Abstract
The coagulations of soft-shell turtle egg white at high pressure or at high temperature were investigated and compared to chicken egg white. Unlike chicken egg white, no coagulation of soft-shell turtle egg white was observed after high-pressure processing (HPP; 200–600 MPa) or heating treatment (75°C). Basic amino acids, histidine, lysine, and arginine were present at higher percentages in soft-shell turtle egg white proteins than in chicken egg white proteins. Sodium dodecyl sulfate/polyacrylamide gel electrophoresis showed that the intensities of soft-shell turtle egg white components were balanced, there were more components with high molecular mass in soft-shell turtle egg white, and that aggregates formed in both soft-shell turtle egg white and chicken egg white. This work revealed some differences in soft-shell turtle egg white compositions. The low protein content in egg white was responsible for the non-coagulation of soft-shell turtle egg white in response to high pressure or temperature.
INTRODUCTION
Coagulation is an important functional property of protein that plays an important role in food processing, particularly in the quality of some foods, such as meat, and in the production of food, including tofu. Avian egg white is a major protein source in the human diet, and its properties including coagulating have been widely studied. The coagulation of egg white appears to be heat-induced and depend on protein concentration, ionic strength, pH, and interactions with other egg white components.[Citation1–Citation5] Bridgman[Citation6] found that high-pressure processing (HPP) induces coagulation of chicken (Gallus gallus) egg white (CEW). Since then, extensive research[Citation7–Citation13] has focused on the possible application of HPP as a new food processing method.
With the rapid development of aquaculture in recent years, the number of eggs from the Chinese soft-shelled turtle (Pelodiscus sinensis), which have been used as luxury food and a nutritional supplement, has increased.[Citation14] However, there is limited information regarding the functional properties of this reptile egg, particularly on soft-shelled turtle egg white (STEW) coagulation. The objectives of this study were to investigate the functional properties of STEW and to compare the effects of high pressure and temperature on the coagulation properties of STEW and CEW.
MATERIALS AND METHODS
Materials
Pelodiscus sinensis eggs were donated by Zhejiang Yueteng Aquatic Foods Co. Ltd. (Xiaoshan District of Hangzhou, China). Chicken eggs were purchased from a local market in Hangzhou and stored at 4°C. Eggs were broken by hand, and egg whites were separated from yolks. The egg whites were gently mixed manually using a glass rod and then analyzed. All chemicals used in this study were of analytical grade.
Thermal Treatment
Samples of egg white (3 ml each) were heated at 75 ± 1°C in 10 mL tubes in a temperature -controlled water-bath. After 10 min, the tubes were removed from the water-bath, transferred immediately to an ice-bath, and then stored at 4°C for 24 h before analysis.
HPP
HPP was conducted using a laboratory-scale unit (UHPF-750 MPa, vessel 112 mm diameter, 304 mm deep; Baotou Kefa, China) at a maximum pressure of 600 MPa. Distilled water was used as a medium during pressurization. Approximately 10 mL of egg white was packed into a polyethylene pouch. Sample pouches were repacked in polyethylene bags, which were filled with water for better pressure transmission. Samples were subjected to pressures of 200, 400, and 600 MPa at ambient temperature for 10 min. Triplicate samples were used for pressure treatment, and average values are reported. Following HPP, samples were stored at 4°C.
Differential Scanning Calorimety (DSC)
Samples of egg white (4 mg each) were transferred into aluminum pans, which were then sealed hermetically; an empty cell was used as a reference. Samples were analyzed by temperature ramping from 20 to 100°C at a heating rate of 5°C min−1 in a differential scanning calorimeter (DSC 7, Perkin-Elmer, Waltham, MA, USA). Samples were analyzed in duplicate.
Rheological Measurement
A rotational rheometer (RS6000, Haake, Germany) was used to measure changes in viscoelasticity of egg white. Samples of egg white were heated from 20 to 75°C at a rate of 2°C min−1. A moisture trap and low-viscosity siloxane oil were used to prevent moisture loss during heating. Measurements were conducted in duplicate using the rheometer in oscillation mode at a frequency of 1 Hz and a strain of 0.05 Pa chosen from the linear viscoelastic region of the samples.
Proximate Analysis and Determination of Amino Acid Composition
Moisture content of egg white was determined gravimetrically by the mass change in samples after drying in a vacuum freeze-drier (ALPHA 1-4 LSC, Martin Christ, German) for 24 h (when mass is constant). The amount of protein was estimated from the total nitrogen content determined using the Kjeldahl method with a fully automatic azotometer (UDK152, Karson Technology Ltd, Hong Kong) and a conversion factor of × 6.25.[Citation15] Amino acid composition of egg whites was measured using an amino acid analyzer (S-433D, Sykam, Germany). All analyses were conducted in triplicate and the results are presented as mean ± standard deviation.
Determination of Solubility
Samples of egg whites (3 g each) were homogenized with 27 mL of buffer A (0.086 M Tris, 0.09 M glycine, 4mM Na2EDTA, pH 8.0) using a homogenizer at 12,000 rpm for 2 min in an ice-bath, and then centrifuged at 4°C (Hettich Universal 320, Germany) for 15 min at 20,000 × g. Protein content of the supernatant was determined by the method of Peterson,[Citation16] using bovine serum albumin as a standard. Solubility is expressed as the mass of protein per liter of supernatant.
Determination of Sulfhydryl (SH) Content
Free SH groups were measured using Ellman’s reagent.[Citation17] A 4 mL sample of protein supernatant obtained using the method described above was added to 0.04 mL (4 mg mL−1) of DTNB (5,5′-dithiobis(2-nitrobenzoic acid)(in 1 mL of buffer A), and the absorbance at 412 nm was measured after incubating the sample for 15 min at ambient temperature. Buffer A used as a reagent blank; a protein blank was used in which Ellman’s reagent solution was replaced with 0.4 mL of buffer A. A molar extinction coefficient of 1.36 × 104 M−l cm−l was used to calculate SH content (in micromoles of SH g−1 of protein). Measure of total SH groups was necessary to maintain the sample of protein supernatant at 40°C in a water-bath for 15 min.
Electrophoresis
Sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS-PAGE) was conducted as described by Laemmli[Citation18] with minor modifications using a 4% (w/v) acrylamide stacking gel and 12.5% (w/v) separating gel. Treatment of samples was similar to that used to determine solubility, but with centrifugation at 7500 × g. The precipitate in buffer A was collected and dissolved in buffer B (buffer A containing 8 M urea and 5 mg mL−1 SDS). The supernatant and the precipitate were adjusted using buffer A and buffer B, respectively, so that the protein concentrations of samples were approximately 1.25 mg mL−1. Samples were mixed 4:1 (v/v) with sample buffer (0.5M Tris-HCl, pH 6.8 containing 4% (w/v) SDS, 20% (v/v) glycerol, and 0.3% (w/v) bromophenol blue) in the presence and absence of 10% (v/v) 2-mercaptoethanol (2-ME); samples were then boiled for 2 min. The prepared sample (20 μL) was loaded onto the gel. Protein standards (rabbit phosphorylase b (97.4 kDa), bovine serum albumin (66.2 kDa), rabbit actin (43 kDa), bovine carbonic anhydrase (31 kDa), trypsin inhibitor (20.1 kDa), and chicken albumin lysozyme (14.4 kDa)) were used to estimate molecular mass. Electrophoresis was conducted in a vertical gel electrophoresis unit (Mini PROTEA-N Tetra; Bio-Rad, Richmond, CA) at a constant current of 15 mA/plate..Gels were stained with Coomassie brilliant blue R-125 (0.125% (w/v) in 25% (v/v) methanol and 10% (v/v) acetic acid. Destaining was conducted using 40% methanol (v/v)/10% acetic acid (v/v), and gels were scanned using an APB Image Scanner (Amersham Pharmacia Biotech, Uppsala, Sweden).
Statistical Analysis
One-way analysis of variance was used to determine the difference among treatment means at the 5% significance level. Duncan’s new multiple range comparison was followed to evaluate difference between treatment means.
RESULTS AND DISCUSSION
Coagulation of Albumin at High Pressure or at High Temperature
First, the coagulation property of STEW induced by high pressure or high temperature was examined. STEW showed non-coagulating behavior induced by high pressure or heating, even at 100°C for 15 min (data not shown). High pressure (600 MPa) induced a large decrement of transparency in STEW, which remained in a fluid state, but CEW was changed from a transparent liquid into a semi-solid white material resembled jellied bean curd (, left). Upon heating, STEW became entirely opaque, accompanied by emergence of a precipitate, and CEW coagulated thoroughly (, right). Visual changes result from transformation of the inner framework of system, and proteins denaturing plays a key role in the state alteration of egg white. Shen et al.[Citation19] reported that most proteins are more sensitive to temperature than to pressure.
Figure 1 Visual changes in STEW and CEW after treatments with high temperature or high pressure. Left: pressure treatment; Right: heating treatment.
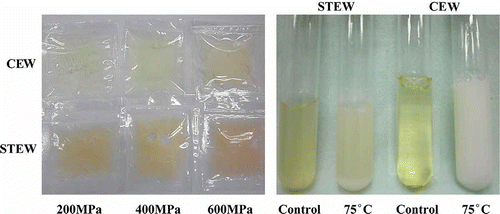
The effects of various levels of pressure on the coagulation properties of both kinds of egg white are shown in (left). The transparency of CEW decreased with increasing pressure but remained relatively unaffected even when treated with 600 MPa. This relatively high pressure stability could be due to the presence of four disulfide bonds in ovalbumin, the major protein in CEW, and strong non-covalent interactions stabilizing the three-dimensional structure of the protein.[Citation20] However, coagulation of CEW does occur at higher pressures. STEW reacted differently when treated with pressure; the sample was clear at 200 MPa, obscure at 400 MPa, and nearly opaque at 600 MPa. CEW remained in the liquid state until pressure reached 400 MPa, when it coagulated. In contrast, STEW underwent a change from liquid to the appearance of sol, to a milky phase and finally, to glue-like state. Protein compositions of reptile egg whites differs from that in CEW,[Citation21] while protein coagulation induced by high pressure or heating is correlated with the protein system, so the STEW showed different coagulation properties than CEW at high pressure and high temperature.
Difference in Denaturation Enthalpy
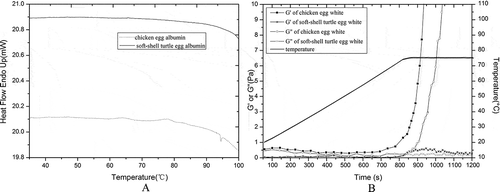
The coagulation properties of egg white are correlated to the conformational stability of protein under stress, such as high pressure and temperature. Denaturation thermograms of STEA and CEA were obtained by DSC. As shown in , the STEW thermogram was smooth with a negligible peak at 64.9°C. In contrast, CEW showed major thermal transitions at 63.9 and 77.7°C during heating from 30 to 100°C. Generally, the status of materials other than inert substances will be changed and present endothermic or exothermic peaks in a DSC thermogram when heated to a given temperature.[Citation22] The difference of denaturation thermograms between STEW and CEW indicate that there is little substance that can be denatured, but an abundance of material with high thermostability in STEW.
Viscoelastic Properties
Observation of the variation in the viscoelastic properties during temperature programming is the most common approach to studying the kinetics of protein coagulation. In this study, the elastic module G′ and viscous module G′′ were monitored during heating to investigate the coagulation properties of STEW. There were only minimal changes in the value of G′ or G′′ for STEW, with only slight fluctuations in the curves of elastic modulus versus time accompanied by laggard enhancement of G′ and G′′ at high temperature compared to those for CEW, which increased suddenly when heating time was 700 and 860 s and temperature was approximately 65 and 72°C, respectively. These results indicate that a pronounced difference exists in the coagulation properties of STEW and CEW under heating. The values of G′ were higher those of G′′ for CEW at different times and temperatures. However G′ values were lower than those for G′′ values for STEW before a heating time of 800 s and temperatures under 73.9°C. G′ values were higher than those for G′′ values at longer treating times. Both observations further revealed the large difference between compositions of STEW and CEW.
Contents of Moisture and Protein
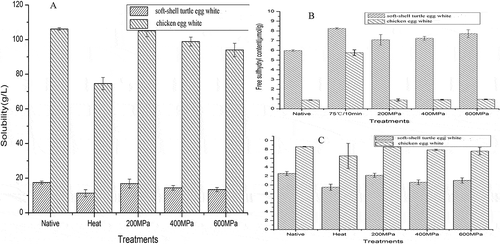
As shown in , the percentage water content of native STEW is higher than that of CEW; turtle egg white contains more water than avian egg white, which agrees with data reported by Booth[Citation15] for two freshwater turtle species. However, Wallace et al.[Citation23] reported that the water content of the leather-back turtle egg white is approximately 840 g kg−1 of egg white wet mass, which is lower than that in CEW. The protein content in STEW is very low, approximately 17.8 g kg−1, compared to that in CEW (115.8 g kg−1), which may explain the absence of notable endothermic peaks in DSC and little change in the rheological properties of STEW. It is known that coagulation of protein solution required a certain concentration of protein; thus, this data appears to explain why STEW did not coagulate under high pressure or high temperature ( and ).
Table 1 Content of moisture and protein in soft-shell turtle egg white and chicken egg white.
Amino Acid Composition
It is well-known that many features of proteins are related to amino acid composition of the protein; thus, analysis of amino acid composition is an important tool for predicting the properties of unfamiliar proteins. There is little information regarding the amino acid composition of STEW in the literature; thus, composition was measured in this study. As shown in , the content of 16 amino acids (aspartic acid, threonine, serine, glutamic acid, proline, glycine, alanine, valine, methionine, isoleucine, leucine, tyrosine, phenylalanine, histidine, lysine, and arginine) is lower in STEW than in CEW; total ˜ 16.5 g kg−1 in STEW compared to ˜ 112.4 g kg−1 in CEW. However, the percentages of threonine, serine, glycine, tyrosine, histidine, lysine, arginine, and particularly histidine (˜ 12.36%) were higher in STEW than in CEW and lower for the other nine amino acids in STEW compared to CEW. The discrepancy of amino acids composition implies that the properties of STEW differ from those of CEW. Shimada and Matsushita[Citation3] suggested that a protein with a low hydrophobic amino acid content (valine, proline, leucine, isoleucine, phenylalanine, and tryptophan) likely do not coagulate at high temperature, even at high protein concentration.
Table 2 Amino acid composition of protein derived from soft-shell turtle egg white and chicken egg white.
Protein Solubility
Change of proteins solubility is a prerequisite of gel formation, and analyzing changes will contribute to the understanding of differences in coagulation properties between STEW and CEW when subjected to heating or high pressure. In this study, the solubility of STEW and CEW decreased upon heating and exposure to high pressure in the following order: 75°C > 600 MPa > 400 MPa > 200 MPa (). However, no significant differences between treatments for STEW or CEW were detected. The number of hydrophobic groups on the protein surface has an important effect on protein solubility. In the native state, most hydrophobic groups are buried in the interior of the molecule, and exposing these groups requires energy input. It has been suggested that heating provides more energy than pressure[Citation24] and, thus, results in greater denaturation of protein and a more striking decrease in protein solubility than high pressure. Additionally, with increasing pressure, more energy is supplied, and hydrophobic groups buried in the protein core become exposed, resulting in loss of water coating due to incompatibility of a protein surface with water, causing protein molecules to aggregate accompanied and become less soluble.[Citation25] However, the increase in surface hydrophobicity is less pronounced at elevated pressure compared to elevated temperature at ambient pressure, particularly when pressure is below 400 MPa.[Citation9] Decreases for STEW and CEW were different (). Responses of protein solutions to external stresses were varied due to differences in structure of protein molecular and effects of other components in system on the protein. Several studies[Citation3,Citation4,Citation26,Citation27] have found that the source of proteins affected changes in protein solubility induced by high pressure or temperature. A possible explanation for these behaviors is that the protein surface hydrophobicity of STEW is more susceptible than that of CEW to heating and to high pressure.
Changes in SH Group Content
SH groups are the precursors of disulfide bonds, which play important roles in maintaining proteins tertiary structure. Disulfide bonds can be broken and reformed, resulting in protein denaturation. Thus, changes in protein structure can be examined estimating conversions between SH groups and S–S bonds. shows that the number of free SH groups was increased in STEW at high pressure and high temperature, which had a more pronounced effect and was correlated with pressure increase (whereas insignificant statistically between various pressures). All increases in exposed SH groups induced by high pressure or high temperature were correlated with a decrease in the total number of SH groups of STEW. Plancken et al.[Citation28] suggested that increased pressure or heating resulted in increased exposure of buried SH groups of CEW proteins and, at the same time, there was oxidation of SH groups into S–S bonds; as a result, total SH groups decreased. However, the increase in exposure of buried SH groups and decrease in total SH was less pronounced at elevated pressure due to the weak acting of high pressure. This may explain why pressure treatment induced protein solubility loss to a lesser extent compared to heat treatment.[Citation9] This may be similar in STEW. Native and treated (high temperature or pressure) STEW had more free SH groups but fewer total SH groups than CEW. This indicates that proteins derived from STEW and CEW are different, which agrees with observations regarding differences in their amino acid compositions.
Electrophoresis
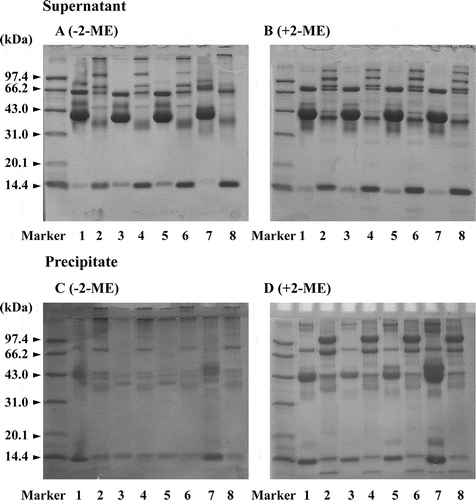
SDS-PAGE showed that more components with high molecular weight exist in STEW compared to CEW, and the intensity of protein constituents (with molecular mass from above 97.4 kDa to 43.0 kDa) in STEW were balanced, unlike in CEW, in which ovalbumin is predominant with very small amounts of other protein components ( and ). This result was similar to that of Prajanban et al.[Citation21] SDS-PAGE showed that high molecular weight aggregates exist in STEW after treatment with high pressure or heating ( and ). Formation of STEW aggregates under high pressure or high temperature appeared to result from formation of an S–S bond between protein molecules because differences of protein patterns under various treatments disappear in the presence of 2-ME ( and ). These results suggest that SH–disulfide interchange reactions occurred in STEW during pressurization and heating. There was little influence on egg white of pressure at low level (< 400 MPa), in good agreement with results reported by Ahmed et al.,[Citation23] whereas a pronounced effect appeared with increasing pressure, as shown in , corresponding to changes of SH groups. The thermocoagulation of proteins with low molecular weight does not depend on protein concentration; but coagulation of CEW, with a higher average molecular weight (> 60.0 kDa), is dependent on the concentration of protein.[Citation4] SDS-PAGE showed, however, that STEW has a larger molecular mass than CEW and that a disulfide cross-link was formed in STEW under high pressure or high temperature, indicating that coagulation of STEW at high pressure or high temperature is unrelated to molecular size and amino acid composition of STEW protein.
Figure 4 Non-reducing (a and c) and reducing (b and d) SDS-PAGE patterns of the supernatant and precipitate from the homogenate of STEW and CEW samples treated by heating (75°C) or high pressure (600 MPa). (1) Native CEW; (2) native STEW; (3) heated CEW; (4) heated STEW; (5) pressurized CEW; and (6) pressurized STEW.
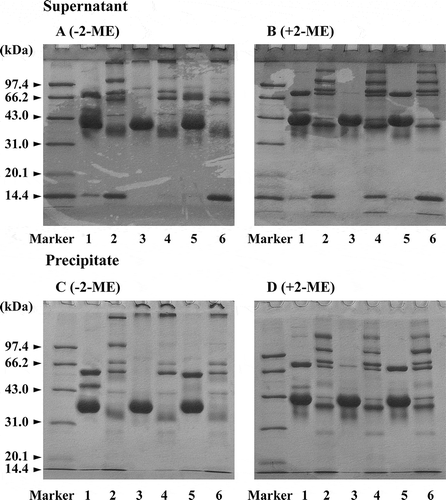
Figure 5 Non-reducing (a and c) and reducing (b and d) SDS-PAGE patterns of the supernatant and precipitate from the homogenate of STEW and CEW samples treated with various pressures. (1) Native CEW; (2) native STEW; (3) CEW with 200 MPa; (4) STEW with 200 MPa; (5) CEW with 400 MPa; (6) STEW with 400 MPa; (7) CEW with 600 MPa; and (8) STEW with 600 MPa.
CONCLUSION
It was found that STEW did not coagulate when heated or treated with high pressure, and STEW showed striking differences with CEW in denaturation enthalpy and viscoelasticity. STEW contained more water and less protein than CEW, and its amino acid composition was not similar to that of CEW, which may explain the above results. Variations in solubility and SH content existed between STEW and CEW, when the proteins were subjected to high pressure or high temperature. Moreover, the SDS-PAGE pattern of protein showed that aggregates formed in STEW at high pressure or high temperature. Thus, it can be concluded that the low protein content in egg white is solely responsible for non-coagulation of STEW under high pressure or high temperature.
FUNDING
This project was supported by Zhejiang Provincial Natural Science Foundation of China (No. Z3090301 and No. LY12C20015) and the Zhejiang Key Scientific and Technological Innovation Team Project (2009R50001).
REFERENCES
- Seideman, W.E.; Cotterill, O.J.; Funk, E.M. Factors affecting heat coagulation of egg white. Poultry Science 1963, 42, 406–417.
- Kaewmanee, T.; Benjakul, S.; Visessanguan, W. Effect of NaCl on thermal aggregation of egg white proteins from duck egg. Food Chemistry 2011, 125, 706–712.
- Shimada, K.; Matsushita, S. Thermal coagulation of egg albumin. Journal of Agriculture and Food Chemistry 1980a, 28, 409–412.
- Shimada, K.; Matsushita, S. Relationship between thermocoagulation of proteins and amino acid compositions. Journal of Agriculture and Food Chemistry 1980b, 28, 413–417.
- Mine, Y.; Noutomi, T.; Haga, N. Thermally induced changes in egg white proteins. Journal of Agriculture and Food Chemistry 1990, 38, 2122–2125.
- Bridgman, P.W. The coagulation of albumen by pressure. Journal of Biological Chemistry 1914, 19, 511–512.
- Al-Nabulsi, A.; Shaker, R.; Osaili, T.; Clark, S.; Harte, F.; Barbosa-Cánovas, G. Impact of high hydrostatic pressure and heat treatments on milk gel properties: A comparative rheological study. International Journal of Food Properties 2012, 15, 613–627.
- Hoppe, A.; Jung, S.; Patnaik, A.; Zeece, M.G. Effect of high pressure treatment on egg white protein digestibility and peptide products. Innovative Food Science and Emerging Technologies 2013, 17, 54–62.
- Van der Plancken, I.; Van Loey, A.; Hendrickx, Marc. E. Foaming properties of egg white proteins affected by heat or high pressure treatment. Journal of Food Engineering 2007, 78, 1410–1426.
- Sahu, J.K. Coagulation kinetics of high pressure treated acidified milk gel for preparation Chhana (an Indian soft cottage cheese). International Journal of Food Properties 2010, 13, 1054–1065.
- Okamoto, M.; Kawamura, Y.; Hayashi, R. Application of pressure to food processing, textural comparison of pressure and heat induced gels of food proteins. Agricultural Biological Chemistry 1990, 54, 183–189.
- Denys, S.; Ludikhuyze, L.R.; Van Loey, A.M.; Hendrickx, M.E. Modeling conductive heat transfer and process uniformity during batch high-pressure processing of foods. Biotechnology Progress 2000, 16, 92–101.
- Ngarize, S.; Adams, A.; Howell, N. A comparative study of heat and high pressure induced gels of whey and egg albumen proteins and their binary mixtures. Food Hydrocolloids 2005, 19, 984–996.
- Shi, H.; James, F.P.; Fan, Z.; Hong, M.; Yin, F. Evidence for the massive scale of turtle farming in China. Oryx (Cambridge University Press) 2008, 42, 147–150.
- Booth, D.T. Composition and energy density of eggs from two species of fresh water turtle with two fold ranges in egg size. Comparative Biochemistry and Physiology 2003, 134, 129–137.
- Peterson, G.L. A simplification of the protein assay method of 1 Lowry et al. which is more generally applicable. Analytical Biochemistry 1977, 83, 346–356.
- Beveridge, T.; Toma, S.J.; Nakai, S. Determination of SH- and SS-groups in some food proteins using Ellman’s reagent. Journal of Food Science 1974, 39, 49–51.
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685.
- Shen, C.; Hsu, S.; Chang, C.J. Co-solvent-modified supercritical carbon dioxide extractions of cholesterol and free amino acids from soft-shell turtle fish egg. Separation and Purification Technology 2008, 60, 215–222.
- Hayakawa, I.; Kajihara, J.; Morikawa, K.; Oda, M.; Fujio, Y. Denaturation of bovine serum albumin (BSA) and ovalbumin by high pressure, heat, and chemicals. Journal of Food Science 1992, 57, 288–292.
- Prajanbana, B.; Shawsuan, L.; Daduang, S.; Kommanee, J.; Roytrakul, S.; Dhiravisit, A.; Thammasirirak, S. Identification of five reptile egg whites protein using MALDI-TOF mass spectrometry and LC/MS-MS analysis. Journal of Protemics 2012, 75, 1940–1959.
- Huang, X.; Han, J.; Wang, Y.; Zhang, Y. Application development of differential scanning calorimetry in meat research. Science and Technology of Food Industry 2009, 30, 353–357. (In Chinese with English abstract)
- Wallace, B.P.; Sotherland, P.R.; Tomillo, P.S.; Bouchard, S.S.; Reina, R.D.; Spotila, J.R.; Paladino, F.V. Egg components, egg size, and hatchling size in leather back turtles. Comparative Biochemistry and Physiology 2006, 145, 524–532.
- Hayakawa, I.; Linko, Y.Y.; Linko, P. Mechanism of high pressure denaturation of proteins. LWT-Food Science and Technology 1996, 29, 756–762.
- Van der Plancken, I.V.; Van Loey, A.; Hendrickx, M.E. Kinetic study on the combined effect of high pressure and temperature on the physico-chemical properties of egg white proteins. Journal of Food Engineering 2007, 78, 206–216.
- He, J.S.; Azuma, N.; Hagiwara, T.; Kanno, C. Effects of sugars 1 on the cross linking formation and phase separation of high-pressure-induced gel of whey protein from bovine milk. Bioscience, Biotechnology, and Biochemistry 2006, 70, 615–625.
- Puppo, C.; Chapleau, N.; Speroni, F.; Lamballerie-Anton, M.D.; Michel, F.; Añón, C.; Anton, M. Physicochemical modifications of high pressure treated soybean protein isolates. Journal of Agricultural and Food Chemistry 2004, 52, 1564–1571.
- Van der Plancken, I.; Van Loey, A.; Hendrickx, M.E. Combined effect of high pressure and temperature on selected properties of egg white proteins. Innovative Food Science and Emerging Technologies 2005, 6, 11–20.