Abstract
The polyphenolic profile and antioxidant activity of peel, pomace, and juice of ‘Verde Doncella’, a Spanish apple cultivar, is presented. Phenolic profile of the worldwide cultivated ‘Red Delicious’ cultivar was used for comparison. Flavanols, hydroxycinamic acids, flavonols, phloridzin, procyanidin B2, and gallic acid were quantified by HPLC. Larger concentrations of polyphenolics were found in the peel, which is in agreement with the total phenolic content and antioxidant activity (FRAP) values. ‘Verde Doncella’ expressed lower concentrations of flavanols and quercetin derivates in peel, pomace, and juice when compared to ‘Red Delicious’. ‘Verde Doncella’ was richer in p-coumaric acid and procyanidn B2 in the peel.
INTRODUCTION
The production area of ‘Verde Doncella’ (Malus domestica), a lesser-known, high market value Spanish apple cultivar, is mainly located in the Aragón region, in northeastern Spain. ‘Verde Doncella’ has a relatively long history in this area, stretching back as far as the 19th century.[Citation1] The fruit possesses a pinkish-yellow color and is highly appreciated by consumers due to its juicy, sweet, and aromatic characteristics. Since the 1950s, important transformations in the Aragón agricultural sector have led to the abandonment of primitive agricultural practices in favor of mechanical-based production. These changes have resulted in the replacement of traditional cultivars with others from diverse origins, to increase demand and production. However, in the last decade, an increasing trend to reintroduce local varieties into the marketplace, products reflecting the local region has been observed.[Citation1]
The general perception that apples are good for human health, together with the consumer’s increasing demand for functional foods, has encouraged researchers to study in depth the polyphenolic profiles and antioxidant properties of many apple cultivars. It is well known that apples are one of the most important natural sources of polyphenols, exhibiting antioxidant activity, which can potentially prevent chronic diseases.[Citation2,Citation3]
During the past few years, a lot of research has been devoted to polyphenols, their occurrence in apples,[Citation4−Citation9] and apple derivates or by-products.[Citation10−Citation13] These studies have contributed to elucidate the major polyphenolic groups and many individual polyphenolic compounds in a variety of cultivars. According to the studies mentioned above, the major phenolic groups that are present in different apple cultivars belong to the hydroxycinnamic acids, flavanols, flavonol anthocyanins, and dihydrochalcons families. With respect to individual compounds, the major apple phenolics are chlorogenic acid, quercetin glycosides, procyanidins, and phloridzin. Distribution of these compounds vary considerably among apple cultivars, and seem to be regulated by environmental and post-harvest factors, including fruit season, fruit maturity, light exposure, storage, and processing.[Citation14]
Major phenolics are well characterized in commercially important cultivars, such as ‘Red Delicious,’ Golden Delicious, Fuji, and Granny Smith, but little or no data is available for traditional, secondary varieties specific to small production areas, such as ‘Verde Doncella’. To the best of our knowledge, only one study carried out more than 20 years ago,[Citation15] has analyzed the phenolic composition in ‘Verde Doncella’ apples. In this study, four major groups of compounds (catechins, procyanidins, hydroxycinnamic acid esters, and flavonoid glycosides) in the peel, pomace, and juice of five apple cultivars, were quantified using high performance liquid chromatography (HPLC).
The lack of information with respect to phenolic composition and antioxidant properties for ‘Verde Doncella’ has motivated the present work. This article, therefore, provides a preliminary insight into the phenolic profile (including color measurements and quantification of major phenolics), and antioxidant activity (ferric reducing ability of plasma [FRAP]) for ‘Verde Doncella’. For comparison purposes, the present article also includes data for ‘Red Delicious’ apples.
MATERIALS AND METHODS
Chemicals
Folin-Ciolcalteu reagent, sodium carbonate anhydrous, gallic acid monohydrate, 2,4,6-tris(2-pyridyl)-s-triazine, (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid, sodium acetate trihydrate, iron (III) chloride hexahydrate, procyanidin B2, chlorogenic acid, (+)-catechin, (+)-epicatechin, kaempferol, caffeic acid, quercetin, quercetin 3-galactoside, quercetin 3-glucoside, quercetin 3-rhamnoside, p-coumaric acid, phloridzin, and rutin hydrate were all obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Sodium hydroxide 0.1 mol/L, hydrochloric acid 1.0 mol/L, malic acid, sodium fluoride, and iron (II) sulfate 7 hydrate were all obtained from Panreac Química S.A.U. (Barcelona, Spain). LiChrosolv methanol for liquid chromatography and acetic acid (glacial) anhydrous GK for analysis were obtained from Merck KGaA (Darmstadt, Germany).
Plant Material
The apple cultivars evaluated in this study were Malus domestica ‘Verde Doncella’ and Malus domestica ‘Red Delicious’. All fruits were commercially harvested in 2010, in an orchard belonging to Frutas Villalengua S.L., located in Zaragoza, Spain. Apples remained in the suppliers packaging cartons and were cold stored at 2–3°C, for 2 weeks until analysis.
Color Measurements
Apple cartons were removed from cold storage and allowed to acclimate to room temperature for 1 h. Colorimetric measurements were performed according to a previously published group article[Citation16] on each apple of both cultivars (measuring peel only) using an Instrument System spectroradiometer IS CAS 140 (München, Germany) with a TOP 100 probe with an AF Nikkor 200 mm 1:4 lens. The spectroradiometer equipment was controlled by ISCOLOR software (Version 2.53, 1996; Instrument Systems Optische Messtechnik GmbH; München, Germany). Illumination was supplied by a 12V-100W projection lamp (type 6834, Royal Philips Electronics, Amsterdam, the Netherlands) attached to a DC power supply (Diamond Antenna, San Marcos, CA, USA). Illumination equipment was operational for 40 min until light spectrum stabilized. White standard was calibrated using a Spectralon® reflectance standard (NIST certified, Labsphere Inc., North Sutton, NH, USA). Apples were measured on a rotating sample platform. Reflectance spectra were measured every 4 s allowing the apple to revolve 360°. Approximately 200 measurements were collected around the latitude of each apple in 4 s, averaged into one measurement. Spectra were measured between 380 and 900 nm every 1 nm. From these spectra, CIELAB (CIE 2004) coordinates L*, a*, b*, C*, and hab were calculated with the CIE64 Standard Observer and the D65 Illuminant.
Sample Processing (Phenolics Extraction from Peel, Pomace, and Juice)
Fresh apple samples from ‘Red Delicious’ and ‘Verde Doncella’ cultivars were peeled with a hand peeler (1–2 mm thickness). Apple pomace and juice were collected after processing the remaining apples through a juicer (Sammic, Azkoitia, Spain). Apple peel, pomace, and juice were processed separately. Five grams for each sample was added into a sterile 50-mL conical centrifuge tube. Approximately 10 mL of an 80% aqueous methanol extraction solution containing sodium fluoride in order to slow oxidation was added to each centrifuge tube. The tubes were shaken for 30 min and then stored at −32°C for 24 h. After 24 h, sample solutions were centrifuged at 2600× g for 40 min at 0°C ± 1°C and then filtered. In order to optimize sample clarification, a total of 13 different filters (in terms of pore size and supplier) were tested to filtrate the supernatant. Additionally, several methods of filtration (syringe, gravity, vacuum) were assayed as shown in . The supernatant was finally filtered through a Pall Life Sci. Corp. 0.45-μm Acrodisc syringe filter and the filtrate was stored at −32°C prior to analysis. These extracts (for apple peel, pomace, and juice) were employed in further chemical analysis (total phenolic content [TPC], FRAP, and HPLC). Apple dry weight was determined gravimetrically, based on sample weight loss after being heated in an oven at 38°C for several days.[Citation17] Samples were dried in labeled brown paper bags.
Total Phenolic Content (TPC)
TPC was determined by a modified Folin-Ciocalteu method.[Citation18,Citation19] Briefly, a 1-mL aliquot of peel, pomace, or juice extract was mixed with 5 mL of Folin-Ciocalteu reagent. After 30 s and before 8 min, 4 mL of 7.5% sodium carbonate (Na2CO3) was added into volumetric flasks. Flasks were incubated in the dark for 60 min at room temperature. Absorbance was measured at 760 nm against a blank extraction solution (80% aqueous methanolic solution with NaF) in an UV/visible spectrophotometer (model 6506, Jenway). The standard curve was prepared with gallic acid (0 to 200 mg/L solutions) in 80% methanol. Total phenolic content of samples were expressed in Gallic acid equivalents (GAE) (mg/100 g). Experiments were performed in triplicate.
Antioxidant Activity: Ferric Reducing Ability of Plasma/Antioxidant Power (FRAP) Assay
The FRAP method was modified from protocol.[Citation20] This method is based on the reducing power of an antioxidant, which will reduce the ferric ion (Fe3+) to the ferrous ion (Fe2+); the latter will form a blue-violet complex (Fe2+/TPTZ), which will increase the absorption at 595 nm.
The FRAP reagent (150 μL) and 20 μL of apple extract (peel, pomace, or juice) or standard (Trolox), were added into each well of a 96-well TPP (TPP AG, Trasadingen, Switzerland) tissue culture plate. Plates were then read at 595 nm using a Tecan GENios multifunction micro plate reader (Tecan Trading AG, Männedorf, Switzerland). Replications were made in triplicate of each treatment. Standard curves were prepared for each plate. The antioxidant capacity is mentioned as Trolox equivalents.
Determination of Phenolic Compounds by HPLC
Phenolics were identified and quantified with a HPLC system from Agilent Technologies (1200 Series) equipped with a quaternary pump, a degasser, a thermostatic auto-sampler, and a UV-diode array detector. Injection volume for each apple extract (peel, pomace, or juice) was 10 μL. Chromatographic separation was performed using a Zorbax SB-C18 column (150 mm × 4.6 mm i.d.; particle size 3.5 μm). The binary phase was performed according to a modified Tsao and Yang procedure.[Citation7] Solvent A consisted of 6% acetic acid in 2 mM sodium acetate (final pH 2.55, v/v) and solvent B, was pure acetonitrile. All solvents were filtered and degassed through a 0.45-μm nylon filter before analysis. Flow rate was set at 0.6 mL/min for a total run time of 48 min. The system was run with a gradient program: 0–15% B in 27 min, 15–30% B in 9 min, 30–50% B in 3 min, and 50–100% B in 3 min. A post-run of 6 min at initial conditions for equilibrium was also performed. This program permitted the analysis of the major apple phenolics in a relatively short chromatographic run (≅ 30 min). Phenolics were detected at 280, 320, 360, and 520 nm ( and ).
Figure 1 ‘Verde Doncella’ apple peel HPLC chromatograms, measured at different wavelengths (a = 280, b = 320, c = 360, d = 520).
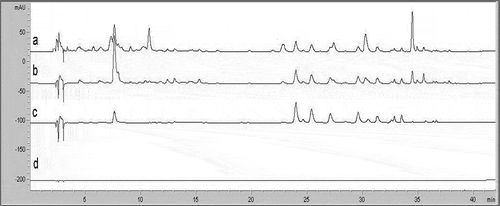
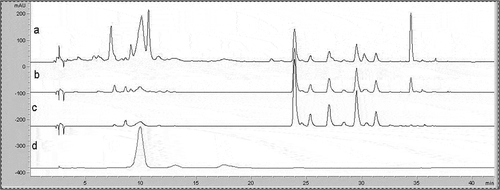
Figure 2 ‘Red Delicious’ apple peel HPLC chromatograms, measured at different wavelengths (a = 280, b = 320, c = 360, d = 520).
Chromatograms and UV-Vis spectra were acquired with Chemstation software (Agilent Technologies, Santa Clara, CA, USA). Phenolics identification was achieved by comparing retention times and UV-Vis spectra with available standard reference compounds. Unknown peaks were tentatively identified by comparison with known polyphenol group profiles of similar apple cultivars previously described in the literature.[Citation7,Citation21] Concentration of phenolics was determined by interpolating in pure compound standard curves. All samples were prepared and analyzed in triplicate.
Data Analysis
The values obtained in the analysis of TPC, antioxidant capacity, and quantitative data derived from HPLC analysis were subjected to analysis of variance (ANOVA) using GraphPad Prism (Version 5.00, GraphPad Software, La Jolla, CA, USA). When significance was observed (p ≤ 0.05) a Tukey’s test was performed for separation of means. Additionally, the relationship between the total phenolics (measured by both TPC and HPLC) and the antioxidant activity were examined by Pearson correlations.
RESULTS AND DISCUSSION
Color
CIELAB color coordinate measurements are presented in . Previous publications have reported measured CIELAB color coordinates in apples.[Citation22,Citation23] However, in each experiment, color was measured using a hand-held pistol, recording individual random points of a sample. In this experiment, samples were placed on a rotating platform (360°) and evaluated with a fixed camera, recording constant color value measurements (n = 200) during a single revolution. Use of a rotational platform allowed samples to be read homogenously and precisely, avoiding possible errors related to light position source or measurement angle. Presented color measurements of ‘Red Delicious’ are consistent with other investigations measuring color of the same cultivar.[Citation23,Citation25] Color parameters of ‘Verde Doncella’ are reported for the first time.
Table 1 Color coordinates obtained in the initial characterization of apples.
Table 2 Filters’ commercial name, type of filtration, pore size, and average absorbance of filtrate extracts assayed for filtration optimization.
Optimization of Extract Filtration for Absorbance-Based Measurements
During preliminary tests it was observed that phenolic extractions with methanol provided turbid supernatants with small particles in suspension. Such turbidity presented a problem for further spectrometric measurements, as it lead to unstable and high absorbance values. Therefore, an optimization effort was made to select a filter that could provide greater clarity for the supernatant, while still being efficient and fast (to limit sample oxidation). lists the 13 filters assayed, the type of filtration (vacuum, gravity, and syringe), the pore size, and the average absorbance obtained after juice filtration. Vacuum filtration resulted in rapid sample recovery; however, vacuum produced more turbid extracts (and therefore greater absorbance values) when compared to gravity and syringe filtration. On the other hand, gravity filtration was slow and the time required in collecting enough sample filtrate risked increasing sample oxidation. Taking into account both quality of absorbance measurements and filtration time, the Pall Life. Sci. Corp. Acrodise Syringe Filter was chosen.
Total Phenolic Content (TPC)
When comparing between cultivars (), the most outstanding result is that peel from ‘Red Delicious’ contained a TPC (12.7 mg/GAE/g DW) more than twice as large as the TPC of ‘Verde Doncella’ (13 mg/GAE/g DW). The TPC obtained from ‘Red Delicious’ peel in our work corresponded well with previously reported studies.[Citation2] On the contrary, only a few differences were found between ‘Red Delicious’ and ‘Verde Doncella’ when comparing TPC for pomace and juice samples, as no significant differences were observed, respectively, between means.
Table 3 Mean and standard deviation (n = 3) of total phenolic content (TPC) and antioxidant activity of ‘Verde Doncella’ and ‘Red Delicious’ fruit extracts using Folin-Ciocalteu and FRAP methods.*
For both varieties, TPC varied significantly between collected peel and pomace. TPC values for apple peel provided the largest values, which is in agreement that phenolics will accumulate in dermal tissues of plant bodies, thus increasing TPC.[Citation7] Previous studies[Citation3,Citation26] explained that TPC in peel was greater than in juice or pomace due to the presence of phenolic compounds, such as anthocyanins and quercetin glycoside molecules, found only in the peel region. Included in TPC is phloridzin, a dihydrochalcone that is up to three times more concentrated in the skin than in the flesh.[Citation4] With regard to ‘Verde Doncella’, TPC values were also greater in peel samples when compared to pomace and juice samples. The single study that we have found reporting data from ‘Verde Doncella’ has been conducted by Perez-Ilzarbe and co-workers.[Citation15] In this study, the phenolic compounds in flesh, juice, and skins of five apple varieties (Starking red, Reineta, Golden Delicious, ‘Verde Doncella’ and Granny Smith), were identified by HPLC. Major compounds quantified were cathechins, procyanidins, hidroxycinnamic acids, and flavonoid derivates. The study concluded that the phenolic content showed different patterns depending on the part and cultivar of the fruit, highlighting that concentrations of ‘Red Delicious’ polyphenols are significantly higher than ‘Verde Doncella’ in peel, pomace, and juice, a result in agreement with our data.
Antioxidant Activity: Ferric Reducing Ability of Plasma/Antioxidant Power (FRAP) Assay
Protocols used were based on studies by Benzie and Strain[Citation20] with some modifications. To prepare the FRAP reagent, these studies used a mixture of three solutions (TPTZ, Acetone buffer, and FeCl2∑6H2O). In every publication describing FRAP preparation, ethanol or acetone was used to dissolve TPTZ powder, followed by water. In our study, neither water nor ethanol was fully able to dissolve TPTZ; however, by using methanol, a more polar solvent, better results were obtained. Antioxidant properties of apple extracts were evaluated to identify their capacity to reduce iron from ferric (Fe+3) to ferrous (Fe+2). Antioxidant results are presented in for ‘Red Delicious’ and ‘Verde Doncella’ apple peel, juice, and pomace samples.
Antioxidant activity measured from peel appeared greater than pomace for both cultivars, which is in accordance with previously observed TPC contents. Oxygen radical absorbance capacity (ORAC) values of ‘Red Delicious’ with skin greater than ‘Red Delicious’ without skin have been reported.[Citation27]. This Institution also reported ‘Red Delicious’ ORAC values greater than all other fresh apple varieties tested, a result in accordance with our TPC data.
Significant differences were found between antioxidant activity in apple peel and juice in both varieties. ‘Red Delicious’ peel extract displayed significantly greater antioxidant activity (143 μmol eq. Trolox) when compared to ‘Verde Doncella’ (52 μmol eq. Trolox); this result was consistent with the TPC contents found in both cultivars. On the contrary, antioxidant activities in both apple juices were not significantly different (6.6 μmol eq. Trolox found in both cultivars). Antioxidant activity data presented in our study is in agreement with data published by other authors.[Citation28]
Determination of Phenolics by HPLC
The characteristic HPLC chromatographic profile of apple samples in ‘Red Delicious’ and ‘Verde Doncella’ cultivars are presented in to . Of the four wavelengths (λ) tested for separating apple peel phenolic compounds, λ monitored at 280, 320, and 360 nm yielded UV-spectra similar to pure compounds tested for detecting hydroxybenzoic acid derivatives, flavan-3-ols, dihydrochalcone, and hydroxycinnamic acid derivatives. Tsao and Yang[Citation7] reported similar results when analyzing ‘Red Delicious’ apple peel. They also reported that the variation in wavelength provides advantages for simultaneous detection of major polyphenolics in fruit.
Figure 3 ‘Verde Doncella’ peel, pomace, and juice HPLC chromatograms read at 280 nm. (1) Gallic acid; (2) Procyanidin B1; (3) Unknown procyanidin dimer; (4) Catechin; (5) Procyanidin B2; (6) Chlorogenic acid; (7) Unknown procyanidin dimer; (8) Caffeic acid; (9) Anthocyanin; (10) Epicatechin; (11) Cyanidin-3-rutinoside; (12) Unknown procyanidin dimer; (13) p-Coumaric acid; (14) Unknown procyanidin dimer; (15) 3-Hydroxyphloretin 2-xyloglucoside; (16) Quercetin 3-galactoside; (17) Rutin; (18) Quercetin 3-glucoside; (19) Quercetin derivative; (20) Unknown phloretin derivative; (21) Phloretin 2’-xyloglucoside; (22) Quercetin 3-rhamnoside; (23) Unknown phloretin derivative; (24) Unknown phloretin derivative; (25) Phloridizin; (26) Unknown; (27) Hyperin; (28) Avicularoside; (29) Quercetin.
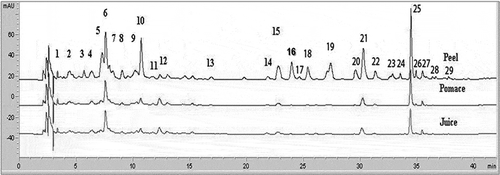
Figure 4 ‘Red Delicious’ peel, pomace, and juice HPLC chromatograms read at 280 nm. (1) Gallic acid; (2) Procyanidin B1; (3) Unknown procyanidin dimer; (4) Catechin; (5) Procyanidin B2; (6) Chlorogenic acid; (7) Cyanidin-3-galactoside; (8) Caffeic acid; (9) Unknown procyanidin dimer; (10) Epicatechin; (11) Cyanidin-3-rutinoside; (12) Procyanidin dimer; (13) p-Coumaric acid; (14); Procyanidin dimer; (15) 3-Hydroxyphloretin 2’-xyloglucoside; (16) Quercetin 3-galactoside; (17) Rutin; (18) Quercetin 3-glucoside; (19) Quercetin 3-xyloside; (20) 3’-Hydroxyphloretin 2’-glucoside; (21) Quercetin 3-arabinoside; (22) Phloretin 2-xyloglucoside; (23) Quercetin 3-rhamnoside; (24) Phloridizin; (25) Unknown; (26) Quercetin 3-α-L-arabofuranoside; (27) Quercetin.
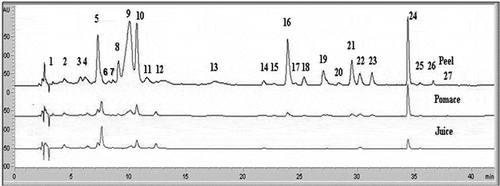
‘Verde Doncella’ chromatograms from peel samples contained a greater number of peaks when compared to ‘Red Delicious’. In regard to ‘Verde Doncella’, a total of four minor peaks were found after phloridzin (). As expected, HPLC profiles were more complex (in terms of number of compounds and peak area) for peel than for pomace and juice in both cultivars. The flavanol epicatechin (peak number 10), and the dihydrochalcone phloridzin (peaks 24 in ‘Red Delicious’ and 25 in ‘Verde Doncella’), were the greatest peaks in the chromatographic profile of both cultivars. These results are in agreement with previous studies[Citation7,Citation21] that pointed out epicatechin and phloridzin as the most abundant compounds in apple peel.
A total of 14 compounds belonging to the five major families of phenolic compounds (flavanols, hydroxycinnamic acids, flavonols, dihydrocalcones, and procyanidins) were determined by HPLC. Method sensitivity was achieved by using wavelengths at the maximum UV absortion (λmax) for different families of polyphenols. All standards gave high linearity within the calibration range. Data with the optimum λ used for measurements, and the mean concentrations of compounds in peel, pomace, and juice samples are presented in .
Table 4 Mean concentrations (μg/g fresh apple) and standard deviations (n = 3) of individual and total polyphenols determined by HPLC.
Apple peel
As shown in and , the chromatographic profile of apple peel was more complex in terms of the number of compounds and peak areas. Although different phenolic distribution patterns can be observed among apple cultivars, it is known that apple peel has substantially higher phenolic content and antioxidant activity than other fruit parts. For example, Boyer and Liu[Citation2] reported that apple peel contains the most phytochemical compounds, including procyanidins, catechin, epicatechin, chlorogenic acid, phloridzin, and quercetin conjugates.
Epicatechin arose as the major phenol in both varieties, although ‘Red Delicious’ content (273 μg/g DW) was much higher than the one found in ‘Verde Doncella’ (135 μg/g DW). Other compounds presenting relatively high concentrations for both cultivars were catechin, chlorogenic acid, and quercetin-3-glucoside. Two compounds presented a greater concentration in ‘Verde Doncella’: p-coumaric acid (reaching a value of 59 μg/g DW) and procyanidin B2 (118 μg/g fresh apple). On the contrary, the two other hydroxycinnamic acids were more concentrated in ‘Red Delicious’. Quercetin derivates were almost exclusively found in the peel of both cultivars. However, major differences were observed between their contents as ‘Red Delicious’ presented a total concentration of flavonols (333 μg/g fresh apple), more than twice as large as those found in ‘Verde Doncella’ (135 μg/g fresh apple). Data clearly illustrates that even if apple peel represents a minor percentage (around 10%) of the whole fruit weight, it is a major source of phenolic compounds. Total phenol content was by far greater in the peel, with 1173 and 894 μg/g DW for ‘Red Delicious’ and ‘Verde Doncella’, respectively, which highlights the significance of apple peel as a polyphenol source in both varieties.
Apple pomace
Pomace chromatograms contained fewer peaks when compared to peel samples. ‘Red Delicious’ chromatograms included 21 peaks, while ‘Verde Doncella’ had 16 peaks. Phenolic compounds quantified as having the greatest concentration in apple pomace were flavanols (catechin and epicatechin), chlorogenic acid, and procyanidin B2. With respect to quercetin derivates, only quercetin-3-galactoside and quercetin-3-O-rutinoside could be quantified in the pomace of ‘Red Delicious’, whereas no quercetin derivate could be detected in ‘Verde Doncella’ pomace. Burda et al.[Citation8] previously reported that quercetin glycosides were found only in peel samples, after testing skin and flesh samples. Schieber et al.[Citation6] reported the presence of quercetin 3-rhamnoside in dried apple seeds (nearly twice as much as the next quercetin glycoside). Despite our processed pomace samples including the seeds, quercetin-3-rhamnoside could not be detected in any of the pomace samples tested for either cultivar. Other compounds found in smaller quantities in both cultivar pomaces included phloridzin and gallic acid. It is important to point out that the total content of flavanols, hydroxycinnamic acids, flavonols, phloridzin, procyanidin B2, and gallic acid content was always less in ‘Verde Doncella’ than in ‘Red Delicious’.
Apple Juice
Total phenolics were much higher in ‘Red Delicious’ (89 μg/g FW) than in ‘Verde Doncella’ juice (58 μg/g FW). ‘Red Delicious’ juice contained greater polyphenol compound concentrations when compared to ‘Verde Doncella’ juice (87 μg/g versus 58 μg/g). Valles et al.[Citation29] reported that Spanish varieties had fewer polyphenol compounds, namely, epicatechin, phloridzin, procyanidin B2, and trimer and tetramer procyanidins when compared to English apple varieties, results that are reflected by our data. Interestingly, no p-coumaric acid was found in juice (nor in pomace) of ‘Verde Doncella’, although a relatively high content of this compound was present in the peel. Catechin and epicatechin, together with chlorogenic acid and procyanidin B2, were identified as polyphenol compounds containing greater concentrations in juices from both cultivars. Previous studies[Citation21,Citation30,Citation31] have identified catechin, epicatechin, chlorogenic acid, caffeic acid, and phloridzin in apple juices and ciders.
Relationship between Total Phenolics and Antioxidant Activity
The relationship between the total phenolics (measured by both TPC and HPLC) and the antioxidant activity were examined by Pearson correlations. ‘Red Delicious’ peel had the greatest antioxidant activity according to the FRAP method, results that are consistent with the TPC values found for this cultivar. The FRAP activity of peel, pomace, and juice of both cultivars showed positive linear correlations with TPC (r = 0.99) and total phenolics determined by HPLC (r = 0.96 for ‘Red Delicious’; r = 0.99 for ‘Verde Doncella’). When calculated against the major groups of polyphenols, the FRAP values were found to have the best linear correlation with the flavanols (r = 0.99) and quercetin derivates (r = 0.98) for ‘Red Delicious,’ and ‘Verde Doncella’ (r = 0.95 and r = 0.99, respectively). This assay clearly showed, therefore, that flavanols and flavonols (quercetin derivates) were the most important contributors to the antioxidant activity of both apple cultivars.
CONCLUSIONS
This study provides a database for color CIELAB coordinates, qualitative and quantitative phenolic composition, and antioxidant activity of ‘Verde Doncella’, a valuable apple cultivar from northeast Spain that has received little to no attention in previous works focused on apple phenolic composition. ‘Verde Doncella’ results were compared to data from ‘Red Delicious’, a worldwide-cultivated variety. Our results highlight that the phenolic distribution patterns as well as the antioxidant activity were quite different among cultivars. ‘Verde Doncella’ demonstrated lower TPC and total phenolics values measured by HPLC, especially with respect to total flavanols and quercetin derivates in the three parts of the fruit evaluated (peel, pomace, and juice). These observations agreed with the low antioxidant activity values acquired for this variety. For both cultivars, the qualitative and quantitative distribution of phenolic compounds varied significantly between the peel, pomace, and juice. At the individual compound level, flavanols and flavonols (quercetin derivates) were the most important contributors to the antioxidant activity of both apple cultivars. The high polyphenolic potential and antioxidant activities of ‘Verde Doncella’ in apple pomace, comparable to those of ‘Red Delicious,’ point out the possible health benefits in the consumption of this variety. This study shows that ‘Verde Doncella’ cultivar presents an interesting polyphenolic profile. The high total phenol content, especially p-coumaric acid and procyanidin B2 in peel as well as phloridzin in pomace, make this apple cultivar a valuable source of natural antioxidants.
FUNDING
This study was supported by the project AGL2009-59 08501 (Programa Nacional de Proyectos de Investigación Fundamental), co-financed by the European Social Fund and by the Departamento de Ciencia, Tecnología y Universidad del Gobierno de Aragón, Research Group “Foods from Plant Origin.” Fellowship funding JAE-Predoc/CSIC was supported by the Ministerio de Ciencia e Innovación (MICCIM).
REFERENCES
- Errea-Abad, P. Recuperación de frutales en peligro de extinción. Revista Aragón Investiga. 2009. http://www.aragoninvestiga.org
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutrition Journal 2004, 3, 5.
- Wolfe, K.; Wu, X.; Liu, R.H., Antioxidant activity of apple peels. Journal of Agricultural and Food Chemistry 2003, 51, 609–614.
- Guyot, S.; Marnet, N.; Laraba, D.; Sanoner, P.; Drilleau, J.F. Reversed-phase HPLC following thiolysis for quantitative estimation and characterization of the four main classes of phenolic compounds in different tissue zones of a French cider apple variety (Malus domestica var. Kermerrien). Journal of Agricultural and Food Chemistry 1998, 46, 1698–1705.
- Spanos, G.A.; Wrolstad, R.E. Phenolics of apple pear and white grape juice and their changes with processing and storage a review. Journal of Agricultural and Food Chemistry 1992, 40, 1478–1487.
- Schieber, A.; Hilt, P.; Streker, P.; Endreß, H.-U.; Rentschler, C.; Carle, R. A new process for the combined recovery of pectin and phenolic compounds from apple pomace. Innovative Food Science & Emerging Technologies 2003, 4, 99–107.
- Tsao, R.; Yang, R. Optimization of a new mobile phase to know the complex and real polyphenolic composition: Towards a total phenolic index using high-performance liquid chromatography. Journal of Chromatography A 2003, 1018, 29–40.
- Burda, S.; Oleszek, W.; Lee, C.Y. Phenolic-compounds and their changes in apples during maturation and cold-storage. Journal of Agricultural and Food Chemistry 1990, 38, 945–948.
- Leccese, A.; Bartolini, S.; Viti, R. Genotype, harvest season, and cold storage influence of fruit quality and antioxidant properties of apricot. International Journal of Food Properties 2012, 15, 864–879.
- Dineiro Garcia, Y.; Suarez Valles, B.; Picinelli Lobo, A. Phenolic and antioxidant composition of by-products from the cider industry: Apple pomace. Food Chemistry 2009, 117, 731–738.
- Dragovic-Uzelac, V.; Pospisil, J.; Levaj, B.; Delonga, K. The study of phenolic profiles of raw apricots and apples and their purees by HPLC for the evaluation of apricot nectars and jams authenticity. Food Chemistry 2005, 91, 373–383.
- Karaman, S.; Tutem, E.; Baskan, K.S.; Apak, R. Comparison of total antioxidant capacity and phenolic composition of some apple juices with combined HPLC-CUPRAC assay. Food Chemistry 2010, 120, 1201–1209.
- Lata, B.; Trampczynska, A.; Paczesna, J. Cultivar variation in apple peel and whole fruit phenolic composition. Scientia Horticulturae 2009, 121, 176–181.
- Kahle, K.; Kraus, M.; Richling, E. Polyphenol profiles of apple juices. Molecular Nutrition & Food Research 2005, 49, 797–806.
- Perez-Ilzarbe, J.; Hernandez, T.; Estrella, I. Phenolic compounds in apples—Varietal differences. Zeitschrift fur Lebensmittel-Untersuchung und-Forschung 1991, 192, 551–554.
- Marquina, P.; Venturini, M.E.; Oria, R.; Negueruela, A.I. Monitoring colour evolution during maturity in Fuji apples. Food Science and Technology International 2004, 10, 315–321.
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC: Arlington, Virginia, 1990 ; 1298.
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology 1999, 299, 152–178.
- Singleton, V.L.; Rossi Jr., J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture 1965, 16, 144–158.
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Analytical Biochemistry 1996, 239, 70–76.
- Schieber, A.; Keller, P.K.; Carle, R. Determination of phenolic acids and flavanoids of apple and pear by high-performance liquid chromatography. Journal of Chromatography A 2001, 910, 265–273.
- Abbott, J.; Saftner, R.; Gross, K.; Vinyard, B.; Janick, J. Consumer evaluation and quality measurement of fresh-cut slices of ‘Fuji,’ ‘Golden Delicious,’ ‘GoldRush,’ and ‘Granny Smith’ apples. Postharvest Biology and Technology 2004, 33, 127–140.
- Iglesias, I.; Salvia, J.; Torguet, L.; Cabús, C. Orchard cooling with overtree microsprinkler irrigation to improve fruit color and quality of ‘Topred Delicious’ apples. Scientia Horticulturae 2002, 93, 39–51.
- McGuire, R.G. Reporting of objective color measurements. Hort Science 1992, 27, 1254–1255.
- Chauhan, O.P.; Singh, A.; Singh, A. Effects of osmotic agents on colour, textural, structural, thermal, and sensory properties of apple slices. International Journal of Food Properties 2010, 14, 1037–1048.
- Vieira, F.G.K.; Borges, G.D.C.; Copetti, C.; Gonzaga, L.V.; Nunes, E.D.; Fett, R. Activity and contents of polyphenolic antioxidants in the whole fruit, flesh and peel of three apple cultivars. Archivos Latinoamericanos De Nutricion 2009, 59, 101–106.
- Haytowitz, D.B., Bhagwat, S.A., USDA database for the oxygen radical capacity (ORAC) of selected foods, release 2. USDA National Nutrient Database for Standard Reference. 2010. http://www.ars.usda.gov/nutrientdata
- Lotito, S.B.; Frei, B. Relevance of apple polyphenols as antioxidant in human plasma: Contasting in vitro and in vivo effects. Free Radical Biology & Medicine 2004, 36, 201–211.
- Valles, B.S.; Victorero, J.S.; Alonso, J.J.M.; Gomis, D.B. High-performance liquid-chromatography of the neutral phenolic-compounds of low-molecular weigh in apple juice. Journal of Agricultural and Food Chemistry 1994, 42, 2732–2736.
- Karaman, Ş.; Tütem, E.; Sözgen Başkan, K.; Apak, R. Comparison of total antioxidant capacity and phenolic composition of some apple juices with combined HPLC–CUPRAC assay. Food Chemistry 2010, 120, 1201–1209.
- Wu, J.H.; Gao, H.Y.; Zhao, L.; Liao, X.J.; Chen, F.; Wang, Z.F.; Hu, X.S. Chemical compositional characterization of some apple cultivars. Food Chemistry 2007, 103, 88–93.
