (CE) per gram of extract.
Determination of anthocyanins
The anthocyanins content in ethanol extracts was determined according to the modified method of Padmavati et al.[Citation16] Four milliliters of acidifed methanol (1% v/v, HCl/methanol) containing extract stored in dark at 4°C for 24 h in screw capped test tube, and then centrifuged at 800 rpm for 15 min. The anthocyanin concentration in the supernatant was measured spectrophotometrically at 530 and 657 nm. The absorbance values for 530 and 657 nm were indicated as A530 and A657, respectively. The extinction coefficient of 31.6 M−1cm−1 was used to convert the absorbance values into anthocyanin concentration. The concentration was calculated using the following equation:
anthocyanin concentration (μmol/g) = ([A530 – 0.33×A657]/31.6) × (volume [mL]/weight [g]).
DPPH radical scavenging activity
The reaction mixture (total volume, 3 mL), consisting of 0.5 mL of 0.5 M acetic acid buffer solution at pH 5.5, 1 mL of 0.2 mM DPPH in ethanol, and 1.5 mL of 50% (v/v) ethanol aqueous solution, was shaken vigorously with the ethanol or pentane extracts.[Citation17] After incubation at room temperature for 30 min, the amount of DPPH remaining was determined by measuring absorbance at 517 nm. Mean values were obtained from triplicate experiments.
Nitric oxide (NO) radical scavenging activity
The scavenging effect of ethanol or pentane extracts on NO was measured according to the slightly modified method of Marcocci et al.[Citation18] Four milliliters of extract solutions of different concentrations were then added in the test tubes to 1 mL sodium nitroprusside solution (10 mM), and the test tubes were incubated at 37oC for 3 h. The same reaction mixture, without the extracts but with an equivalent amount of ethanol/DMSO, served as control. An aliquot (0.5 mL) of the incubation solution was removed and then diluted with 0.5 mL Griess reagent (1% sulfanilamide in 5% H3PO4 and 0.1% naphthylethylenediamine dihydrochloride) and finally the absorbance of the chromophore was immediately read at 570 nm.
Reducing power activity
The reducing power of ethanol extracts was determined according to the method of Oyaizu.[Citation19] Briefly, different concentrations of ethanol extracts were mixed with 2.5 mL of 0.2 M phosphate buffer, pH 6.6, and 2.5 mL of 1% potassioum ferricyanide solution. After incubation at 50oC for 20 min, the mixtures were mixed with 2.5 mL of 10% trichloroacetic acid followed by centrifugation at 650 g for 10 min. The supernatant (2.5 mL) was mixed with 2.5 mL of distilled water and 0.5 mL of 0.1% ferric chloride. The absorbance of this solution was measured at 700 nm. Ascorbic acid served as the positive control.
Determination of total antioxidant capacity
The assay was done according to the method described by Prieto et al.[Citation20] The tubes containing ethanol extract and reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate were incubated at 90oC for 90 min. After cooling at room temperature, the absorbance was taken at 695 nm against a blank. The antioxidant capacity was expressed as ascorbic acid equivalent (AAE) and GAE.
Statistical analysis
Results are expressed as mean ± SD for a given number of observations (n = 3–5). The level of significance was set at p value of 0.05.
RESULTS AND DISCUSSION
Polyphenols, Flavonoids, and Anthocyanins Contents
Polyphenols, flavonoids, and anthocyanins are bioactive components of plants and show various beneficial health promoting activities in humans. showed their content in ethanol extracts of common fruity vegetables in Bangladesh. Depending on polyphenols content, the top five vegetables were graded as M. oleifera > L. acutangula > A. esculentus > L. siceraria, L. purpureus, F. hispida > S. melongena. Polyphenols content in M. oleifera was 85.05 ± 1.08 mg GAE/g extract. In previous studies, polyphenol content of various edible common fruits,[Citation10] mangrove plants,[Citation11,Citation21,Citation22]and common antidiabetic medicinal plants[Citation23] in Bangladesh was reported. However, the extraction of phenolic compounds from the fruits or vegetables is commonly achieved with methanol or aqueous methanol.[Citation24,Citation25] Flavonoids, and anthocyanins mainly present as coloring pigments in fruits also function as potent antioxidants at various levels. L. acutangula had high content of flavonoids (14.46 ± 0.47 mg CE/g extract) followed by L. siceraria, A. esculentus, and S. melongena. Total flavonoid content in Bangladeshi pineapple ranges from 39.4 to 55.2 mg quercetin/g weight[Citation26] whether that was 14.6 mg CE/g in pericarp and 30.6 mg CE/g extract in seed of S. apetala.[Citation22] The highest anthocyanin content was in F. hispida (2.22 μmol/g extract) followed by S. melongena (1.04 μmol/g extract) whether it was found to be 0.3 μmol/g and 2.3 μmol/g in pericarp and seed extracts, respectively for S. apetala fruit.[Citation22] Anthocyanin limits the development of cancers, cardiovascular diseases, neurodegenerative diseases, and diabetes.[Citation27] Reportedly, fruits and vegetables are the main sources of antioxidant vitamins such as vitamin E, vitamin C, precursor of vitamin A, i.e., β-carotene, which acts as an antioxidant. Therefore, antioxidant activity of these fruity vegetables might be the collective result of their content of polyphenols, flavonoids, anthocyanins, and other antioxidant components.
Table 1 Phenolics, flavonoids, anthocyanins contents, and DPPH and NO radical scavenging activities and reducing power of ethanol extracts of common Bangladeshi fruity vegetables
Table 2 IC50 values of ethanol extracts of fruity vegetables potential as antioxidant
Table 3 DPPH and NO free radical scavenging activities of lipophilic extracts of fruity vegetables
Antioxidant Activity
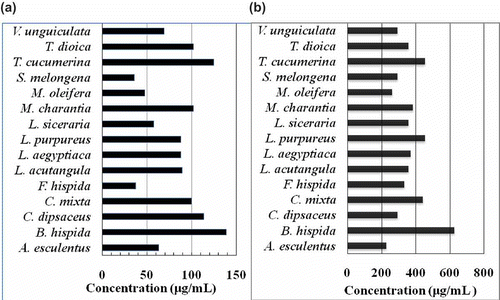
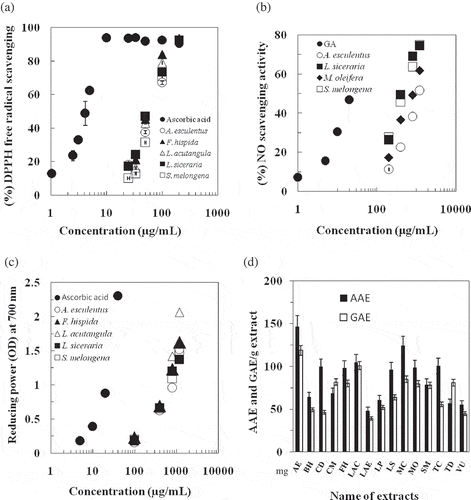
DPPH and NO free radical scavenging and reducing power of ethanol extracts of 15 fruity vegetables were shown in . Among them A. esculentus, F. hispida, L. acutangula, L. siceraria, and S. melongena showed high activity in scavenging of DPPH free radical and reducing power. showed dose-dependent scavenging of DPPH free radical by these potential extracts. The inhibitory concentration 50 (IC50), which means the concentration of extract that was needed to scavenge 50% of total DPPH free radicals. The IC50 values for DPPH radical scavenging of A. esculentus, F. hispida, L. acutangula, L. siceraria, and S. melongena were 70.4, 64.9, 70.4, 64.9, and 94.3 μg/mL, respectively, and that for ascorbic acid was 4.6 (). F. hispida and L. siceraria showed same as well as lowest IC50, which means that among all the extracts tested, they had the strongest DPPH radical scavenging activity. It has been found that cysteine, glutathione, ascorbic acid, tocopherol, polyhydroxy aromatic compounds, and aromatic amines reduce and decolorize DPPH by their hydrogen donating ability.[Citation17] Therefore, these extracts possess hydrogen donating capabilities to act as antioxidants. Hossain et al.[Citation10] reported that the IC50 values for the fruits namely Phyllanthus emblica, Syzygium cumini, Dillenia indica, Spondias dulcis, and P. acidus were 2.1, 8.6, 57, 81, and 93 μg/mL, respectively. showed DPPH radical scavenging activity of lipophilic (pentane) extracts of common fruity vegetables and their IC50 values were shown in . Depending on DPPH free radical scavenging of lipophilic extracts of fruity vegetables, they were graded as S. melongena, F. hispida > M. oleifera > L. siceraria > A. esculentus > V. unguiculata > L. acutangula, L. aegyptiaca, L. purpureus > C. mixta, M. charantia, T. dioica > C. dipsaceus > T. cucumerina > B. hispida. NO is synthesized in many different mammalian cells types such as endothelial cells, vascular smooth muscular cells, neurons, platelets, macrophages, and neutrophils. NO, or some related reactive nitrogen species, act as neurotransmitters, preventing platelet aggregation and they are one of the defense molecules of immune system against tumor cells, parasites, and bacteria. However, large amounts of NO, peroxynitrite, and other reactive nitrogen oxide species are considered to be potentially cytotoxic and capable of injuring the surrounding cells.[Citation28] The dose-dependent inhibition of nitrite production of potential ethanol extracts of A. esculentus, L. siceraria, M. oleifera, and S. melongena was shown in with IC50 values of 974.7, 438.6, 684.9, and 450.5 μg/mL respectively (). Similar reports were found for lotus seed extract[Citation29] and leaves of Pistacia atlantica.[Citation28] showed NO free radical scavenging activity of lipophilic extracts of fruity vegetables and their IC50 values were shown in . Depending on their effects on NO scavenging, they were graded as A. esculentus > M. oleifera > C. dipsaceus, S. melongena, V. unguiculata > F. hispida > L. acutangula, L. siceraria, T. dioica > L. aegyptiaca > M. charantia > C. mixta > L. purpureus, T. cucumerina > and B. hispida. Reportedly, the activity of antioxidants is concomitant with the development of reducing power.[Citation30] showed the reducing power of the ethanol extracts determined using the potassium ferricyanide reduction method. At a concentration of 400 μg/mL extract in phosphate buffer, A. esculentus, F. hispida, L. acutangula, L. siceraria, and S. melongena had high reducing activity (O.D., optical density) of 0.62, 0.69, 0.67, 0.66, and 0.65, respectively (). Since these extracts displayed high reducing power and their dose-dependent increase of reducing power was shown in . Furthermore, total antioxidant capacity, which was expressed as the AAE, and GAE of ethanol extracts, was shown in . The highest antioxidant capacity was in A. esculentus followed by L. acutangula and M. charantia. However, when considered TPH content versus DPPH, and NO scavenging activity (%) or reducing power of ethanol extracts, the correlation coefficient (r) was 0.61, 0.58, or 0.64, respectively. In a previous study on Egyptian plants, it was reported that correlation between total polyphenol and DPPH radical scavenging activity, reducing power or total antioxidant capacity was 0.73, 0.65, or 0.79 respectively.[Citation12] For Bangladeshi fruits, correlation between total polyphenol and DPPH radical scavenging activity and reducing power was 0.54 and 0.97, respectively.[Citation10] In general, antioxidant activity relates with total phenolic content[Citation31] but the notion does not always hold true.[Citation32] Therefore, it is remarkable that unknown components other than polyphenols, especially lipophilic components, in these fruity vegetables may contribute a part to their antioxidant activity. However, among the plant-originated dietary intake of antioxidants, polyphenols are at the top. Reportedly polyphenols have various beneficial effects on health of humans.[Citation2−Citation4,Citation27,Citation33,Citation34] Attention should be paid when polyphenols are used to prepare functional foods and dietary supplements since some polyphenols perturb the membrane structure.[Citation33−Citation35]
Figure 1 Antioxidant activity of ethanol extracts of common fruity vegetables in Bangladesh. Dose-dependency of (a): the DPPH and (b): NO free radical scavenging activities of ethanol extracts (AA: ascorbic acid, GA: gallic acid, positive control); (c): Dose-dependent increase of reducing power of the extracts (AA: ascorbic acid, positive control); (d) Comparison of total antioxidant capacity of the extracts (AAE: ascorbic acid equivalent, GAE: gallic acid equivalent). Data are presented as mean ± SD (bar), n = 3-5. AE = A. esculentus, BH = B. hispida, CD = C. dipsaceus, CM = C. mixta, FH = F. hispida, LAC = L. acutangula, LAE = L. aegyptiaca, LP = L. purpureus, LS = L. siceraria, MC = M. charantia, MO = M. oleifera, SM = S. melongena, TC = T. cucumerina, TD = T. dioica, and VU = V. unguiculata.
CONCLUSION
Present observations revealed that among the 15 common fruity vegetables, F. hispida > M. oleifera > A. esculentus, L. acutangula > L. siceraria > and S. melongena were the top that should be considered as potential sources of both hydrophilic and lipophilic antioxidant(s). Therefore, these potential fruity vegetables could be used as a natural treasure of dietary antioxidants to prevent oxidative damage of both hydrophilic and lipophilic organs. In addition, they may be used to prepare functional foods and nutraceuticals. Moreover, depending on the results of this study, health conscious consumers may choose their dietary vegetables. However, further in vitro and in vivo experiments are essential with the potential fruity vegetables to confirm the present observations.
FUNDING
This research work was partially supported by the grants from the Khulna University Research Cell (KURC) in 2011, and University Grants Commission (UGC) of Bangladesh in 2012, which are gratefully acknowledged.
REFERENCES
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, Oxford Science Publications: Oxford, 2000; 617–624.
- Terao, J.; Piskula, M.; Yao, Q. Protective effect of epicatechin, epicatechin gallate, and quercetin on lipid peroxidation in phospholipid bilayers. Archives of Biochemistry and Biophysics 1994, 308, 278–284.
- Sawa, T.; Nakao, M.; Akaike, T.; Ono, K.; Maeda, H. Alkylperoxyl radical-scavenging activity of various flavonoids and other polyphenolic compounds: Implications for the anti-tumor-promoter effect of vegetables. Journal of Agricultural and Food Chemistry 1999, 47, 397–402.
- Kahkonen, M.P.; Hopia, A.I.; Vuorela, H.J; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. Journal of Agricultural and Food Chemistry 1999, 47, 3954–3962.
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular diseases and cancer. The American Journal of Medicine 2002, 113, 71S–88S.
- Di Matteo, V.; Esposito, E. Biochemical and therapeutic effects of antioxidants in the treatment of Alzeimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Current Drug Targets-CNS and Neurological Disorders 2003, 2, 95–107.
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proceedings of the National Academy of Sciences of the USA 1993, 90, 7915–7922.
- Gulcin, I.; Elmastas, M.; Aboul-Enein, H.Y. Determination of antioxidant and radical scavenging activity of Basil (Ocimum basilicum L., Family Lamiaceae) assayed by different methodologies. Phytotherapy Research 2007, 21, 354–361.
- Pokorny, J. Natural antioxidant for food use. Trends in Food Science and Technology 1991, 2, 223–227.
- Hossain, S.J.; Tsujiyama, I.; Takasugi, M.; Islam, Md. A.; Biswas, R.S.; Aoshima, H. Total phenolic content, antioxidative, anti-amylase, anti-glucosidase, and antihistamine release activities of Bangladeshi fruits. Food Science and Technology Research 2008, 14, 261–268.
- Hossain, S.J.; Aoshima, H.; El-Sayed, M.; Ahmed, F. Antioxidative and anti-histamine-release activities of Excoecaria agallocha L. Pharmacology Online 2009, 2, 927–936.
- Hossain, S.J.; El-Sayed, M.; Aoshima, H. Antioxidative and anti-α-amylase activities of four wild plants consumed by pastoral nomads in Egypt. Oriental Pharmacy and Experimental Medicine 2009, 9, 217–224.
- Hossain, S.J.; El-Sayed, M.A.; Mohamed, A.H.; Sheded, M.G.; Aoshima, H. Phenolic content, antioxidative, anti-α-amylase, and anti-α-glucosidase activities of Solanum diphyllum L. Bangladesh Journal of Botany 2009, 38, 139–143.
- Ough, C.S.; Amerine, M.A. Methods for Analysis of Musts and Wine, Wiley & Sons: New York, 1988; 196–221.
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry 1999, 64, 555–559.
- Padmavati, M.; Sakthivel, N.; Thara, K.V.; Reddy, A.R. Differential sensitivity of rice pathogens to growth inhibition by flavonoids. Phytochemistry 1997, 46, 449–502.
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200.
- Marcocci, L.; Maguire, J.J.; Droy-Lefaix, M.T.; Packer, L. The nitric-oxide scavenging properties of Ginkgo biloba extract EGb 761. Biochemical and Biophysical Research Communications 1994, 201, 748–755.
- Oyaizu, M. Studies on products of browning reaction: Antioxidative activity of products of browning reaction prepared from glucosamine. Japanese Journal of Nutrition 1986, 44, 307–315.
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Analytical Biochemistry 1999, 269, 337–341.
- Mubassara, S.; Takasugi, M.; Iga, R.; Hossain, S.J.; Aoshima, H. Inhibition of the histamine and leukotriene B4 release from rat peritoneal exudates cells by six Bangladeshi plants. Pharmacology Online 2011, 2, 76–85.
- Hossain, S.J.; Basar, M.H.; Rokeya, B.; Arif, K.M.T.; Sultana, M.S.; Rahman, M.H. Evaluation of antioxidant, antidiabetic, and antibacterial activities of the fruit of Sonneratia apetala (Buch.-Ham.). Oriental Pharmacy and Experimental Medicine 2013, 13, 95–102.
- Basar, M.H.; Hossain, S.J.; Sadhu, S.K.; Rahman, M.H. A comparative study of antioxidant potential of commonly used antidiabetic plants in Bangladesh. Oriental Pharmacy and Experimental Medicine 2013, 13, 21–28.
- Antolovich, M.; Prenzler, P.; Robards, K.; Ryan, D. Sample preparation in the determination of phenolic compounds in fruits. The Analyst 2000, 125, 989–1009.
- Bhat, R.; Min-Tze, L.; Abdorreza, M.N.; Karim, A.A. Evaluation of free radical scavenging activity and antioxidant potential of a few popular green leafy vegetables of Malaysia. International Journal of Food Properties 2013, 16, 1371–1379.
- Hossain, M.A.; Rahman, S.M.M. Total phenolics, flavonoids, and antioxidant activity of tropical fruit pineapple. Food Research International 2011, 44, 672–676.
- Scalbert, A.; Manach, C.; Morand, C.; Remesy, C.; Jimenes, L. Dietary polyphenols and the prevention of diseases. Critical Reviews in Food Science and Nutrition 2005, 45, 287–306.
- Peksel, A.; Arisan-Atac, I.; Yanardag, R. Evaluation of antioxidant and antiacetylcholinesterase activities of the extracts of Pistacia atlantica Desf. leaves. Journal of Food Biochemistry 2010, 34, 451–476.
- Yen, G.C.; Duh, P.D.; Su, H.J.; Yeh, C.T.; Wu, C.H. Scavenging effects of lotus seed extracts on reactive nitrogen species. Food Chemistry 2006, 94, 596–602.
- Duh, P.D.; Tu, Y.Y.; Yen, G.C. Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat.). Lebensm Wiss Technology 1999, 32, 269–277.
- Zujko, M.E.; Witkowska, A.M. Antioxidant potential and polyphenol content of beverages, chocolates, nuts, and seeds. International Journal of Food Properties 2014, 17, 86–92.
- Chinnici, F.; Bendini, A.; Gaiani, A.; Riponi, C. Radical scavenging activities of peels and pulps from cv. Golden Delicious apples as related to their phenolic composition. Journal of Agricultural and Food Chemistry 2004, 52, 4684–4689.
- Hossain, S.J.; Kato, H.; Aoshima, H.; Yokoyama, T.; Yamada, M.; Hara, Y. Polyphenol-induced inhibition of the response of Na+/glucose cotransporter expressed in Xenopus oocytes. Journal of Agricultural and Food Chemistry 2002, 50, 5215–5219.
- Hossain, S.J.; Aoshima, H.; Koda, H.; Kiso, Y. Review of functional studies of beverage components acting on the recombinant GABAA neuroreceptor, and Na+/glucose cotransporter-response using the Xenopus oocyte expression system and electrophysiological measurements. Food Biotechnology 2007, 21, 237–270.
- Aoshima, H.; Okita, Y.; Hossain, S.J.; Fukue, K.; Mito, M.; Orihara, Y.; Yokohama, T.; Yamada, M.; Kumagai, A.; Nagaoka, Y.; Uesato, S.; Hara, Y. Effect of 3-o-octanoyl-(+)-catechin on the responses of GABAA receptors and Na+/glucose co-transporters expressed in Xenopus oocytes and on the oocyte membrane potential. Journal of Agricultural and Food Chemistry 2005, 53, 1955–1959.