Abstract
Four loose-curd cauliflower varieties, and one common cauliflower cultivar, were studied to investigate the appearance, antioxidant capacity, and levels of ascorbic acid, chlorophylls, carotenoids and glucosinolates in the florets. The loose-curd cauliflower was typically characterized by a loose curd and long florets with green pedicels and a yellow surface. The levels of total glucosinolates, sinigrin, and neoglucobrassicin were lower in the loose-curd cauliflower varieties than in the common cauliflower. The amount of glucoiberin, major aliphatic glucosinolate, present in loose-curd cauliflower varieties, was 147 to 241 nmol/g dry weight (average: 147 nmol/g dry weight). In contrast, the common cauliflower contained a much higher amount of sinigrin (249 nmol/g dry weight) than glucoiberin (56 nmol/g dry weight). All cauliflower varieties contained minor amounts of progoitrin and neoglucobrassicin. Furthermore, the loose-curd cauliflower varieties exhibited comparably higher levels of ascorbic acid, chlorophylls, and carotenoids, than total phenolics. The ferric reducing antioxidant power values in the loose-curd varieties ranged from 92 ± 7 to 107 ± 8 μmol Fe2+/g dry weight, which were also higher than that in the common cauliflower. The results indicated that the loose-curd cauliflower can provide higher levels of health-promoting compounds than the common cauliflower.
INTRODUCTION
Loose-curd cauliflower (Brassica oleracea var. botrytis) is a special type of cauliflower with a loose curd and long florets with green pedicels.[Citation1] This special type of cauliflower is popular in China and Southeast Asia, possibly because the florets are tender and crisp, and the flavor is good. In recent years, the area under cultivation and the production of this kind of cauliflower have greatly increased in some provinces of China (Zhejiang and Fujian) and become even higher than those of the common cauliflower.
Numerous epidemiological studies have indicated that diets based on cruciferous vegetables could slow down the development of chronic diseases such as various cancers and coronary heart disease.[Citation2,Citation3] Previous studies have also demonstrated that Brassica oleracea vegetables (e.g., broccoli and cauliflower) possess high levels of phytochemicals, including phenolics, glucosinolates, vitamins, and carotenoids,[Citation4,Citation5] that contribute to the chemopreventive activity against chronic diseases.[Citation6,Citation7] Ascorbic acid (vitamin C), an antioxidant and free-radical scavenger, is closely linked to the reduced risk of various chronic diseases that have their origins in oxidative stress.[Citation8] Phenolic compounds act as the major antioxidants in Brassica crops,[Citation9] and play important roles in the prevention of degenerative diseases such as cancers and cardiovascular diseases.[Citation6,Citation7,Citation10] Moreover, several phenolic compounds have the potential to prevent diabetes mellitus by inhibiting certain starch-digesting enzymes.[Citation10] The role of carotenoids in human health has been recently reviewed by some authors.[Citation11,Citation12] These natural lipophilic antioxidants are free-radical scavengers and singlet oxygen quenchers. Some components such as β-carotene, γ-carotene, and β-cryptoxanthin are precursors of vitamin A. Moreover, certain carotenoids exert protective effects against cardiovascular diseases cancers, and aging-related diseases. Dietary chlorophyll derivatives present in both fresh and processed foods and dietary supplements also have antioxidant and antimutagenic activities.[Citation13] Glucosinolate breakdown products have received a great deal of attention mainly because of its anti-cancer activity. For example, some isothiocyanates (ITCs), notably sulforaphane and iberin, can strongly induce the activity of phase II detoxification enzymes, and thereby inhibit carcinogenesis and tumor growth by inducing apoptosis and causing cell cycle arrest in cancer cell lines.[Citation7] In addition, Cabello-Hurtado et al.[Citation14] recently reported that some glucosinolates (glucobrassicin, glucoiberin, and gluconapin) and their breakdown products extracted from cauliflower also contributed to its overall antioxidant capacity. However, oxazolidine-2-thione, a hydrolysis product of 2-hydroxy-3-butenyl glucosinolate is abundant in rapeseed and may cause goiter and other harmful effects in mammals.[Citation15] In addition, a recent study reported that the neoglucobrassicin/myrosinase complex showed strongly mutagenic properties in bacterial and mammalian cells.[Citation16] Thus, the intake of large amounts of these potentially harmful compounds should be restricted. In addition to the health effects, the breakdown products of neoglucobrassicin and sinigrin glucosinolates were responsible for the bitter taste in cauliflower and, thereby, they could influence the consumers acceptance.[Citation17,Citation18] Recent studies have reported that the common cauliflower, an important vegetable in our diet, supplies a multitude of health-related phytochemicals such as glucosinolates, polyphenols, and ascorbic acid.[Citation4,Citation5,Citation18,Citation19] However, to the best of the authors knowledge, there is no reliable data on the nutritional value and morphological characteristics of the new cauliflower variety (loose-curd cauliflower), even though it is highly popular in China and Southeast Asia.
The objective of this study was to characterize the appearance of, and determine the antioxidant capacity and health-related phytochemicals (ascorbic acid, chlorophylls, carotenoids, and glucosinolates) in four loose-curd cauliflower varieties and one common cauliflower variety. The study was undertaken in an attempt to provide reliable data on the nutritional value of this vegetable to the people and researchers.
MATERIALS AND METHODS
Culture and Sampling
Four loose-curd cauliflower varieties, including three commercial cultivars (‘ZHE017’, ‘ZHE091’, and ‘Qingnong65tian’ [QN65]) and one trial hybrids (‘ZHE047’), and one common cauliflower cultivar (‘Limin70’ [LM70]), were analyzed in this study. In July 2011, the seeds were germinated in growth matrix for plug seedlings in a greenhouse under natural day light. After growing them for four weeks in the greenhouse, the seedlings were transplanted into other greenhouses at the Zhejiang Academy of Agricultural Science Experimental Station in Hangzhou, China. At least ten seedlings were planted a in each line of a two-row plot, maintaining a distance of approximately 40 cm between adjacent plants. All lines were cultivated under the same environmental conditions with consistent agronomic measurements before harvest.
Between 4:00 and 5:00 PM, four healthy mature cauliflower heads of uniform size were harvested, delivered to the lab, and immediately stored in a refrigerator at 4°C. For the loose-curd cauliflower, seven or eight small lateral florets of length of 5–6 cm were selected and cut from each loose-curd cauliflower head for analysis. For the ‘LM70’ cultivar, small lateral florets were selected and cut directly from each curd for analysis. Fresh samples were used for chlorophyll, ascorbic acid, and total carotenoid analyses, while samples were frozen, lyophilized, and stored at –20 °C for total phenolics and glucosinolates determination.
Appearance of the Curd
Color of curd surface was measured using a chromameter (CHROMA METER CR400, Minolta Camera Co. Ltd., Osaka, Japan). After harvest, the color of five curds from each variety at the same location, was recorded and denoted as L*, a*, and b*. The meter was calibrated using the standard white plate provided by the manufacturer. The values were expressed by the International Commission on Illumination (CIE) system and converted to C* = SQRT (a*2 + b*2) and hue angle by, using the formula: h° = (ATAN(b*/a*)/ 6.2832) × 360 + 180, when a* < 0 and b* > 0.[Citation20,Citation21] The photographs of curds were taken by a camera (PowerShot G12, Canon, Japan).
Chlorophyll and Carotenoid Contents
The compounds were determined according to the method described by Lichtenthaler and Buschmann.[Citation22] Small balls (fresh weight [FW], 4–5 g) were weighed and ground in 3 ml of 96% ethanol, and then washed with 17 ml of 96% ethanol in a 50-ml tube. The extraction solution was centrifuged at 7000 × g at 4°C for 5 min. The residue was then re-extracted in 20 ml of 96% ethanol. The supernatants were collected and combined. Chlorophylls a and b and total carotenoids were measured by reading the absorbance at 664, 649, and 470 nm, respectively, with a UV-Vis spectrophotometer (DU-800, Beckman Coulter, Inc., Brea, USA). The concentrations of Chl a (Ca), Chl b (Cb), and total carotenoids C(x+c) were calculated using the following equations: Ca = 13.36A664 – 5.19A649, Cb = 27.43A649 – 8.12A664, C(x + c) = (1000A470 – 2.13Ca – 97.64Cb)/ 209, and the final concentrations of total chlorophylls and carotenoids were further expressed as μg/g FW.
Ascorbic Acid Content
Fresh small balls (4–5 g) were ground thoroughly in 1.0% (w/v) oxalic acid on ice, then extracted twice with 40 ml of 1.0% (w/v) oxalic acid and centrifuged at 7000 × g for 5 min at 4°C. Each sample was filtered through a 0.45-μm cellulose acetate filter. Ascorbic acid content was estimated using the high performance liquid chromatography (HPLC) method developed by Sun et al.[Citation23] HPLC analysis was performed using a Waters 600 system with a 717 UV-detector (Waters Inc., Milford, USA). Samples (20 μl) were separated at room temperature on an Elite Spherisorb C18 column (5 μm, 250 mm × 4.6 mm I.D.; Elite Analytical Instruments Co., Ltd., Dalian, China) using a solvent of 0.1 % oxalic acid at a flow rate of 1.0 ml/min. The absorbance was at 243 nm. The amount of ascorbic acid was calculated from ascorbic acid standard curves. Results were expressed as mg/100 g FW.
Total Phenolic Contents
Methanolic extractions for the analysis of total phenolics and antioxidant capacity were based on the protocols described by Volden et al.,[Citation4] with some modifications. Lyophilized samples (200–300 mg) were extracted with 15 ml of cold methanol and placed in an orbital shaker overnight at 180 rpm at 4°C under darkness. The suspension was centrifuged at 31,000 × g for 10 min at 4°C, and the residue was extracted again as described above. The supernatants were combined for the detection of total phenolics and antioxidant activity. Total phenolics were determined using Folin-Ciocalteu’s reagent by reading the absorbance at 760 nm with the spectrophotometer (DU-800, Beckman Coulter, Inc., Brea, USA). Gallic acid was used as a standard and the results were expressed as milligram Gallic acid equivalents (GAE)/gram dry weight (DW).
Ferric Reducing Antioxidant Power (FRAP)
FRAP assays were conducted according to the method described by Volden et al.[Citation4] The working FRAP reagent was freshly prepared by mixing 300 mM acetate buffer (pH 3.6), 20 mM ferric chloride, and 10 mM 2,4,6-tripyridyl-s-triazine in 40 mM HCl at a ratio of 10:1:1 (v/v/v). The methanolic extractions (300 μl) were added to 2.7 ml of the FRAP working solution, vortexed, and incubated at 37°C for 10 min in darkness. The absorbance was then recorded at 593 nm by using the UV-Vis spectrophotometer (DU-800, Beckman Coulter, Inc., Brea, USA) after the incubation and vortex thoroughly. FRAP values were calculated from FeSO47H2O standard curves and expressed as μmol Fe2+/g DW.
Glucosinolate Composition and Content
Glucosinolates were extracted and analyzed according to the previous protocol with minor modification.[Citation24] Briefly, freeze-dried samples (100 mg) were boiled in 10 ml of water for 10 min, and centrifuged at 7000 × g for 5 min. Each supernatant was purified with DEAE-Sephadex A-25 (Sigma-Aldrich, USA). The glucosinolates were converted into their desulpho analogues by overnight treatment with aryl sulphatase (Sigma-Aldrich, USA) at 25°C, and the desulphoglucosinolates were eluted with water. The elution was filtered through a 0.45 μm syringe-filter for HPLC analysis. The HPLC system was a Waters 600 system with a 717 UV-detector (Waters Inc., Milford, USA). A column of Elite Spherisorb C18 column (5 μm, 250 mm × 4.6 mm I.D.; Elite Analytical Instruments Co., Ltd., Dalian, China) protected by a guard column was used for separation at 25°C. The mobile phases were (A) water and (B) acetonitrile, with an isocratic elution of 1.5% B in A in the first 5 min, a linear gradient to 20% B from 5 to 20 min, followed by an isocratic elution of 20% B from 20 to 35 min. The flow rate was 1.0 ml/min. Absorbance was detected at 229 nm. Sinigrin (Sigma-Aldrich, USA) was used as the external standard for HPLC analysis. The glucosinolate concentration was expressed as nmol/g DW.
Data Analysis
Three replicates were performed for fresh sample assays and four replicates for dried sample assays. Statistical analysis was performed using the SPSS software, version 16.5 (SPSS Inc. Chicago, USA). Data were analyzed using a one-way ANOVA model and an independent-samples t-test (95% confidence interval). The means were compared using Tukey’s honest significance test at a significance level of 0.05. The values were reported as means with standard deviations.
RESULTS
Appearance of the Curd
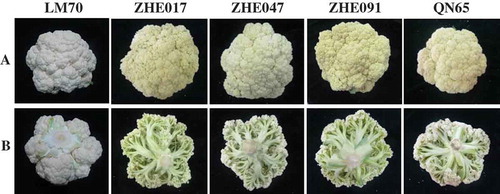
Compared to ‘LM70’ with a tight white curd, all loose-curd cauliflower varieties have long and green pedicels and exhibit visible spacing among the small florets (). Moreover, these loose-curd cauliflower varieties were yellow on the surface, while ‘LM70’ was white (). According to a previous report by McGuire,[Citation21] negative a* indicates a hue of bluish-green; positive b* yellow; and the 90 and 180° values of h° yellow and bluish green, respectively. In this study, the h° values of the curds ranged from 93 ± 0 to 97 ± 1 (). In addition, the L* value of the curd surface of each loose-curd variety was significantly lower than that of ‘LM70’. In contrast, the C* value, an index somewhat analogous to color saturation or intensity, was significantly higher in all of loose-curd cauliflower varieties than in the common cauliflower. These color measurements correlated well with the curd phenotypic features ( and ).
TABLE 1 Analysis and comparison of curd color among five cauliflower varieties
Glucosinolate Composition and Content
Twelve glucosinolates consisting of eight methionine-derived and four tryptophan-derived profiles were detected in all the five cauliflower varieties (). The total glucosinolate content was 716 ± 4 nmol/g DW in ‘LM70’, of which sinigrin (249 ± 3 nmol/g DW) and glucorbrassicin (217 ± 1 nmol/g DW) were the major aliphatic and indole glucosinolates, respectively. The total glucosinolate content in the loose-curd cauliflower varieties ranged from 374 ± 25 to 487 ± 4 nmol/g DW, with an average value of 443 nmol/g DW. Glucoiberin (174 nmol/g DW) and glucorbrassicin (79 nmol/g DW) were the predominant aliphatic and indole glucosinolates, respectively, in the loose-curd cauliflower. In this study, two important anticancer active glucosinolates, glucoiberin, and glucoraphanin, were identified in the loose-curd cauliflower varieties, ranging from 147 ± 34 to 241 ± 11 nmol/g DW (average value, 174 nmol/g DW) and from 5 ± 1 to 11 ± 2 nmol/g DW (average value, 8.9 nmol/g DW), respectively. The loose-curd cauliflower varieties showed higher levels of glucoiberin and glucoraphanin than ‘LM70’. In contrast, progoitrin levels in the loose-curd cauliflowers varied from 17 ± 2 to 29 ± 1 nmol/g DW, with an average of 20 nmol/g DW, which was lower than that in ‘LM70’. The contents of the predominant aliphatic glucosinolates, indole glucosinolates, and total glucosinolates in ‘LM70’ were much higher than those in all the loose-curd cauliflower varieties. In addition, the ratio of aliphatic glucosinolates to indole glucosinolates in the loose-curd varieties was higher than that in ‘LM70’.
TABLE 2 Glucosinolates composition and contents (nmol/g DW) in loose-curd and common cauliflower varieties
TABLE 3 Contents of ascorbic acid (mg/100 g FW), total phenolics (mg GAE/g DW), FRAP (μmol Fe2+/g DW), chlorophylls (μg/g FW), and carotenoid (μg/g FW) in five cauliflower varieties
Chlorophyll and Carotenoid Contents
The loose-curd cauliflower varieties contained more total chlorophylls (ranging from 9.7 ± 1.3 to 16.1 ± 0.4 μg/g FW, average 12.8 μg/g FW), amounting to about three-fold of that in the common cauliflower ‘LM70’ (3.7 ± 0.1 μg/g FW) (). Significant variations in total chlorophyll were also observed among the loose-curd cauliflower varieties. The highest level of carotenoids was found in ‘ZHE017’ (4.3 ± 0.1 μg/g FW), followed by ‘ZHE091’ (3.8 ± 0.3 μg/g FW), ‘QN65’ (3.4 ± 0.1 μg/g FW), and ‘ZHE047’ (2.7 ± 0.2 μg/g FW). The lowest content of carotenoids in the loose-curd cauliflower varieties was two-fold (1.3 ± 0.1 μg/g FW) in ‘LM70’.
Ascorbic Acid Content
The ascorbic acid content ranged from 59 ± 13 to 77 ± 1 mg/100 g FW, with an average of 69 mg/100 g FW in the loose-curd cauliflower varieties (); this was about 15% higher than that in ‘LM70’ (60 ± 4 mg/100 g FW). The highest level among all varieties was observed in ‘ZHE047’, and the lowest was observed in ‘ZHE017’. With the exception of ‘ZHE017’, the other three varieties contained significantly higher levels of ascorbic acid than ‘LM70’.
Total Phenolics Content
As shown in , the total phenolic content ranged from 6.1 ± 0.2 to 8.6 ± 0.6 mg/g GAE DW, averaging 7.4 mg/g GAE DW in the loose-curd varieties. The average level of total phenolics in the loose-curd varieties was slightly lower than that in ‘LM70’ (7.7 ± 0.5 mg/g GAE DW) (). There was no significant difference in total phenolic content among ‘LM70’, ‘ZHE047’, and ‘QN65’. In all, significant variations in total phenolic content were found among these cauliflower varieties.
Antioxidant Capacity
In , FRAP values are also listed as a measurement of antioxidant activity. ‘ZHE047’ and ‘ZHE017’ possessed the highest FRAP values (107 ± 8 and 100 ± 7 μmol Fe2+/g DW, respectively), followed by the two loose-curd cauliflower varieties ‘ZHE091’ (96 ± 16 μmol Fe2+/g DW) and ‘QN65’ (92 ± 7 μmol Fe2+/g DW). ‘LM70’ showed the lowest FRAP value (89 ± 2 μmol Fe2+/g DW). However these varieties did not significantly differ from each other with respect to their FRAP values.
DISCUSSION
The green pedicel of the curd is one distinct characteristic of the loose-curd cauliflower, compared to the common cauliflower with white curd pedicels. Therefore, many loose-curd cauliflower cultivars are also labeled as the “green-pedicel types.” Apart from the green pedicels of the curd, the yellow color of the surface of the loose-curd cauliflower can be observed clearly in some varieties such as ‘ZHE017’ and ‘ZHE091’ (), indicating the accumulation of pigments such as carotenoids or flavonoids. Moreover, the green pedicel of the curd may also contain higher amount of carotenoids than the white florets of the common cauliflower. Nevertheless, obvious differences in the color of the curd pedicels are observed among the loose-curd varieties at harvest. Moreover, the results also showed that the variations in chlorophyll and carotenoid contents are significant among these loose-curd varieties. Therefore, the variations in color are probably also associated with genotypic factors. It is possible that these factors could mediate the metabolism of these pigments or control other appearance characteristics of the curd (e.g., density of the head). In turn, the structure of the curd may affect the passage of light.[Citation1]
The aliphatic glucosinolate composition of the cauliflower varieties was basically in accordance with the previous findings for common white cauliflower varieties,[Citation25−Citation27] but it was different from that in some cultivars from Norway such as ‘Aviso’, ‘Flamenco’, and ‘Dania’, in which the predominant aliphatic glucosinolate was progoitrin rather than sinigrin or glucoiberin.[Citation4] In this study, the composition of aliphatic glucosinolates in the loose-curd varieties was different from that in the common cauliflower. The predominant component in loose-curd cauliflower was glucoiberin rather than sinigrin. Although the contents of total glucosinolates, aliphatic glucosinolates, and indole glucosinolates were lower in loose-curd cauliflowers, the content of glucoiberin was approximately three times higher than that in the common cauliflower. In comparison to the ITCs hydrolysis from sinigrin and progoitrin, iberin showed stronger quinone reductase-inducing activity in Hepa 1c1c7 murine hepatoma cells.[Citation28,Citation29] Thus, it is possible that the anticarcinogenic effect of this new type of cauliflower does not reduce, compared to the common cauliflower. Compared to the two predominant glucosinolate profiles in these cauliflower varieties, other individual glucosinolates were found to have lower levels, but with varying concentrations. In addition, sinigrin and neoglucobrassicin were reported to be related to bitterness in cauliflower, but alkyl glucosinolates such as glucoerucin, glucoiberin, and glucoraphanin do not contribute to the bitter taste.[Citation17,Citation18] Therefore, these results may partially explain the good flavor of this new vegetable and its quick consumer acceptance. Significant variations in individual glucosinolate profiles among many cauliflower cultivars were initially reported by Sones et al.[Citation27] and Carlson et al.[Citation25] To date, the critical factors that determine the variations in glucosinolates in cauliflower curds have not been identified, even though the glucosinolate metabolism and regulation network have been elucidated in fine detail in Arabidopsis.[Citation30] Moreover, two essential components, AT3G47960 Arabidopsis Thaliana Glucosinolatetransporter-1 (GTR1) and AT5G62680 Arabidopsis Thaliana Glucosinolatetransporter-2 (GTR2), have been identified and characterized in Arabidopsis, and they contribute to the transport of glucosinolates from the biosynthetic sites (such as leaves) to storage organs, (flowers and seeds).[Citation31]
In the present study, significant variations in antioxidants, such as ascorbic acid, total phenolics, chlorophylls, and carotenoids were observed among these cauliflower varieties. The range of ascorbic acid levels corresponded well with the values previously reported in common cauliflower varieties,[Citation4,Citation5,Citation19] but was higher than that reported by Kurilich et al.[Citation32] The average level of ascorbic acid in the loose-curd cauliflower is about 15% higher than that in ‘LM70’. The results from authoritative testing organizations also confirmed that the ascorbic acid content detected in the florets of loose-curd cauliflower varieties was generally higher than that in the common cauliflower in the same batch (data not shown). In the common cauliflower, lower chlorophyll and carotenoid contents were detected. Other reports also showed that the tight cauliflower varieties contained trace amounts of carotenoids.[Citation19,Citation32] The results of this study showed that the chlorophyll and carotenoid contents of the loose-curd cauliflower with green pedicels was 2-3 fold that in the common cauliflower. Another class of important antioxidants is polyphenols, which control the digestibility of starch and have a high antioxidant activity.[Citation6,Citation7,Citation10] Loose-curd cauliflower florets usually contain about 93–94% water (data not shown). Therefore, the total phenolic contents observed in these cauliflower varieties () were basically consistent with that reported in other cauliflower varieties.[Citation4,Citation5,Citation19] Volden et al.[Citation4] found that the white cultivars, such as ‘Aviso’ and ‘Flamenco’, had lower total phenolic content than the colored varieties such as ‘Celio’ (green pyramidal/romanesco), ‘Emeraude’ (green), and ‘Grafitti’ (purple). The lower amounts of total phenolics (about 2.0 mg/g DW) in the ‘Emeraude’ cauliflower florets grown under different managements were reported by Pichi et al.[Citation5] In general, the level of total phenolics in the common cauliflower was comparable to that in the loose-curd cauliflower. Nevertheless, it was also found that the total phenolic values varied significantly among these loose-curd cauliflower varieties. The recent research demonstrated that the levels of anthocyanin pigments were significantly higher than that in the common cauliflower (data not shown). These results indicate that the components of phenolic compounds may be different in the common cauliflower and loose-curd cauliflower.
FRAP analysis showed that the antioxidant activity of the loose-curd cauliflower universally is higher than that in the tight cauliflower ‘LM70’. In addition, the results of this study suggested that the significant variations in these antioxidants levels did not result in significant differences in the overall antioxidant activity among the samples. Similar results in other cauliflower varieties were reported by Volden et al.[Citation4] They found that the green pyramidal cultivars with higher levels of ascorbic acid and total phenolics did not show significantly higher FRAP values than two white cultivars. Sommano, Caffin, and Kerven even found a negative correlation between antioxidant capacity and total phenolic content in bush plants.[Citation33] It is well-known that the compounds with antioxidant activities are beyond ascorbic acid, carotenoids, total phenolics, and glucosinolates in cauliflower. Moreover, antioxidant activities may differ greatly between members within a single class of antioxidants such as phenolic compounds, carotenoids, and glucosinolates. These components may also display antagonistic or synergistic effects on the overall antioxidant capacity.[Citation8,Citation9,Citation14]
CONCLUSIONS
The curd appearance, glucosinolates, and some antioxidants, as well as antioxidant capacity of loose-curd cauliflower and common cauliflower were compared. The results of this study revealed that the loose-curd cauliflowers exhibited yellow curd with green pedicels, and were enriched with ascorbic acid, carotenoids, and chlorophylls compared to the common cauliflower. The antioxidant capacity of this vegetable was generally higher. The total glucosinolate content in the loose-curd cauliflower was lower than that in the common cauliflower, especially the components related with bitterness. However, the most abundant glucosinolate in this new type of cauliflower was glucoiberin, which has high anticarcinogenic activity. These results provide reliable data on some potential health-promoting compounds present in the loose-curd cauliflower, which will encourage its increased consumption. These findings will encourage new lines of research for determining more natural compounds in this new vegetable and using various food technologies to improve its nutritional value.
FUNDING
This study was funded by the Science and Technology Department of Zhejiang Province, National Natural Science Foundation of China, and Zhejiang Academy of Agricultural Sciences.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Lingping Wang (Zhejiang Academy of Agricultural Sciences, Hangzhou) for technical assistances.
REFERENCES
- Zhao, Z.; Gu, H.; Wang, J.; Sheng, X.; Yu, H. Development and comparison of quantitative methods to evaluate the curd solidity of cauliflower. Journal of Food Engineering 2013, doi: 10.1016/j.jfoodeng.2013.06.025.
- Higdon, J.V.; Delage, B.; Williams, D.E.; Dashwood, R.H. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacological Research 2007, 55, 224–236.
- Zhang, X.; Shu, X.O.; Xiang, Y.B.; Yang, G.; Li, H.; Gao, J.; Cai, H.; Gao, Y.T.; Zheng, W. Cruciferous vegetable consumption is associated with a reduced risk of total and cardiovascular disease mortality. American Journal of Clinical Nutrition 2011, 94, 240–246.
- Volden, J.; Bengtsson, G.B.; Wicklund, T. Glucosinolates, L-ascorbic acid, total phenols, anthocyanins, antioxidant capacities, and colour in cauliflower (Brassica oleracea L ssp. botrytis); effects of long-term freezer storage. Food Chemistry 2009, 112, 967–976.
- Picchi, V.; Migliori, C.; Lo Scalzo, R.; Campanelli, G.; Ferrari, V.; Di Cesare, L.F. Phytochemical content in organic and conventionally grown Italian cauliflower. Food Chemistry 2012, 130, 501–509.
- Butt, M.S.; Sultan, T. Selected functional foods for potential in disease treatment and their regulatory issues. International Journal of Food Properties 2013, 16, 397–415.
- Traka, M.; Mithen, R.F. Health benefits of dietary plant natural products. In: Plant-Derived Natural Products; Osbourn, A.E.; Lanzotti, V.; Eds.; Springer Science+Business Media: New York, 2009; 285–403.
- Davey, M.W.; Montagu, M.V.; Inzé, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability, and effects of processing. Journal of the Science of Food and Agriculture 2000, 80, 825–860.
- Podsedek, A. Natural antioxidants and antioxidant capacity of Brasscia vegetables: A review. LWT-Food Science and Technology 2007, 40, 1–11.
- Asgar, Md. A. Anti-diabetic potential of phenolic compounds: A review. International Journal of Food Properties 2013, 16, 91–103
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Molecular Aspects of Medicine 2005, 26, 459–516.
- Rao, A.V; Rao, L.G. Carotenoids and human health. Pharmacological Research 2007, 55, 207–216.
- Ferruzzi, M.G.; Böhm, O.; Courtney, P.D.; Schwartz, S. Antioxidant and antimutagenic activity of dietary chlorophyll derivatives determined by radical scavenging and bacterial reverse mutagenesis assays. Journal of Food Science 2002, 67, 2589–2595.
- Cabello-Hurtado, F.; Gicquel, M.; Esnault, M.A. Evaluation of the antioxidant potential of cauliflower (Brassica oleracea) from a glucosinolate content perspective. Food Chemistry 2012, 132, 1003–1009.
- Tripathi, M.K.; Mishra, A.S. Glucosinolates in animal nutrition: A review. Animal Feed Science and Technology 2007, 13, 21–27.
- Glatt, H.; Baasanjav-Gerber, C.; Schumacher, F.; Monien, B.H.; Schreiner, M.; Frank, H.; Seidel, A.; Engst, W. 1-Methoxy-3-indolylmethyl glucosinolate; a potent genotoxicant in bacterial and mammalian cells: Mechanisms of bioactivation. Chemico-Biological Interactions 2011, 192, 81–86.
- Engel, E.; Baty, C.; Le Corre, D.; Souchon, I.; Martin, N. Flavor-active compounds potentially implicated in cooked cauliflower acceptance. Journal of Agriculture and Food Chemistry 2002, 50, 6459–6467.
- Schonhof, I.; Krumbein, A.; Brückner, B. Genotypic effects on glucosinolates and sensory properties of broccoli and cauliflower. Nahrung/Food 2004, 1, 25–33.
- Lo Scalzo, R.; Bianchi, G.; Genna, A.; Summa, C. Antioxidant properties and lipidic profile as quality indexes of cauliflower (Brassica oleracea L. var. botrytis) in relation to harvest time. Food Chemistry 2007, 100, 1019–1025.
- Zou, Y.; Yang, Y.; Zeng, B.; Gu, Z.; Han, Y. Comparison of physicochemical properties and antioxidant activities of melanins from fruit-bodies and fermentation broths of Auricularia auricular. International Journal of Food Properties 2013, 16, 803–813
- McGuire, R. Reporting of objective color measurements. HortScience 1992, 1254–1255.
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. In: Current Protocols in Food Analytical Chemistry; Wrolstad, R.E. et al.; Eds.; John Wiley & Sons: New York, 2001, F4.3.1--F4.3.8.
- Sun, J.; Yan, H.; Liu, N.; Wei, J.; Wang, Q. Effect of 1-MCP treatment on postharvest quality characters, antioxidants, and glucosinolates of Chinese kale. Food Chemistry 2012, 131, 519–526.
- Wang, J.; Gu, H.; Yu, H.; Zhao, Z.; Sheng, X.; Zhang, X. Genotypic variation of glucosinolates in broccoli (Brassica oleracea var. italica) florets from China. Food Chemistry 2012, 133, 735–741.
- Carlson, D.G.; Daxenbichler, M.E.; Van Ettern, C.H.; Kwolek, W.F.; Williams, P.H. Glucosinolates in crucifer vegetables: Broccoli, brussels sprouts, cauliflower, collards, kale, mustard greens, and kohlrabi. Journal of the American Society for Horticultural Science 1987, 112, 173–178.
- Gratacós-Cubarsí, M.; Ribas-Agustí, A.; García-Regueiro, J.A.; Castellari, M. Simultaneous evaluation of intact glucosinolates and phenolic compounds by UPLC-DAD-MS/MS in Brassica oleracea L. var. botrytis. Food Chemistry 2010, 121, 257–263.
- Sones, K.; Heaney, R.H.; Fenwick, R. Glucosinolates in Brassica vegetables. Analysis of twenty-seven cauliflower cultivars (Brassica oleracea L. var. botrytis subvar. Cauliflora DC). Journal of the Science of Food and Agriculture 1984, 35, 762–766.
- Munday, R.; Munday, C.M. Induction of phase II detoxification enzymes in rats by plant-derived isothiocyanates: Comparison of allyl isothiocyanate with sulforaphane and related compounds. Journal of Agricultural and Food Chemistry, 2004, 52, 1867–1871.
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proceedings of the National Academy of Sciences of the United States of America 1997, 94, 10367–10372.
- Sønderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of glucosinolates-gene discovery and beyond. Trends in Plant Science 2010, 15, 283–290.
- Nour-Eldin, H.H.; Andersen, T.G.; Burow, M.; Madsen, S.R.; Jøgensen, M.E.; Olsen, C.E.; Dreyer, I.; Hedrich, R.; Geiger, D.; Halkier, B.A. NRT/PTR transporters are essential for translocation of glucosinolate defence compounds to seeds. Nature 2012, 488, 531–534.
- Kurilich, A.C.; Tsau, G.J.; Brown, A.; Howard, L.; Klein, B.P.; Jeffery, E.H.; Kushad, M.; Walig, M.A.; Juvik, J.A. Carotene, tocopherol, and ascorbate contents in subspecies of Brassica oleracea. Journal of Agriculture and Food Chemistry 1999, 47, 1576–1581.
- Sommano, S.; Caffin, N.; Kerven, G. Screening for antioxidant activity, phenolic content, and flavonoids from Australian native food plant. International Journal of Food Properties 2013, 16, 1394–1406.