Abstract
The characterization of bioactive components of virgin olive oil from nine varieties (Cuquillo, Empeltre, Manzanilla, Cornicabra, Picual, Arbequina, Lechin, Picudo, and Hojiblanca) has been carried out. Carotenoids, chlorophylls, α-tocopherol, fatty acids, total phenols, and individual phenols were determined. The antioxidant capacity was evaluated by 2,2′-diphenyl-1-picrylhydrazyl and 2,2′-azinobis (3-ethylbenzothiaziline-6-sulfonate) radical scavenging capacity assay, ferric reducing antioxidant potential assay, and oxygen radical absorbance capacity assay. Twelve phenolic compounds were detected and quantified by high-performance liquid chromatography with diode-array detection. Dialdehydic form of elenolic acid linked to tyrosol and hydroxytyrosol, oleuropein, and ligstroside aglycones were the major components in all the samples. Principal component analysis confirmed that Manzanilla, Lechin, Cuquillo, and Hojiblanca var. were the most similar. Furthermore, these results showed that the antioxidant capacity measured by different assays was highly influenced by the phenolic content, especially the dialdehydic form of elenolic acid linked to tyrosol and hydroxytyrosol.
INTRODUCTION
Bioactive compounds are being intensively studied to evaluate their effects on health, including antioxidant, antiallergic, antimicrobial, antithrombotic, antiatherogenic, hypoglycaemic, anti-inflammatory, antitumor, cytostatic, immunosuppressive properties, and hepatoprotective activities.[Citation1] Due to its fatty acid composition and other functional food components, such as polyphenols, tocopherols, and pigments, a growing interest in olive oil as a healthy food has been observed especially in the nonproducing regions. The main antioxidants of virgin olive oil are carotenoids and phenolic compounds. The phenolic fraction of olive oils contains several types of chemical compounds, each of which varies in chemical properties and impacts on the quality of virgin olive oil. It is important to note that the bitter and pungent sensory notes of virgin olive oils are related to the presence of these compounds. In addition to their sensory implications, the importance of these compounds lies in their antioxidant and health-promoting properties.[Citation2] The virgin olive oil composition depends on numerous factors, such as the interaction between the cultivar and the environment, cultivation techniques, fruit ripeness, and the oil extraction system.
As mentioned earlier, the olive oil hydrophilic extract contains a large number of phenolic compounds, including phenolic acids, phenyl ethyl alcohols, hydroxy-isochromans, flavonoids, lignans, and secoiridoids, with antioxidant properties, which have been studied using different methods, such as the 2,2′-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azinobis (3-ethylbenzothiaziline-6-sulfonate) (ABTS) tests,[Citation3] ferric reducing antioxidant potential (FRAP), and oxygen radical absorbance capacity (ORAC).[Citation4,Citation5] Typically, these methods have been applied to monitoring differences between olive varieties and changes during ripening and oil processing. The aim of this study is to characterize the composition on bioactive components of different monovarietal virgin olive oils, in order to know the variability between varieties of these compounds that are of great importance to human health and to evaluate the antioxidant capacity by four different methods (DPPH, ABTS, FRAP, and ORAC).
MATERIALS AND METHODS
Samples
A total of nine samples of virgin olive oil, including Cuquillo (Sierra del Solán, Murcia), Empeltre (Alcañiz, Teruel), Manzanilla (Cáceres, Extremadura), Cornicabra (Noez, Toledo), Picual (Vilches, Jaén), Arbequina (Mequinenza, Zaragoza), Lechín (Montellano, Sevilla), Picudo (Priego de Córdoba, Córdoba), and Hojiblanca (Vilches, Jaén), were studied. This study was conducted using commercial virgin olive oils obtained in online olive oil stores, cooperatives, and supermarkets from Seville, Spain. All samples were filtered with filter paper and stored under refrigeration (below 5°C) in the dark using amber glass bottles without headspace until analysis. Samples were analyzed within 1 week.
Chlorophylls and Carotenoids Determination
Chlorophylls and carotenoids contents were determined by spectrophotometric measurements.[Citation6] The quantitative assessment of the chlorophylls and carotenoids fraction was carried out by measuring the absorption of the olive oil at 670 and 470 nm, respectively. Measurements were made on a Hewlett Packard 8452 UV–vis diode array spectrophotometer (Hewlett–Packard, Palo Alto, CA, USA).
Fatty Acids and α-Tocopherols Determination
Fatty acids analysis was carried out by gas chromatography. Fatty acid methyl esters were prepared by transmethylation according to the Official Journal of the European Union.[Citation7] About 0.3 g of virgin olive oil was dissolved in 5 mL of hexane and 0.6 mL of a 2 N solution of KOH/CH3OH. The mixture was vigorously shaken for 30 s. The sample (1 μL) was injected into the gas chromatograph (Varian 3900, Netherlands) equipped with a split-splitless injector and a flame ionization detector (FID). An SPTM-2380, 60 m × 0.25mm i.d. and 0.25-μm film thickness column (Supelco, Bellefonte, PA, USA) was used. The temperatures of the injector and detector were set at 250°C. The oven temperature was maintained at 170°C for 10 min and was then programmed from 170 to 200°C at 1.5°C/min and kept at isothermal for 8 min. Hydrogen was used as the carrier gas at a flow rate of 1.0 mL/min. Each sample was analyzed in duplicate. The content of α-tocopherol was determined by luminescence.[Citation8] The emission spectrum was collected between 350 and 450 nm at intervals of 1 nm, and the excitation wavelength was set at 350 nm. The quantitative evaluation was based on five wavelengths (370, 371, 378, 414, and 417 nm), which are attributed to the fluorescence of this compound. All luminescence measurements were carried out with a Shimadzu RF-1501 spectrofluorophotometer (Shimadzu Co, Kyoto, Japan).
Total Phenolics and Individual Phenolics Determination
The total phenolic compounds content was estimated using the Folin-Ciocalteu colorimetric method.[Citation9,Citation10] Spectrophotometric analysis was performed at 765 nm. The concentration of total phenolics was expressed as mg gallic acid equivalent (GAE)/kg. The individual phenolics from virgin olive oil were performed by liquid-liquid extraction, and syringic acid solution (0.01 mg/mL) dissolved in CH3OH:H2O (80:20 v/v) was used as the internal standard. The analysis was performed as follows: 5 g of virgin olive oil was diluted with 2.5 mL of hexane and 2.5 mL of internal standard (IS), the extraction was performed with 2.5 mL of CH3OH:H2O (80:20 v/v). The hydro-alcoholic extract was concentrated under vacuum at room temperature. The polar fraction was filtered and analyzed with a HPLC LaChrom Elite® System (Hitachi Corporation, Japan). The column was a Superspher® 100 RP-18 column (4.0 mm inner diameter × 250 mm; 5 μm particle size) (Merck). The gradient system used was based on the procedure described by García-González et al.[Citation2]
Determination of Hydrophilic Antioxidant Capacity
The DPPH assay was performed according to the method described by Nenadis and Tsimidou[Citation11] with a few modifications. Briefly, a 100 μmol/L DPPH solution was freshly prepared in methanol. Then the absorbances were taken at 516 nm. The ABTS assay was described by Arnao et al.[Citation12] A stock solution of 7.4 mmol/L ABTS solution and 2.6 mM potassium persulfate solution were mixed in equal quantities and then the reaction was developed for 12 h. Then the absorbances were taken at 734 nm. The FRAP assay was performed according to the method by Cheung et al.[Citation13] The antioxidant capacity was measured using ferric reducing/antioxidant and ascorbic acid. The solutions used included: 300 mmol/L of pH 3.6 acetate buffer, 10 mmol/L 2,4,6-Tripyridyl-s-Triazine in 40 mmol/L HCl and 20 mmol/L FeCl3 anhydrous solution. The readings of the reaction were taken at 593 nm. The ORAC assay was performed according to the method by Cao et al.[Citation14] with a few modifications.[Citation5] The conditions used in the measurement of fluorescence were as follows: excitation wavelength at 485/20 nm and emission filter at 520/20 nm. The final reaction (2 mL) was performed in fluorometry cells (10 mm path length): Fluorescein (1600 μL; 4 nmol/L) and sample or standard (trolox) (200 μL). The mixture was pre-incubated for 10 min at 37°C, before the addition of 2,2′-azobis(2-methilpropionamide) dihydrochloride (200 μL; 221 mM). The results in all assays were expressed as mmol trolox equivalent (TE)/kg.
Statistical Analysis
The analytical determinations of fatty acids and individual phenols were carried out in duplicate; all other measurements were performed in triplicate. An analysis of variance (ANOVA) was performed to compare the data. Significant differences were calculated according to Fisher’s least significant difference (LSD) test, with p < 0.05 being considered significant in all analyses. Principal component analysis (PCA) involves a mathematical procedure that identifies patterns in data and expresses them in such a way as to highlight similarities and differences. PCA was applied to the samples according to their values of carotenoids, chlorophylls, α-tocopherol, oleic acid, linoleic acid, linolenic acid, hydroxytyrosol, tyrosol, dialdehydic form of elenolic acid linked to tyrosol and hydroxytyrosol (p-HPEA-EDA and 3,4-DHPEA-EDA), oleuropeine aglycon (p-HPEA-EA) and ligstroside aglycon (3,4-DHPEA-EA), total phenolics, and antioxidant capacity (DPPH, ABTS, FRAP, ORAC). Varimax rotation was applied to maximize the variance in each loading vector. Statistical analysis was performed using SPSS software for Windows (release 18.0).
RESULTS AND DISCUSSION
Chlorophylls and Carotenoids Content
Virgin olive oil has a color from green-yellow to gold, depending on the variety and the stage of maturity. As shown in , the compositions of pigments of the samples were found to be between 0.31–5.48 and 0.98–4.33 mg/kg, respectively. These results show significant differences between samples (p < 0.05) and are in agreement with the findings of Gandul-Rojas and Mínguez-Mosquera,[Citation15] who reported that the composition of chlorophylls and carotenoids in virgin olive oil is subject to a wide range of variations, such as variety, fruit ripening, latitude, environmental conditions, processing techniques, and storage conditions. Mínguez-Mosquera et al.[Citation16] found high levels of chlorophylls in Verdial, Hojiblanca, and Picudo var. virgin olive oils, the former being far superior to the latter two. Lechin var. had lower chlorophylls levels, however, was more stable over time while the Hojiblanca var. was the least stable. The levels of carotenoids are also variable (), which agrees with the findings of other authors. Moyano et al.[Citation6] found that Verdial, Pico limón, Picual, and Hojiblanca var. virgin olive oils had a higher content of carotenoids than Picudo and Arbequina var.
TABLE 1 Composition of chlorophylls, carotenoids, and α-tocopherol in virgin olive oil
TABLE 2 Composition of fatty acids in virgin olive oil samples
α-Tocopherol and Fatty Acids Content
shows the α-tocopherol contents of the oils studied and the significant differences between varieties (p < 0.05). In fact, α-tocopherol contents are ranging from 165.88 mg/kg (Cuquillo var.) to 344.31 mg/kg (Empeltre var.). These results are in agreement with those previously found suggesting that the levels obtained indicate a wide range of α-tocopherol milligrams per kg oil that depends on the potential varieties and technological factors. Escuderos et al.[Citation8] obtained values for Hojiblanca, Picual, Royal, Coratina, Frantoio, Koroneiki, and Chemlali var. between 147–329 mg/kg. García et al.[Citation17] found an α-tocopherol content for Picual, Cornicabra, and Hojiblanca var. between 174–252 mg/kg. The fatty acid composition is a quality parameter and an authenticity indicator of virgin olive oils. shows the fatty acid composition. The oleic acid, the major monounsaturated fatty acid, is especially high in Cornicabra virgin olive oil (82.46%), while it is low in Arbequina (70.14%). Concerning linoleic acid, a relatively low percentage was observed in Cornicabra (3.28%), and the highest value was observed in Empeltre (11.64%). The level of linolenic acid in the analyzed samples varies between 0.61–0.95%, corresponding to Arbequina var. and Picudo var., respectively. Oleic, linoleic, and linolenic fatty acids showed significant differences between the different virgin olive oil varieties (p < 0.05). These results agree with the observations by Aranda et al.[Citation18] for Cornicabra, Picual, Hojiblanca, and Arbequina var. The results show that Arbequina var. contains more total content of saturated fatty acids (ΣSFA) (17%) mainly for their high content of palmitic acid. With respect to the total content of monounsaturated fatty acids (ΣMUFA), Cornicabra var. contains a high percentage (83%) due to the presence of oleic acid and Empeltre var. contains a high percentage of polyunsaturated fatty acids (ΣPUFA) (13%) due to the high content of linoleic acid.
Total Phenolics and Individual Phenolic Content
shows the total phenols content (TPC) and individual phenolic compounds (IPC), recorded at λ = 280 nm of virgin olive oil samples of different varieties and the concentration of 12 phenolic compounds. According to the bibliography, the concentration of phenols varies, from a few to approximately 1200 mg/kg, and it depends on the cultivar and environmental variables, such as the irrigation regimes and the olive ripeness, among others.[Citation2] It is important to note that Picudo and Arbequina var. had the highest concentrations of total phenols (339.86 and 367.95 mg/kg, respectively) and Picual var. had the lowest one (86.91 mg/kg). The most representative individual phenolic components were p-HPEA-EDA and 3,4-DHPEA-EDA, p-HPEA-EA and 3,4-DHPEA-EA. The concentrations of p-HPEA-EDA and 3,4-DHPEA-EDA were higher in Arbequina (17.42 and 26.62 mg/kg) and lower in Cornicabra (6.42 and 1.10 mg/kg) and Picual (5.78 and 0.13 mg/kg), respectively. With regard to simple phenols, such as hydroxytyrosol and tyrosol, these compounds are more abundant in the oil from Cornicabra var. The Hojiblanca var. showed the lowest concentration of hydroxytyrosol and in Picual var. this compound was detected at significant levels. However, Cornicabra, Picudo, Cuquillo, and Lechin were the most representative. It is important to remark that virgin olive oil from Picual var. is characterized by a high concentration of phenols.[Citation2] Other phenolic compounds, such as vanillin, p-coumaric acid, vanillic acid, and pinoresinol, showed less variability; the concentrations of these compounds were relatively similar in all varieties. Similar results were detected by Allalout et al.[Citation19] in Arbequina, Arbosana, and Koroneiki var. Brenes et al.[Citation20] reported that the degree of ripeness of the olives is related to the content of phenols in olive oil, the levels of hydroxytyrosol, and tyrosol increase with the degree of maturity, while the secoiridoid compounds decrease during the ripening process.[Citation2]
TABLE 3 Concentrations of individual phenols and total phenols of virgin olive oil
Hydrophilic Antioxidant Capacity
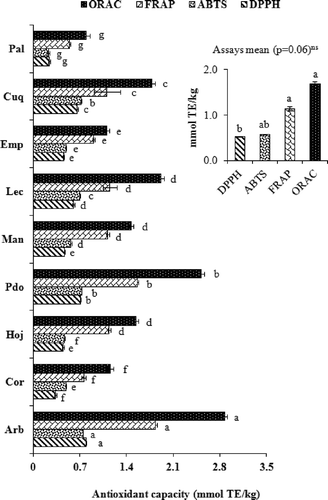
shows the antioxidant capacity values obtained by using different methods (DPPH, ABTS, FRAP, and ORAC) for the different varieties of olive oil. The results showed that Arbequina and Picudo var. had the greatest antioxidant capacity assessed by all methods used, but Picual var. showed less activity, since the sample of this variety contains the lowest concentration of phenols (). These results, however, cannot be generalized because a limited number of samples for each variety have been used. The difference in the levels of antioxidant responses found may reflect a relative difference in the ability of the phytochemical components present in the hydrophilic extracts from virgin olive oil to protect the peroxyl-fluorescein radical interaction, reduction of DPPH and ABTS free radicals, and the reduction of the FeCitation3+-tripyridyltriazine complex. Moreover, the assays’ mean was not significant (p = 0.06) and the ANOVA showed no interaction between variety and assays (p = 1.00). Previous studies on antioxidant capacity using the ORAC and FRAP methods in virgin olive oils showed values between 183–949 and 38–167 μmol TE/100 g, respectively.[Citation4] For comparison, the ORAC values for extra virgin olive oils reported by other authors were in the range of 178–620 μmol TE/100 g.[Citation5]
Principal Component Analysis (PCA)
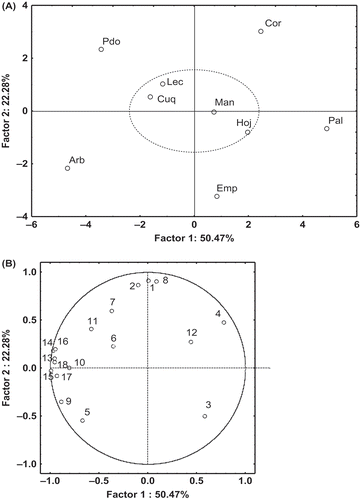
When PCA was applied, samples were separated along the first principal component (PC) by differences observed in α-tocopherol, oleic acid, linoleic acid, 3,4-DHPEA-EDA, p-HPEA-EDA, ΣIPCHPLC, TPCUV/vis, and antioxidant capacity (DPPH, ABTS, FRAP, and ORAC assays). The second PC separated the samples on the basis of carotenoids, chlorophylls, hydroxytyrosol, and tyrosol. The eigenvalues express the amount of the total variance explained by each principal component. The first PC explains 50.47% and the second PC explains 22.28%; therefore, the first two factors accounted for 72.75%. Four factors were selected corresponding to eigenvalues greater than 1, and the total percentage of variance explained by four factors selected was 88.58%. The scores of the first two principal components for nine virgin olive oils are presented in and the loadings plots for variables in . The analysis of the whole data set by PCA confirmed that Manzanilla, Lechin, Cuquillo, and Hojiblanca var. presents more similarity.
FIGURE 2 Scatter diagram of PC1 vs. PC2 of the main sources of variability between the virgin olive oil samples (A) and loadings plots obtained of the first two principal components for variables (B). 1: Carotenoids; 2: Chlorophylls; 3: α-Tocopherols; 4: Oleic acid; 5: Linoleic acid; 6: Linolenic acid; 7: Hydroxytyrosol; 8: Tyrosol; 9: 3,4-DHPEA-EDA; 10: p-HPEA-EDA; 11: 3,4-DHPEA-EA; 12: p-HPEA-EA; 13: ΣIPCHPLC; 14: TPCUV/vis; 15: DPPH; 16: ABTS; 17: FRAP; 18: ORAC.
CONCLUSIONS
The oils from Arbequina and Picudo var. presented major composition of phenolic compounds. The hydrophilic antioxidant capacity was also major for these varieties, considering that the lipophilic fraction was not analyzed and that the measurement of the nonpolar fraction would give us a greater understanding of this activity. With regard to carotenoids and chlorophylls content, these components were low in most of the samples, while the content of α-tocopherol and fatty acids was within the limits established by the regulation. On the other hand, the total and individual phenolic content was highly variable in the oils analyzed, which was reflected in the antioxidant capacity of the hydrophilic fraction. In fact, secoiridoids compounds, such as 3,4-DHPEA-EDA, p-HPEA-EDA, 3,4-DHPEA-EA, and p-HPEA-EA, were the major components of this fraction. The principal component analysis applied to the samples, according to the contents of the variables, showed that the oils from Manzanilla, Lechin, Hojiblanca, and Cuquillo var. are more similar. Furthermore, these results showed that the antioxidant capacity measured by DPPH, ABTS, FRAP, and ORAC is highly influenced by the phenolic content.
FUNDING
One of the authors (FR-E) is thankful to the Program on Science and Technology (FINCyT) for the award of Doctoral Research Fellowship (124-2009-FINCyT- BDE).
REFERENCES
- Patil, B.S.; Jayaprakasha, G.K.; Chidambara-Murthy, K.N.; Vikram, A. Bioactive compounds: Historical perspectives, opportunities, and challenges. Journal of Agricultural and Food Chemistry 2009, 57 (18), 8142–8160.
- García-González, D.; Tena, N.; Aparicio, R. Quality characterization of the new virgin olive oil var. Sikitita by phenols and volatile compounds. Journal of Agricultural and Food Chemistry 2010, 58 (14), 8357–8364.
- Samaniego, S.C.; Troncoso, A.M.; García-Parrilla, M.C.; Quesada, J.J.; López, H.; López, M.C. Different radical scavenging tests in virgin olive oil and their relation to the total phenol content. Analytica Chimica Acta 2007, 593 (1), 103–107.
- Szydłowska-Czerniak, A.; Karlovits, G.; Dianoczki, C.; Recseg, K.; Szłyk, E. Comparison of two analytical methods for assessing antioxidant capacity of rapeseed and olive oils. Journal of the American Oil Chemists’ Society 2008, 85 (2), 141–149.
- Ninfali, P.; Bacchiocca, M.; Biagiotti, E.; Servili, M.; Montedoro, G. Validation of the oxygen radical absorbance capacity (ORAC) parameter as a new index of quality and stability of virgin olive oil. Journal of the American Oil Chemists’ Society 2002, 79 (10), 977–982.
- Moyano, M.J.; Meléndez-Martínez, A.J.; Alba, J.; Heredia, F.J. A comprehensive study on the colour of virgin olive oils and its relationship with their chlorophylls and carotenoids indexes (I): CIEXYZ non-uniform colour space. Food Research International 2008, 41 (5), 505–512.
- European Economic Community. Commission Regulation (EU) No 61/2011, Amending Regulation (EEC) No 2568/91 on the Characteristics of Olive Oil and Olive-Residue Oils and on the Relevant Methods of Analysis. In: Official Journal of the European Union; Brussels, Belgium, 2011; L23/1–L23/14.
- Escuderos, M.E.; Sayago, A.; Morales, M.T.; Aparicio, R. Evaluation of α-tocopherol in virgin olive oil by a luminescent method. Grasas y Aceites 2009, 60 (4), 336–342.
- Cecchi, T.; Passamonti, P.; Alfei, B.; Cecchi, P. Monovarietal extra virgin olive oils from the Marche region, Italy: Analytical and sensory characterization. International Journal of Food Properties 2011, 14 (3), 483–495.
- Loizzo, M.R.; Di Lecce, G.; Boselli, E.; Menichini, F.; Giuseppe-Frega, N. Radical scavenging, total antioxidant capacity, and antiproliferative activity of phenolic extracts from extra virgin olive oil by cultivar ‘Frantoio’. International Journal of Food Properties 2012, 15 (6), 1345–1357.
- Nenadis, N.; Tsimidou, M. Observations on the estimation of scavenging activity of phenolic compounds using rapid 1,1-diphenyl-2-picrylhydrazyl (DPPH•) tests. Journal of the American Oil Chemists’ Society 2002, 79 (12), 1191–1195.
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chemistry 2001, 73 (2), 239–244.
- Cheung, S.Ch.M.; Szeto, T.Y.; Benzie, I.F.F. Antioxidant protection of edible oils. Plant Foods for Human Nutrition 2007, 62 (1), 39–42.
- Cao, G.; Alessio, H.M.; Culter, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radical Biology and Medicine 1993, 14 (3), 303–311.
- Gandul-Rojas, B.; Mínguez-Mosquera, M.I. Olive processing. In: Handbook of Fruits and Fruits Processing; Hui, Y.H.; Barta, J.; Cano, M.P.; Gusek, T.; Sidhu, J.S.; Sinha, N.K.; Eds.; Blackwell Publishing: Ames, Iowa, 2006; 491–513.
- Mínguez-Mosquera, M.I.; Rejano-Navarro, L.; Gandul-Rojas, B.; Sánchez-Gómez, A.H.; Garrido-Fernández, J. Color-pigment correlation in virgin olive oil. Journal of the American Oil Chemists’ Society 1991, 68 (5), 332–336.
- García, A.; Brenes, M.; García, P.; Romero, C.; Garrido, A. Phenolic content of commercial olive oils. European Food Research and Technology 2003, 216 (6), 520–525.
- Aranda, F.; Gómez-Alonso, S.; Rivera del Álamo, R.M.; Salvador, M.D.; Fregapane, G. Triglyceride, total and 2-position fatty acid composition of Cornicabra virgin olive oil: Comparison with other Spanish cultivars. Food Chemistry 2004, 86 (4), 485–492.
- Allalout, A.; Krichène, D.; Methenni, K.; Taamalli, A.; Oueslati, I.; Daoud, D.; Zarrouk, M. Characterization of virgin olive oil from super intensive Spanish and Greek varieties grown in northern Tunisia. Scientia Horticulturae 2009, 120 (1), 77–83.
- Brenes, M.; García, A.; García, P.; Rios, J.J.; Garrido, A. Phenolic compounds in Spanish olive oils. Journal of Agricultural and Food Chemistry 1999, 47 (9), 3535–3540.