Abstract
The physical properties of coffee solutions were determined for temperatures close to the freezing point. Rheological behaviour, freezing curve, density, and their relationship between coffee mass fraction and Brix degrees were determined for coffee mass fractions between 5 and 50% (wet basis) in the −6 to 20°C temperature interval. Values of viscosity varied from 1.99 to 1037 mPa·s and values of density from 1000 to 1236 kg·m−3. The freezing curve was generated using the undercooling method, giving values within freezing curves for food fluids. The results were used to generate mathematical models to predict viscosity, freezing point depression, and density as a function of coffee mass fraction and temperature.
INTRODUCTION
Coffee is the second most traded commodity in the world after petroleum and one of the most consumed food beverages worldwide.[Citation1,Citation2] In the coffee industry, preservation of quality is highly important; for this reason, low temperature technologies are commonly implemented. Technologies, such as freeze-concentration and freeze-drying, are used to produce soluble coffee thanks to the flavour preservation promoted for using low temperatures.[Citation3−Citation5]
The measurement of physical properties of food fluids at low temperatures is relevant in the designing of processes and equipment for freezing technologies. The freezing curve of food fluids represents the state of food as a function of solid concentration and temperature. The state diagram is useful for process condition’s selection in freezing technologies.[Citation6] Flux behaviour comprehension at temperatures just above the freezing point of fluids is required for sizing freeze-concentration equipment, such as falling film or tubular systems. In a similar way, determining of the viscosity and density is important to establish the power requirements for pumping during fluid processing.[Citation7] Determining of mathematical models for physical properties and rheological behaviour at low temperatures and at different mass fractions allows applying calculation methods for designing and sizing equipment for freeze concentration.[Citation8]
The coffee mass fraction or solid content can be measured by gravimetric techniques; however, °Brix determination can be a faster technique. There is no coincidence between °Brix and solid content because the darker colour of coffee solutions and the minimum fraction of sediments of coffee extract can affect the diffraction of light. A relationship between °Brix and coffee mass fraction has not been described.
There are several mathematical models for viscosity prediction of food fluids.[Citation9−Citation12] However, few studies report mathematical modeling of food fluids viscosity at low temperatures. In this sense, viscosities for sugar solutions, fruit juices, and dairy emulsions at low temperatures were reported by Falguera et al.,[Citation13] Ibarz et al.,[Citation14] Ruiz et al.,[Citation15] Tavares et al.,[Citation16] and Gabriele et al.[Citation17] In the case of coffee solutions, viscosity and some physical properties have been reported by Sobolik et al.[Citation18] and Telis-Romero et al.[Citation19] for temperatures ranging from 20 to 80°C. The freezing curve of coffee extract was obtained by Thijssen[Citation20] and Pardo et al.;[Citation21] nevertheless, the authors report the dependence of data on the type of coffee and extraction methods. Additionally, Telis-Romero et al.[Citation22] studied the physical properties of coffee extract. However, there are no reports of coffee solution’s viscosity and physical properties for temperatures below 0°C. The modeling of viscosity and other physical properties at temperatures close to the freezing point could contribute to the design of processes and equipment for freezing technologies, such as freeze concentration, freeze drying, and coffee extract handling, in the coffee industry. The aim of the present work was to measure and model the relation of coffee mass fraction and °Brix, freezing curve, rheological behaviour, and density of coffee solutions at temperatures close to the freezing point.
MATERIALS AND METHODS
Materials
Colombian freeze-dried coffee (Buencafé, Buencafé Liofilizado de Colombia) provided by the Colombian Coffee Growers Federation was used to prepare aqueous solutions at different coffee mass fractions. Soluble coffee granules were dissolved in distilled water at 30°C to obtain samples at different concentrations.
Relationship between Coffee Mass Fraction and °Brix
Coffee solutions were prepared at different coffee mass fractions: Xs = 0.10, 0.20, 0.30, 0.40, and 0.50. The solutions were stored at 20°C. °Brix and index refraction were measured by refractometry (Atago Pal 100, Japan) at 20°C ± 1°C. The total dry matter was measured by weight-loss after oven drying at 103°C ± 1°C for 4 h according to technical standards.[Citation23] Measurements were performed in quadruplicate.
Freezing Curve
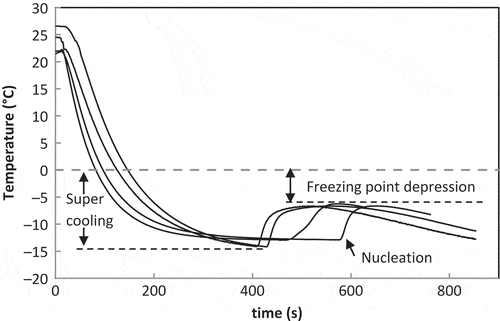
The method of undercooling reported by Auleda et al.,[Citation24] Ayel et al.,[Citation25] and Jie et al.[Citation26] was used to determine the freezing point of coffee solutions. Coffee solutions at Xs = 0.10, 0.20, 0.30, 0.40, and 0.50 were tested. The method consisted of cooling the sample by immersion in a cooling bath. Four test tubes with 10 mL of the sample were immersed in a cooling bath (Polystat, Cole Parmer, USA). The cooling fluid was a mixture of ethylene glycol and water (53% w/w). The bath was temperature controlled at an interval from −35 to 150°C ± 0.01°C. The temperature of the bath was settled at −13°C ± 0.01°C. The test tubes were immersed after the bath reached the temperature. The test tubes contained a PT100-IP65 temperature sensor (Testo, Germany) located in the centre of the sample. The sensor had a 2-mm diameter and a precision of ±0.01°C, and it was connected to a 176 T2 datalogger (Testo, Germany). The temperature profile was stored in a PC. The freezing points were determined based on the cooling curves. The highest temperature reached after the undercooling due to nucleation corresponded to the freezing point. All of the experiments were performed in quadruplicate.
The method was previously standardized by measuring the freezing curve of sucrose solutions and comparing it with reported data.[Citation10,Citation24] The solutions were prepared with sucrose of analytic grade (Panreac Química, Colombia) at solid concentrations of 10–50% w/w and distilled water at 40°C. The solutions were stored and then freezing points were determined. The technique was accepted when the difference between experimental and theoretical data was within 5%. This difference may be attributed to the solute inclusion in ice.[Citation27]
Rheological Measurements
Coffee solutions were prepared at Xs = 0.05, 0.20, 0.35, and 0.50. The solid content was verified by refractometry (Atago Pal 100, Japan) at 20°C ± 0.05°C using the equation obtained in the °Brix and coffee mass fraction measurements. The rheological behaviour of the samples was determined using a viscometer of coaxial cylinders (FungiLab Viscostar L, Barcelona, Spain) equipped with a device for low viscosity measurements, which is able to measure viscosities from 0 to 2000 mPa•s. The 18-mL sample of coffee solution was placed on the device with a concentric inside spindle. The device was immersed in a cryostat (Polysience Model 9505, USA, temperature range: −30 to 150°C; temperature stability ±0.5°C; readout accuracy: ±0.5°C). The viscometer was connected to a PC for data storing. The sample temperature was verified using a thermocouple type K (Precision ±0.5°C; measurement range: −50 to 1000°C) connected to a Datalogger (Testo 174 T4, Spain). Viscosity was measured after the sample reached the desired temperature.
The measurements were performed varying shear rate, sample temperature, and coffee mass fraction. Four shear rates were adjusted for each sample, from 5 to 120 s−1, depending on the solution’s viscosity. The limit was established by the maximum torque of the viscometer. Shear rates were calculated using an equation given by the viscometer to convert rotational speed into shear rate, γ = 1.2236 * ω. Different temperatures above freezing point were tested (−6, −4, −2, 0, 2, and 4°C) depending on Xs, such that the solution remained in a liquid state. Four different coffee mass fractions (0.05, 0.20, 0.35, and 0.50) were tested. Experiments were performed in triplicate. The rheological behaviour of coffee solutions was modeled using the power law shown in Eq. (1):
An Arrhenius type equation (Eq. 2) was used to describe the effect of temperature on the viscosity of coffee solutions:[Citation14]
Activation energy and frequency factor were fitted to the Xs dependent model[Citation9] shown in Eqs. (3) and (4):
Two general models to predict viscosity of coffee solutions as a function of temperature and coffee mass fraction simultaneously were fitted. Mathlouli and Genotelle (cited by [Citation10]) proposed a general model for sucrose solutions shown in Eq. (5):
Moreover, Sobolik et al.[Citation18] proposed a model applied to coffee solutions at room temperatures and higher, as shown in Eq. (7):
Density
The density of coffee solutions at Xs = 0.10, 0.20, 0.30, 0.40, and 0.50 was determined by using a pycnometer at temperatures of 0, 5, 10, 15, 20, and 25°C ± 0.01°C. The pycnometers with the samples were immersed in a cooling bath at the settled temperature (Polystat Cole Parmer, USA). One blank sample had a PT100-IP65 temperature sensor (Testo, Germany) immersed to check the temperature. After the sample reached the temperature, the pycnometers were closed and weighed in an analytical scale (Mettler Toledo, USA). The measures were performed in triplicate.
Statistical Analysis
The average and the standard deviation of all data were calculated by SPSS 20.0 software. The unknown parameters of the models shown in Eqs. (1) to (10) were adjusted from experimental results using a linear regression fitting procedure with SPSS 20.0 for Eqs. (2) to (4) and a non-linear regression procedure for the other intrinsically non-linear models. The goodness of model fit was based on coefficient of determination (R2), defined by the ratio between the regression sum of square and the total sum of squares. For the best fit, the R2 value should be high.
RESULTS AND DISCUSSION
Relationship between Coffee Mass Fraction and °Brix
The °Brix are a measure of the soluble solid content of sugar solutions. The relationship between °Brix and coffee mass fraction is presented in . A linear relation was obtained as seen in Eq. (8). The equation allows measuring coffee mass fraction by refractometry. The equation can be modified to %Solids = 0.87·°Brix, in order to obtain solid percentage. Similar relationships are used in the coffee industry.[Citation28] The refractive index was also measured and its relation with Xs is presented in . The relationship was fitted in Eq. (9). The models allowed calculating coffee mass fraction using a quick technique, such as refractometry.
TABLE 1 Xs and refractive index as a function of °Brix for coffee solutions
Freezing Curve
The cooling curves of coffee solutions were determined in quadruplicate as shown in . The super-cooling can be observed by the temperature decrease until the nucleation process begins. Subsequently, a temperature increase was produced due to the latent heat of the phase change. The highest temperature reached corresponded to the freezing point of the sample.[Citation24] From the cooling curves at different Xs, an average freezing point was calculated and the freezing curve for coffee solutions was obtained (). Data correspond to average and standard deviation. The values are comparable to those reported by Thijssen[Citation20] and Thaler[Citation29] for different types of coffee. The difference with the freezing point of water (0°C) corresponded to the freezing point depression. Data were fitted to Eq. (10) for freezing point depression prediction as a function of coffee mass fraction. The regression coefficient obtained was 0.998, showing a good fitness.
TABLE 2 Freezing point of coffee solutions as a function of coffee mass fraction
The freezing curve of coffee is between the freezing curves of glucose and sucrose, within the typical region of food fluids proposed by Auleda et al.[Citation24] This can be attributed to the polysaccharides content of coffee extract, which varies from 20 to 75% dry basis,[Citation29−Citation31] depending on coffee variety, roasting, and extracting processes.
Rheological Measurements
Rheological behaviour
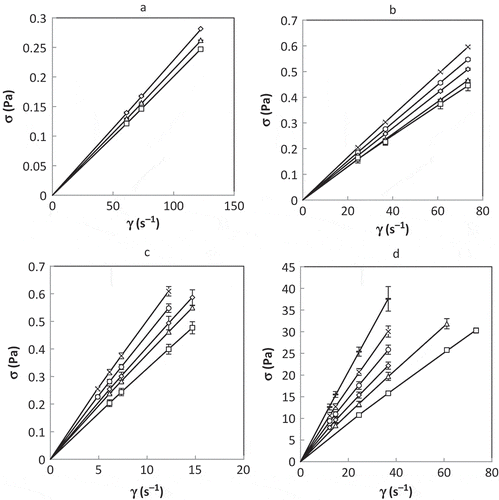
The rheograms of coffee solutions at Xs 5, 20, 35, and 50% and temperatures between −6 and 4°C are presented in , where shear stress (σ) as a function of shear rate (γ) is plotted. The dots correspond to experimental data and the lines were generated from parameters of Eq. (1), shown in . For this regression, coefficients of determination between 0.95 and 1.00 were obtained, suggesting good fitness of the models. A Newtonian behaviour was obtained with a flow index close to 1. Exponents of power law were less than 1 for high Xs and low temperatures, showing a slightly pseudoplastic behaviour. However, this result is not significant according to standard deviation. The Newtonian behaviour was reported by Sobolik et al.[Citation18] for concentrated coffee solutions at higher temperatures in the interval from 0 to 1574 s−1. Moreover, the consistency coefficient of Eq. (1) was increased with Xs and decreased with temperature, as indicated by several researchers.[Citation7,Citation32] Assuming a Newtonian behaviour, the values of viscosity of coffee solutions at the tested shear rates are presented in . As expected, the viscosity increased with increasing Xs and decreasing temperature. It was observed that influence of Xs on viscosity is greater than influence of temperature. These results are comparable with those obtained for other food solutions[Citation15] and for coffee solutions at higher temperatures.[Citation18,Citation19]
TABLE 3 Parameters of power law (Eq. 1) for different coffee mass fractions and temperatures
TABLE 4 Viscosity of coffee solutions at different temperatures (T) and coffee mass fractions (Xs) (mPa·s)
TABLE 5 Parameters of Arrhenius equation (Eq. 2) for coffee solutions at different Xs
Viscosity mathematical modeling
The viscosity dependence on temperature is presented in . As expected, viscosity increased with Xs and decreased with temperature. Data was fitted to Eq. (2) and the parameters for coffee solutions are presented in . The activation energy was increased with Xs, similarly to the result obtained by Telis-Romero et al.[Citation19] for coffee solutions at temperatures between 20 and 80°C. On the other hand, Ko values decreased with Xs. A good fit between experimental and modeled data was obtained. The results are comparable with those reported for other food solutions at temperatures close to freezing.[Citation13−Citation15]
Ea for sucrose solutions was reported by Galmarini et al.,[Citation33] for sucrose concentration of 35% with a value of 22.0 kJ·mol−1 between 20 and 34°C. For fruit juices, activation energy of 42 kJ·mol−1 is reported by Chin et al.[Citation7] Likewise, the reported activation energy of untreated sugar cane juice is 36.79 kJ·mol−1.[Citation34] Ea value for mandarin juice at low temperatures is 33 kJ·mol−1.[Citation13] It is important to remember that Ea for water is 14.4 kJ·mol−1 and this value can be increased until 60 kJ·mol−1 with solid concentration.[Citation35] Ea for coffee solutions obtained in the present study varied from 22.0 to 51.3 kJ·mol−1, giving values within the range for food fluids.
General models
The regression analyses were performed for four different models of viscosity prediction as a function of Xs and temperature. Parameters of models, standard deviation, and coefficients of determination are given in . Values are comparable with those reported by Ibarz et al.,[Citation9] Longinotti and Corti,[Citation10] and Sobolik et al.,[Citation18] although there are some differences in values due to specificity for coffee solutions at the present conditions. Equation (11) had the highest R2 value; thus, this model seems to be capable of adequately describing viscosity of coffee solutions at different temperatures (°C) and coffee mass fraction at the investigated conditions:
TABLE 6 Parameters of mathematical models for prediction of coffee solution’s viscosity
The other two models tested presented a slightly lower regression coefficient, but the adjustment was also satisfactory. Consequently, it is assumed that all models properly describe the viscosity of coffee solutions in the temperature and coffee mass fraction intervals evaluated in this study.
The parameters of Eq. (7) for coffee solutions at temperatures between 0 and 80°C were reported by Sobolik et al.[Citation18] It is possible to compare a coincident point between the reported model and the model fitted in the present work. The viscosity of a coffee solution at Xs 0.20 and T = 0°C, generated by the model reported by Sobolik et al.[Citation18] is 0.0085 Pa s and the corresponding value obtained in this work is 0.0069 Pa s, showing a difference of 18%. Sobolik et al.[Citation18] compared their results with those obtained by Weisser in a previous work and found a maximum difference of 15%. This difference is attributed by the authors to the fact that viscosity depends on the type of coffee and its processing. The generation of parameters of this model at temperatures close to freezing expands the range of application of the model to sub-zero temperatures.
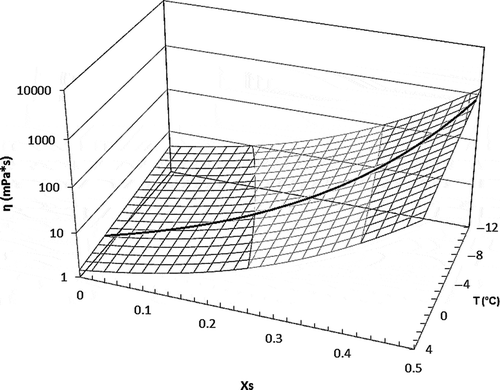
A plot of viscosity values as a function of temperature and Xs was generated using the parameters of Eq. (11) (). This figure showed that viscosity increased with Xs and decreased with temperature. The same result was reported by Diaz-Ocampo et al.[Citation36] The curve on the surface represents the freezing point curve of coffee solutions using values obtained by Eq. (10). The viscosity values beyond the line do not have physical significance because they are below the freezing point. A higher dependence of viscosity on Xs than on T is evident in the studied interval.
Density
The density of coffee extract as a function of coffee mass fraction and temperature is shown in . As expected, density increases with Xs and decreases with temperature.[Citation37] Values between 1036 and 1277 kg·m−3 were obtained. The results are similar to those reported by Telis-Romero et al.[Citation22] between 1000 to 1200 kg m−3 for the same Xs intervals and higher temperatures. The density evidences a stronger dependence on solid content than on temperature. Density can be expressed as an average of coffee solids density and water density;[Citation18] in turn, density of these components is dependent on temperature. The model fitted is shown in Eqs. (12) to (14). The regression coefficient was R2 = 0.989, showing a good data fitting.
TABLE 7 Density of coffee extract (kg·m−3) as a function of coffee mass fraction and temperature
CONCLUSIONS
The viscosity of coffee solutions at temperatures close to the freezing point can be predicted by three general models as a function of temperature and coffee mass fraction. The coffee solutions presented a Newtonian behaviour. A slight pseudoplasticity was found at high concentrations and low temperatures, but this result was not significant. In turn, a linear relationship between coffee mass fraction and °Brix was found; this expression allows measuring coffee mass fraction by a simple technique, such as refractometry. Meanwhile, the freezing curve presented a quadratic behaviour within the zone for food fluids. Finally, the density of coffee solutions can be expressed as an average of coffee solids and water densities. The characterisation of physical properties, rheological behaviour, and freezing curve of coffee solutions is useful for designing operations, such as freeze-concentration and freeze drying. Mathematical models for °Brix, viscosity, freezing point depression, and density of coffee solutions were fitted. These models can contribute in designing technologies, such as freeze concentration and freeze drying in the coffee industry.
NOMENCLATURE
| °Brix | = | Brix degrees |
| a, b, c, d | = | |
| a1, a2, b1, b2, c1 | = | Parameters of Eq. (5) |
| a3, a4, a5, a6, a7 | = | Parameters of Eq. (7) |
| Ea | = | Activation energy (kJ•mol−1) |
| FPD | = | Freezing point depression |
| Γ | = | Shear rate (s−1) |
| K | = | Consistency coefficient (Pa•sn) |
| k0 | = | Frequency factor (mPa•s) |
| n | = | Flow behaviour index |
| Nd | = | Refractive index |
| R | = | Universal gas constant (8.314 kJ•mol−1•K−1) |
| R2 | = | Coefficient of determination |
| T | = | Temperature (°C) |
| Xs | = | Coffee mass fraction (g coffee/g solution) |
| ρ | = | Density |
| ρc | = | Density of coffee solids |
| ρw | = | Density of water |
| σ | = | Shear stress (Pa) |
| Φ | = | Temperature correction |
| Ω | = | Rotational speed (rpm) |
| η | = | Viscosity (mPa·s) |
| η* | = | Standard reference viscosity (1 mPa·s) |
ACKNOWLEDGMENTS
The authors wish to thank Eng. Carlos Eduardo Osorio, Buencafe Liofilizado de Colombia (Colombian Coffee Growers Federation) for providing the coffees and for his help with the research. Author F.L. Moreno wishes to thank COLCIENCIAS for its condonable grant for doctoral studies (2013). Author Y. Ruiz wishes to thank COLCIENCIAS for its condonable grant for doctoral studies (2004).
FUNDING
The research was supported by Universidad de La Sabana and COLCIENCIAS (Project 1230521-28461) (2011).
REFERENCES
- Cheong, M.W.; Tong, K.H.; Ong, J.J.M.; Liu, S.Q.; Curran, P. and Yu, B. Volatile composition and antioxidant capacity of Arabica coffee. Food Research International 2013, 51, 388–396.
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Research International 2012, 46, 488–495.
- MacLeod, C.S.; McKittrick, J.A.; Hindmarsh, J.P.; Johns, M.L.; Wilson, D.I. Fundamentals of spray freezing of instant coffee. Journal of Food Engineering 2006, 74, 451–461.
- Miyawaki, O.; Liu, L.; Shirai, Y.; Sakashita, S.; Kagitani, K. Tubular ice system for scale-up of progressive freeze-concentration. Journal of Food Engineering 2005, 69, 107–113.
- Sánchez, J.; Ruiz, Y.; Auleda, J.M.; Hernandez, E.; Raventos, M. Review. Freeze concentration in the fruit juices industry. Food Science and Technology International 2009, 15, 303–315.
- Rahman, M.S. State diagram of foods: Its potential use in food processing and product stability. Trends in Food Science & Technology 2006, 17, 129–141.
- Chin, N.L.; Chan, S.M.; Yusof, Y.; Chuah, T.G.; Talib, R. Modelling of rheological behaviour of pummelo juice concentrates using master-curve. Journal of Food Engineering 2009, 93, 134–140.
- Auleda, J.; Raventós, M.; Hernández, E. Calculation method for designing a multi-plate freeze-concentrator for concentration of fruit juices. Journal of Food Engineering 2011, 107, 27–35.
- Ibarz, A.; Gonzalez, C.; Esplugas, S.; Vicente, M. Rheology of clarified fruit juices. I: Peach juices. Journal of Food Engineering 1992, 15, 49–61.
- Longinotti, M.P.; Corti, H.R. Viscosity of concentrated sucrose and trehalose aqueous solutions including the supercooled regime. Journal of Physical and Chemical Reference Data 2008, 37, 1503–1515.
- Telis, V.R.N.; Telis-Romero, V.; Mazzotti, H.B.; Gabas, L. Viscosity of aqueous carbohydrate solutions at different temperatures and concentrations. International Journal of Food Properties 2007, 10, 185–195.
- Falguera, V.; Ibarz, A. A new model to describe flow behaviour of concentrated orange juice. Food Biophisycs 2010, 5, 114–119.
- Falguera, V.; Vélez-Ruiz, J.F.; Alins, V.; Ibarz, A. Rheological behaviour of concentrated mandarin juice at low temperatures. International Journal of Food Science and Technology 2010, 10, 2194–2200.
- Ibarz, R.; Falguera, V.; Garvín, A.; Garza, S.; Pagán, J.; Ibarz, A. Flow behavior of clarified orange juice at low temperatures. Journal of Texture Studies 2009, 40, 445–456.
- Ruiz, Y.; Sánchez, J.; Hernández, E.; Auleda, J.M.; Raventós, M. Viscosidad de zumos comerciales de melocotón, manzana y pera a temperaturas cercanas a la congelación. Afinidad 2010, 66, 114–118.
- Tavares, D.; Alcantara, M.; Tadini, C.; Telis-Romero, J. Rheological properties of frozen concentrated orange juice (FCOJ) as a function of concentration and subzero temperatures. International Journal of Food Properties 2007, 10, 829–839.
- Gabriele, D.; Migliori, M.; Baldino, N.; Di Sanzo, R.; de Cindio, B.; Vuozzo, D. Rheological characterisation of dairy emulsions for cold foam applications. International Journal of Food Properties 2011, 14 (4), 786–798.
- Sobolík, V.; Zitny, R.; Tovcigrecko, V.; Delgado, M.; Allaf, K. Viscosity and electrical conductivity of concentrated solutions of soluble coffee. Journal of Food Engineering 2002, 51, 93–98.
- Telis-Romero, J.; Ferreira, R.; Gabas, A.; Niccoletti, V. Rheological properties and fluid dynamics of coffee extract. Journal of Food Process Engineering 2001, 24, 217–230.
- Thijssen, H.A.C. Freeze concentration of food liquids. Food Manufacture 1969, 44, 49–53.
- Pardo, J.M.; Suess, F.; Niranjan, K. An investigation into the relationship between freezing rate and mean ice crystal size of coffee extracts. Transactions of the Institution of Chemical Engineers 2002, 80, 176–182.
- Telis-Romero, J.; Gabas, A.L.; Polizelli, M.A.; Telis, V.R.N. Temperature and water content influence on thermophysical properties of coffee extract. International Journal of Food Properties 2000, 3 (3), 375–384.
- Icontec Standard. Determination of extraction yield and soluble solids in coffee beverage. NTC 4602-1, Colombia, 2009.
- Auleda, J.M.; Raventós, M.; Sánchez, J.; Hernández, E. Estimation of the freezing point of concentrated fruit juices for application in freeze concentration. Journal of Food Engineering 2011, 105, 289–294.
- Ayel, V.; Lottin, O.; Popa, E.; Peerhossaini, H. Using undercooling to measure the freezing points of aqueous solutions. International Journal of Thermal Sciences 2005, 44, 11–20.
- Jie, W.; Lite, L.; Yang, D. The correlation between freezing point and soluble solids of fruits. Journal of Food Engineering 2003, 60, 481–484.
- Chen, X.D.; Chen, P. Freezing of aqueous solution in a simple apparatus designed for measuring freezing point. Food Research International 1996, 29, 723–729.
- Moreno, F.L.; Robles, C.M.; Sarmiento, Z.; Ruiz, Y.; Pardo, J.M. Effect of separation and thawing mode on block freeze-concentration of coffee brews. Food and Bioproducts Processing 2013. DOI:10.1016/j.fbp.2013.02.007.
- Thaler, H. The chemistry of coffee extraction in relation to polysaccharides. Food Chemistry 1978, 4, 13–22.
- Franca, A.S.; Mendonça, J.C.F.; Oliveira, S.D. Composition of green and roasted coffees of different cup qualities. LWT–Food Science and Technology 2005, 38, 709–715.
- De Maria, C.A.B.D.; Trugo, L.C.; Neto, F.R.A.; Moreira, R.F.A.; and Alviano, C.S. Composition of green coffee water-soluble fractions and identification of volatiles formed during roasting. Food Chemistry 1996, 55, 203–207.
- Magerramov, M.A.; Abdulagatov, A.L.; Azizov, N.D.; Abdulagatov, I.M. Effect of temperature, concentration, and pressure on the viscosity of pomegranate and pear juice concentrates. Journal of Food Engineering 2007, 80, 476–489.
- Galmarini, M.V.; Baeza, R.; Sanchez, V.; Zamora, M.C.; Chirife, J. Comparison of the viscosity of trehalose and sucrose solutions at various temperatures: Effect of guar gum addition. LWT–Food Science and Technology 2011, 44, 186–190.
- Astolfi-Filho, Z.; Telis, V.R.N.; de Oliveira, E.B.; Coimbra, J.S.D.R.; Telis-Romero, J. Rheology and fluid dynamics properties of sugarcane juice. Biochemical Engineering Journal 2011, 53, 260–265.
- Saravacos, G.; Maroulis, Z. Food Process Engineering Operations; CRC Press: Boca Raton, FL, 2011; 94.
- Díaz-Ocampo, R.; Sánchez, R.; Franco, J.M. Rheology of commercial and model borojo jam formulations. International Journal of Food Properties 2013 (In press). DOI:10.1080/10942912.2012.665406.
- Gundurao, A.; Ramaswamy, H.; Ahmed, J. Effect of soluble solids concentration and temperature on thermo-physical and rheological properties of mango puree. International Journal of Food Properties 2011, 14 (5), 1018–1036.