Abstract
In the present investigation, biochemical changes in dressed and frozen stored Priacanthus hamrur and their influence on quality of fish sausage have been studied. During the frozen storage period of five months, moisture content decreased and led to the increment on other proximate constituents due to percentage balance. Quality indices such as total volatile base nitrogen and trimethyl amine nitrogen increased significantly (P < 0.05) and reached 44.80 mg N/100 g and 22.00 mg N/100 g of meat, respectively. Non-protein nitrogenous contents were found to decrease due to the loss in thaw drip. Lipid freshness parameters such as peroxide value and free fatty acid value increased and resulted in reduced extractability of salt soluble proteins. Gel strength of fish sausage decreased significantly (P < 0.05) during the storage period and this was also supported by the folding test. Increments in the expressible moisture content clearly indicated the loss of protein functionality, like water holding ability, due to denaturation during frozen storage. From the sensory evaluation, dressed P. hamrur can be stored frozen without any cryoprotective and preservative agents up to four months when it is intended for the preparation of fish sausage and this was also evidence from the changes in biochemical constituents.
INTRODUCTION
Due to the over exploitation of resources, deep sea fishes are the emerging fishery resources for developing countries like India. Priacanthus hamrur is an underutilized deep sea fish widely distributed in the Indo-Pacific region and identified as one of the potential species available for the production of surimi and surimi based products. Surimi is a wet concentrate of myofibrillar proteins used as an intermediate product in the development of various fish mince based products. Fish sausage is a prominent surimi or fish mince based value-added product, which is gaining a wide fame in recent years. Fish sausage, a hybrid product between kamaboko and meat sausage,[Citation1] is prepared from fish mince by mixing with additives, spices, followed by stuffing the mix/paste into synthetic casing and heat processing.
Eating quality or consumer preference of fish sausage is determined by the gel forming ability. Formation of gel involves partial denaturation of proteins with irreversible ordered aggregation leading to the formation of a three dimensional network with the entrapment of water and added ingredients.[Citation2] Gelation is one of the most important functional properties that influence the textural properties of fish products. Gel strength of fish protein gels mainly depends on the quality of raw material used for the production. Species from the same genus (Priacanthus sp) have been reported for the comparable gel forming ability compared to fishes such as threadfin bream, croaker, lizardfish, etc.[Citation3]
The utilization of fish species for the preparation of gelled products like fish sausage calls for deeper understanding on the biochemical changes in fish during various preservation methods and their impact on the quality of products. Freezing and frozen storage is a long term preservation method widely employed in fish processing industry and makes the raw material availability around the year. Quality changes in fish and shellfishes are evaluated for years in the seafood industry by measuring the chemical constituents such as trimethylamine (TMA) and total volatile base nitrogen (TVB-N)[Citation4,Citation5] and limits of these compounds are regulated according to Directive (91/493/EEC) and Directive (95/149/EEC), respectively. Spoilage due to microbial activity is terminated during freezing and frozen storage. But the endogenous enzymes and changes in proteins are limiting the storage life of frozen stored fish.
The gel forming ability of lizard fish, hoki, and silver carp have been reported to decrease during frozen storage due to the denaturation of myofibrillar proteins.[Citation6,Citation7,Citation8] Denaturation and aggregation of myofibrillar proteins are associated with the formation of free fatty acids (FFAs) and lipid oxidation products leading to the loss of functionality and texture.[Citation9,Citation10] Hydrophobic association of oxidized and hydrolysed lipid based products to the surface of proteins decrease the solubility which is a major factor determining the functional properties.[Citation11,Citation12] In the present study, changes in the biochemical constituents of dressed P. hamrur during frozen storage and their influence on the physical and sensory quality of fish sausage have been investigated.
MATERIALS AND METHODS
Preparation of Raw Material for Storage Study
Fresh bull’s eye (Priacanthus hamrur) was procured from the local fish landing center, Mangalore, located along the west coast of India and brought to the laboratory within an hour. During transportation, the raw material was packed in an insulated container with ice in alternative layers. The fish was thoroughly washed with chilled water prior to processing. The processing was carried out under hygienic condition.
Fish was dressed to remove scales, head, and entrails. Dressed fish was washed thoroughly in chilled water, and then packed in polyethylene bags of 2 kg each in a single layer. Around 20 such bags were made and they were frozen in the air blast freezer (Armfield Limited, Ringwood Hampshire, England) at –35°C and then all the packs were stored in the deep freezer (Blue star, India) at –20°C for a period of five months. Monthly, about 6 kg (3 × 2 kg) of dressed fish was taken out and thawed at 4°C in refrigerator for 6–8 h. Meat was separated and analyzed for biochemical changes. Fish sausage preparation was carried out using the recipe formulated by Bhatta, Prabhu, and Reddy.[Citation13] Process flow for the fish sausage preparation and quantity of ingredients used are given in and , respectively. The study was conducted for the frozen storage period of five months.
TABLE 1 Composition of ingredients used in fish sausage preparation
Analysis
Proximate composition
The proximate composition namely moisture, total protein, and total ash of fish meat were determined according to the methods described in AOAC.[Citation14] The moisture content was determined by the standard hot air oven (100 ± 2°C for 18 h) method. Total nitrogen was determined by Kjeldahl method of AOAC and crude protein was calculated by multiplying with the factor of 6.25 and expressed in percentage on wet weight basis. Total ash was determined by heating an incinerated sample in a muffle furnace (550°C for 10 h) and values were expressed on wet weight basis (AOAC). Total lipids were extracted by the procedure of Bligh and Dyer[Citation15] using chloroform and methanol mixture.
Quality indices
Total volatile base nitrogen (TVB-N) and trimethyl amine nitrogen (TMA-N) were estimated by the method described by Beatty and Gibbons.[Citation16]
Lipid freshness evaluation
Peroxide value (PV) and FFA value were determined according to the method described by Jacobs[Citation17] and Takagi, Hayashi, and Itabashi,[Citation18] respectively using chloroform-methanol extract of lipids prepared from fish meat.
Salt soluble proteins (SSP)
SSP were extracted with 5% (w/v) sodium chloride solution buffered with 0.02 M sodium bicarbonate, pH 7.2, using the method of Dyer, Frennch, and Snow.[Citation19] Extraction was carried at 4°C. Protein content in the extract of SSP was determined by the method of Kjeldhal as described in AOAC.[Citation14]
Non-protein nitrogen
The non-protein nitrogen content of meat was analyzed according to the method described by Velankar and Govindan[Citation20] and expressed as mg N/100 g of meat.
Physical Quality Evaluation of Fish Sausage
Gel strength measurement
The gel strength of fish sausage was determined using Okada gelometer (Saitama Keki Seisa Kuso Co. Ltd., Tokyo, Japan) as described by Suzuki.[Citation21]
Expressible moisture content (EMC)
The EMC of the sausage was measured according to the method described by Okada.[Citation22]
Folding test
The folding test of fish sausage in synthetic casing was carried out according to the method described by Suzuki.[Citation21] The method involves the use of round shaped cut piece of fish sausage with 5 mm thickness. While testing, the piece is folded along its diameter. Folding test grades are given based on the place and the extent of damage and is described as follows.
AA: The sausage slice does not break even when it is folded twice in axis of the first fold being perpendicular to that of the second.
A: the slice is intact without any break in the first fold.
B: crack occurs only on the part of the folded edge of the slice.
C: crack runs through the line of folding.
D: the slice breaks apart completely.
Sensory Analysis
Fish sausages prepared in a synthetic casing were subjected to sensory evaluation by a team of trained panelists. The minimum number of ten panelists evaluated the fish sausage. The procedure for organoleptic evaluation was followed as described by Karthikeyen et al.[Citation23] The attributes that were evaluated by trained panelists included appearance, color, texture, flavor, taste, and overall acceptability. The scoring pattern of each attribute is (hedonic scale):
Like extremely–9;
Like very much–8;
Like moderately–7;
Like slightly–6;
Neither like nor dislike–5;
Dislike slightly–4;
Dislike moderately–3;
Dislike very much–2;
Dislike extremely–1.
Statistical Analysis
Analyses were carried out in triplicates for the samples drawn randomly from three different frozen dressed fish packs and each was packed with 2 kg of dressed fish. Correlation coefficient between the variables was analyzed using Pearson correlation coefficient and the significance of correlation was tested using student’s “T” test. Data were subjected to ANOVA and Duncan multiple range mean comparison test to determine the homogeneity of variance and significant differences in biochemical indices, lipid freshness, and sensory scores as a function of storage days. Statistical analysis was performed using statistical program SPSS 16 (SPSS.16.0 for windows, SPSS Inc., Chicago, IL,).
TABLE 2 Changes in proximate composition of meat from bull’s eye during frozen storage*
RESULTS AND DISCUSSION
Changes in Proximate Composition
Changes in the proximate composition of P. hamrur during the frozen storage period of 150 days are given in . Moisture content decreased from 77.28 to 72.87%. The observed decrement of 5.71% from the initial moisture content can be attributed to the structural changes in the protein fractions due to freezing and frozen storage. Protein, lipid, and ash contents varied from 18.95 to 19.53%, 1.27 to 2.30%, and 2.42 to 4.35% during the storage period, respectively. There was no significant (P > 0.05) increment in the protein content throughout the storage period. But the increment in the total lipid and total ash were found to be significant compared to the initial value (P < 0.05). There was a significant correlation between the decrement in the moisture content and increment in the lipid (r = 0.741; P < 0.01). The similar trend was also observed between moisture and ash values (r = 0.650; P < 0.01). Moisture content of surimi from pink perch has been reported to decrease from 78.50 to 76.55% during the frozen storage period of 36 weeks.[Citation24] In the present study, the lipid and ash contents increased and this can be attributed to the decrement in the moisture content (due to the percentage balance between the proximate constituents). A similar relationship between moisture and lipid has been reported for the restructured trout during frozen storage.[Citation25]
Changes in the Chemical Quality Indices
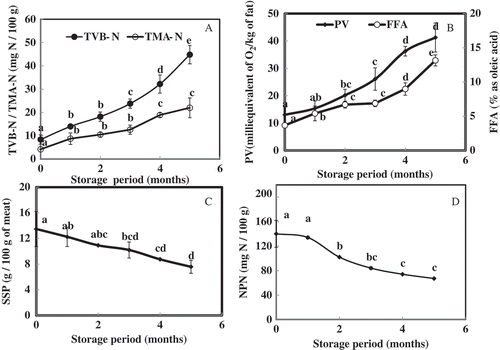
Quality changes of fish during preservation by various methods are commonly evaluated by measuring the TVB-N and TMA-N. Changes in the TVB-N and TMA-N of P. hamrur during 150 days (5 months) of frozen storage are given in . The TVB-N and TMA-N contents were observed to increase significantly from the initial value of 8.40 to 44.80 mg N/100 g and 4.20 to 22.00 mg N/100 g of meat (P < 0.05), respectively. A positive correlation was observed for TVB-N (r = 0.963; P < 0.01) and TMA-N (r = 0.943; P < 0.01) with storage periods and found to be significant. Generally, marine fish with the TVB-N value of 15–20 mg N/100 g is considered as good in quality whereas 50 mg N/100 g is regarded as poor in quality.[Citation26] The acceptable limit of TMA-N varies from 10–15 mg/100 g of meat. In the present investigation, TMA-N value reached 18.90 mg N/100 g of meat on fourth month of frozen storage, whereas the TVB-N content was within the acceptable limit (44.80 mg N/100 g of meat) up to the fifth month. It is well established that accumulation of TMA-N and TVB-N during frozen storage is due to the bacterial activity and endogenous enzyme, TMAOase. Though frozen storage is having detrimental effect on the bacterial proliferation, enzymes mediated changes were likely to be continued and resulted in the increment of TVB-N and TMA-N. Siddaiah et al.[Citation8] reported 32.85 mg of TVB-N for 100 g of mince from silver carp (Hypophthalmichthys molitrix) for the frozen storage period of six months. TMA-N is responsible for the fishy odor of ice stored or frozen stored fish. In the present research, a negative correlation was observed for the changes in the sensory score of odor of the prepared sausage with TMA-N (r = –0.467; P < 0.05) and TVB-N (r = –0.600; P < 0.01) and found to be significant. As the marine fish contains higher amount of TMA compared to fresh water fishes, TMA and TVB-N are considered as better indices of quality which vary greatly between the species and exhibit greater difference.[Citation27]
FIGURE 1 Changes in (A) total volatile base nitrogen and trimethyl amine nitrogen, (B) peroxide value and free fatty acid value, (C) salt soluble proteins, and (D) non-protein nitrogenous contents of dressed P. hamrur during frozen storage. Error bars represent the standard deviation from triplicates. Different small letters indicate that the results are significantly different (P < 0.05).
Changes in Lipid Freshness
The lipid freshness of fish meat during frozen storage is evaluated by measuring FFAs, PV, and thiobarbituric acid (TBA) values. FFAs are formed due to the activity of lipolytic enzymes which are known for their stability during low temperature preservation.[Citation28−Citation30] Peroxides formation is the indication of early oxidation process and formation of TBA reactive substances indicates the severity of oxidative deterioration of lipids.[Citation25] In the present investigation, lipid freshness was evaluated by measuring PV and FFA and results are given in . PV and FFA were observed to increase significantly (P < 0.05) from 13.03 to 41.25 mEq of O2/kg of fat and 3.67 to 13.16% of total lipids as oleic acid, respectively. Mince from silver carp has been studied for the changes in the PV and FFA during frozen storage for the storage period of 180 days and it was reported that the PV and FFA increased from 16.93 to 145.54 mmol of O2/kg of fat and from 7.66 to 30.76% of total lipids as oleic acid, respectively.[Citation8] A significant positive correlation was observed for PV (r = 0.929; P < 0.01) and FFA (r = 0.929; P < 0.01) with storage period. Similarly, a significant positive correlation was observed between FFA and PV (r = 0.937; P < 0.01). Solubility and textural properties of proteins were reported to be influenced by FFA and PV. The interaction between the oxidized products of lipids/FFA with protein molecules lead to the formation of protein aggregates.[Citation31] The interaction occurs mainly with amino acids of fish proteins by binding to reactive sulfhydryl groups of cysteins, amino groups of lysine, aspartic acid, tyrosin, methionine, and arginine.[Citation8,Citation32−Citation34] A significant negative correlation for PV (r = –0.574; P < 0.01) and FFA (r = –0.611; P < 0.01) with texture score of prepared sausage allotted by sensory panelist was observed. Oxidation of lipid and also the oxidation of protein were implicated in textural deterioration. Lipid derived reactive oxygen species, such as peroxy radicals, are potential initiators of protein oxidation. Oxidation of lipids during frozen storage could be due to the release of oxidative enzymes and pro-oxidants from various ruptured cellular organells. These processes are commonly linked to a decrease in muscle protein functionality, leading to increasing expressible moisture and weaker protein gels.[Citation35,Citation36] A significant negative correlation was recorded for the changes in the flavor and odor with PV and FFA (P < 0.05).
Changes in SSP and Non-Protein Nitrogen
SSP are the extractable muscle proteins in intermediate or high ionic strength buffer constitute about 55 to 60% of total muscle proteins, or 10% of the weight of meat.[Citation37] Solubility of SSP mainly is affected during frozen storage due to the formation of protein aggregates.[Citation3] Changes in SSP fraction of P. hamrur during frozen storage for the period of 150 days is given in the . SSP decreased significantly (P < 0.05) from the initial value of 13.47 to 7.58 g/100 g of meat at the end of frozen storage. The observed decrement of about 44% from the initial solubility may be due to protein denaturation/aggregation of proteins mediated by the oxidation of lipids and proteins. During frozen storage, actin and myosin become gradually less extractable in salt solutions forming high molecular weight protein aggregates.[Citation38] A decrease in SSP after frozen storage has been reported for many fish species including red hake mince, farmed rainbow trout, tilapia surimi.[Citation39−Citation41] A significant negative correlation was observed for SSP with the storage days (r = –0.860; P < 0.01), PV (r = –0.714; P < 0.01), FFA (r = –0.760; P < 0.01). SSP are responsible for the textural properties like gel strength of fish paste product, fish sausage. Similar results have been reported for the protein solubility of mince from Sardinella longiceps, Hypothalmichthys molitrix, Ilex argentinus, Saurida micropectoralis.[Citation8,Citation33,Citation42,Citation43]
Measurement of NPN content during frozen storage indicates the proteolytic activity of endogenous muscle proteases which bring the hydrolytic changes in major muscle protein fractions.[Citation44] Changes in NPN content of meat from P. hamrur during the period of study are given in . NPN content decreased significantly from 140 to 67 mg N/100 g meat (P < 0.05). The results contradict the observation made by Singh and Balange[Citation24] on changes in NPN of pink perch surimi during frozen storage, where a sharp increment in NPN from 13.63 to 22.35 mg/100 g of surmi for the storage period of 36 weeks. A significant inverse relationship was observed between the frozen storage period and NPN content (r = –0.918; P < 0.01). Non-protein nitrogenous (NPN) constituents include free amino acids, nucleotides, and also the smaller molecular weight peptides like di- and tri-peptides likely to be generated during frozen storage by the action of muscle proteases. The decreasing trend in NPN content in the present study could be due to the loss of nitrogenous constituents in the thaw drip or due to degradation of NPN by enzymes. Similar result has been reported by Binsi et al.[Citation45] in the study conducted on the physicochemical changes in green muscle during ice storage.
Changes in the Quality of Fish Sausage Prepared from Frozen Stored Priacanthus hamrur
Every month of storage period, meat from frozen stored dressed P.hamrur was picked, minced, and mixed with the additives, stuffed, and heat processed. The product, called fish sausage was studied for the quality attributes by evaluating gel strength, EMC, folding test, and organoleptic characteristics such as appearance, color, texture, odor, and flavor.
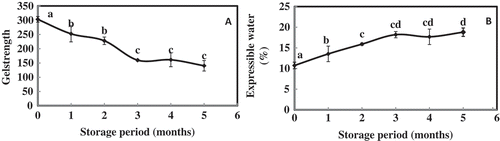
Gel Strength
Quality of mince based products is mainly judged by the attribute called gel strength. In the present investigation, gel strength of prepared sausages was evaluated using Okada gelometer and is given in . Gel strength of fish sausage prepared at zero and the fifth month of frozen stored P. hamrur was 302.89 and 140.17 g.cm, respectively. There was no significant (P > 0.05) difference in the gel strength of fish sausage prepared from three, four, and five months of frozen stored P. hamrur. Similarly, there was no significant difference between the gel strength of fish sausage prepared from the frozen stored fish meat for first two months. However, significantly (P < 0.05) a higher gel strength was recorded for the fish sausage prepared at zero month (frozen and thawed immediately). Freezing and storage time have remarkable influence on the quality of food products prepared from thawed foodstuffs.[Citation46] Gelation of myofibrillar protein is influenced by various factors, like, nature of myosin, source of muscle protein, concentration of protein, pH, ionic strength, and rate of heating.[Citation47] Protein solubility is the prime requirement for the formation of three dimensional network with the entrapment of water. In the present investigation, SSP was found to decrease during the storage period. Prabhu et al.[Citation48] reported a significant decrease in the the kamaboko (gel) forming ability of fish sausage prepared from lesser sardine mince during frozen storage. A similar report has been given for mince from sardine[Citation49] and silver carp.[Citation8] It is more preferable to store the fish as a whole than fish mince as the latter case is established to be more prone to protein denaturation in turn affect the gelation process. In the present study, a significant negative correlation was established between the storage period and gel strength (r = –0.935; P < 0.01). Gel forming ability of different tropical fish species have been reported to decrease during frozen storage.[Citation3] Decrease in the gel forming ability of fish muscles during frozen storage was associated with the freeze denaturation of actomyosin via the aggregation of protein chains, leaving them unavailable for subsequent gel formation during heat processing. This results in an inferior gel network formation, causing a lower elasticity with poor water holding capacity in the gel matrix. The formation of protein aggregates via disulfide bond formation and hydrophobic interaction has been reported to decrease the gel forming ability of fish muscle proteins during frozen storage.[Citation41,Citation43] Gel strength was also found to have a positive correlation with texture (r = 0.727; P < 0.01) and SSP (r = 0.868; P < 0.01).
EMC
Measurement of EMC supports the correlation between the protein denaturation and frozen storage period. In the present investigation, EMC increased from 10.74 to 18.80% (). A similar result has been reported for the effect of pre-freezing icing duration on frozen Nile perch.[Citation50] The water holding capacity of tissue (protein) decreases due to changes brought out in micro-structure of myofibrillar proteins from a continuous filamentous matrix to a globular matrix by freezing and frozen storage.[Citation51,Citation52] Decreasing water holding capacity resulted in increasing EMC.[Citation53] EMC of fish sausage prepared from the mince of silver carp during frozen storage has been reported to increase significantly. In the present investigation, increment in EMC was found to have significant positive correlation with storage period (r = 0.884; P < 0.01) and also a significant negative correlation with SSP (r = –0.643; P < 0.01). An inverse relationship between the gel strength and EMC was recorded. Moisture present inside the gel in the free form can easily be expelled by pressure. Similar correlation has been reported between the gel strength and expressible water content of gels from pelagic fatty fish, Sardinella longiceps.[Citation54]
Folding Test
Folding test of surimi gel is used to grade the quality of surimi in the industry. In the present investigation, folding test revealed that the quality of fish sausage for first two months were of A grade. Based on the folding test, fish sausage prepared on two to five months of frozen storage graded B. From the results obtained it can be inferred that the gel forming ability/elasticity of fish sausage decreases during frozen storage.
Sensory Evaluation
Changes in sensory attributes of fish sausage prepared during every month of frozen storage for a period of five months are given in . There was no significant difference (P > 0.05) in the scores allotted by the panelists for the sensory attributes like appearance, color, flavor, and odor up to the storage period of four months. At the end of the fifth month all the above attributes scored significantly low. Texture of fish sausage prepared from P. hamrur was found not to vary for the first three months of storage period. However, the sensory scores obtained for overall acceptability revealed that the sausages were liked slightly by panelists at the end of four months. A significant negative correlation was recorded between the storage days and the sensory attributes.
TABLE 3 Changes in sensory scores of sausage prepared from frozen bull’s eye during frozen storage*
CONCLUSION
The study was aimed to evaluate the changes in the biochemical properties of P. hamrur during frozen storage for the period of five months and their influence on the quality of mince based product, sausage. Biochemical indices such as TVB-N, TMA-N, FFA, and PV increased significantly. Extractability of salt soluble muscle proteins and NPN constituents reduced during the period of frozen storage. An inverse relationship was observed between EMC and gel strength of meat gels prepared from dressed and frozen stored P. hamrur. Based on the sensory and physical analysis, fish sausage prepared from P. hamrur had better quality for the period of four months. The gel forming quality of fish meat decreased during frozen storage and can be attributed to the denaturation of proteins by the interaction of proteins with lipid oxidized and hydrolyzed compounds.
REFERENCES
- Okada, M. History of surimi technology in Japan. In: Surimi Technology Edt.; Lanier, T.C.; Lee, C.M.; Eds.; Marcel Dekker: New York, 1992; 3–20.
- Lanier, T.C; Carvajal, S.A.; Yongsawatdigul, J. Surimi and Gelation Chemistry. In: Surimi and Surimi Seafood Edt. ; Park, J.W.; Ed.; 2nd Ed; Taylor and Francis: Boca Raton, 2005; 437–477.
- Benjakul, S.; Visessanguan, W.; Thongkaew, C.; Tanaka, M. Comparative study on physicochemical changes of muscle proteins from some tropical fish during frozen storage. Food Research International 2003, 36, 787–795.
- Oehlenschlager, J. Detailed experimental iced-storage characteristics of Barents Sea cod. Inf Fischwirtsch 1998, 45, 35–42.
- Zeng, Q.Z.; Thorarinsdottir, K.A.; Olafsdottir, G. Quality changes of shrimp (Pandalus borealis) stored under different cooling conditions. Journal of Food Science 2005, 70, S459–S466.
- Kurokawa, T. Kamaboko-forming ability of frozen and ice stored lizard fish. Bulletin of the Japanese Society of Scientific Fisheries 1979, 45, 1551–1555.
- MacDonald, G.A.; Lelievre, J.; Wilson, N.D.C. Effect of frozen storage on the gel forming properties of hoki (Macuronus novaezelandiae). Journal of Food Science 1992, 57, 69–71.
- Siddaiah, D.; Vidyasagar Reddy, G.; Raju, C.V.; Chandrasekhar, T.C. Changes in lipids, proteins, and Kamaboko forming ability of silver carp (Hypophthalmichthys molitrix) mince during frozen storage. Food Research International 2001, 34, 47–53.
- Shenouda, S.T.K. Theories of protein denaturation during frozen storage of fish flesh. Advances in Food Research 1980, 26, 275–311.
- Careche, M.; Del Mazo, M.L.; Torrejon, P.; Tejada, M. Importance of frozen storage temperature in the type of aggregation of myofibrillar proteins in cod (Gadus morhua) fillets. Journal of Agriculture and Food Chemistry 1998, 46, 1539–1546.
- Sikorski, Z.E.; Olley, J.; Kostuch, S. Protein changes in frozen fish. Critical Reviews in Food Science and Nutrition 1976, 8, 97–129.
- Sarma, J.; Srikar, L.N.; Vidyasagar Reddy, G. Effect of ice storage on the functional properties of pink perch and oil sardine meat. Journal of the Science of Food and Agriculture 1999, 79, 169–199.
- Bhatta, U.B.; Prabhu, R.M.; Manjunatha Reddy, A. Physical and organoleptic properties of fish sausage prepared from bull’s eye (Priacanthus hamrur). Continental Journal of Food Science and Technology 2012, 6, 33–35.
- AOAC. Official Methods of Analysis, 17th Ed., Association of Official and Analytical Chemists International: Virginia, USA, 2000.
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology 1959, 37, 911–917.
- Beatty, S.A.; Gibbons, N.E. The measurement of spoilage in fishery. Journal of Biological Board of Canada 1937, 3, 77–91.
- Jacobs, M.B. The Chemical Analysis of Foods and Food Products, Krieger Publishing Co. Inc.: New York, 1958; 393–394.
- Takagi, T.; Hayashi, K.; Itabashi, Y. Toxic effect of free unsaturated fatty acids in mouse assay of diarrheatic shellfish toxin by intra peritoneal injection. Bulletin of the Japanese Society of Scientific Fisheries 1984, 50, 1413–1418.
- Dyer, W.J.; Frennch, H.V.; Snow, J.W. Proteins in fish muscle: Extraction of protein fraction in fresh fish. Journal of Fisheries Research Board of Canada 1950, 7, 585–593.
- Velankar, N.L.; Govindan, T.K. A preliminary study of the distribution of non-protein nitrogen in some marine fishes and invertebrates. Proceedings of Indian Academy of Sciences 1958, 47, 202–209.
- Suzuki, T. Fish and Krill protein: Processing Technology, Applied Science Publishers: Barking, Essex, 1981.
- Okada, M. Elastic properties of kamaboko (fish meat jelly). Bulletin of Takai Regional Fisheries 1963, 36, 26–126.
- Karthikeyen, M.; Abraham, T.J.; Shanmugam, S.A.; Indrajasmin, G.; Jeyachandran, P. Effect of washing and chlorine disinfection on the quality and shelf life of iced cultured shrimp. Journal of Food Science and Technology 1999, 36, 173–176.
- Singh, R.K.; Balange, A.K. Characteristics of Pink perch (Nemipterus japonicus) surimi at frozen temperature. Journal of Food Processing and Preservation 2005, 29, 75–83.
- Jittinandana, S.; Kenney, P.B.; Slider, S.D. Cryoprotectants affect physical properties of restructured trout during frozen storage. Journal of Food Science 2005, 70, C35–C42.
- Connell, J.J. Control of Fish Quality, Fishing News Books: Farnham, Surrey, England. 1995; 152–157.
- Sharifian, S.; Zakipour, E.; Mortazavi, M.S.; Arshadi, A. Quality assessment of tiger tooth croaker (Otolithes ruber) during ice storage. International Journal of Food Properties 2011, 14, 309–318.
- Hwang, K.T.; Regenstein J.M. Characteristics of mackerel mince lipid hydrolysis. Journal of Food Science 1993, 58, 79–83.
- Bosund, I.; Ganrot, B. Lipid hydrolysis in frozen Baltic herring. Journal of Food Science 1969, 34, 13–17.
- Huss, H.H. Quality and quality changes in fresh fish. In: FAO Fisheries Technical Paper; No: 334; FAO: Roma, 1995; 76–79.
- Pacheco-Aguilar, R.; Lugo-Sànchez, M.E.; Robles-Burgueˇno, M. Postmortem biochemical and functional characteristic of Monterey sardine muscle stored at 0°C. Journal of Food Science 2000, 65, 40–47.
- Kussi, T.; Nikkila, O.E.; Savolainen, K. Formation of malonaldehyde in frozen Baltic herring and its influence on the changes in protein. Zeitschriftfjir Lebenssmittel-Unters und-for-chung 1975, 159, 285–287.
- Verma, J.K.; Srikar, L.N.; Sudhakara, N.S.; Sarma, J. Effect of frozen storage on lipid freshness parameters and some functional properties of oil sardine (Sardinella longiceps) mince. Food Research International 1995, 28, 87–90.
- Sarma, J.; Reddy, G.V.S.; Srikar, L.N. Effect of frozen storage on lipids and functional properties of dressed Indian oil sardine (Sardinella longiceps). Food Research International 2000, 33, 815–820.
- Xia, X.; Kong, B.; Liu, Q.; Liu, J. Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze thaw cycles. Meat Science 2009, 83, 239–245.
- Xiong, Y.L. Protein oxidation and implication for muscle food quality. In: Antioxidants in Muscle Foods; John Wiley and Sons Inc.: 2000; 85–111.
- Asghar, A.; Samejima, K.; Yasui, T. Functionality of muscle proteins in gelation mechanisms of structured meat products. Critical Reviews in Food Science and Nutrition 1985, 22, 27–106.
- Cheung, I.W.Y.; Liceaga, A.M.; Li-Chan, E.C.Y. Pacific hake (Merluccius productus) hydrolysates as cryoprotective agents in frozen Pacific cod fillet mince. Journal of Food Science 2009, 74, C588–C593.
- Lian, P.Z.; Lee, C.M.; Hufnagel, L. Physicochemical properties of frozen red hake (Urophycis chuss) mince as affected by cryoprotective ingredients. Journal of Food Science 2000, 65, 1117–1123.
- Herrera, R.; Mackie, I.M. Cryoprotection of frozen-stored actomyosin of framed rainbow trout (Oncorhynchus mykiss) by sugars and polyols. Food Chemistry 2004, 84, 91–97.
- Zhou, A.; Benjakul, S.; Pan, K.; Gong, J.; Liu, X. Cryoprotective effects of trehalose and sodium lactate on tilapia (Sarotherodon nilotica) surimi during frozen storage. Food Chemistry, 2006, 96, 96–103.
- Paredi, M.E.; Roldan, H.A.; Crupkin, M. Changes in myofibrillar proteins and lipids of Squid (Illex argentines) during frozen storage. Journal of Food Biochemistry 2006, 30, 604–621.
- Leelapongwattana, K.; Benjakul, S.; Visessanguan, W.; Howell, N.K. Physicochemical and biochemical changes in whole lizard fish (Saurida micropectoralis) muscles and fillets during frozen storage. Journal of Food Biochemistry 2005, 29, 574–569.
- George, C; Gopakumar, K. Spoilage changes in the muscles of crab, Scylla serrata stored at three different temperatures. Proceedings of the 1st Indian Fisheries Forum; Asian Fisheries Society, Mangalore, India, December 4–8, 1987; 347–349.
- Binsi, P.K.; Shamasundar, B.A.; Dileep, A.O. Physico-chemical and functional properties of proteins from green muscle (Perna viridis) during ice storage. Journal of the Science of Food and Agriculture 2007, 87, 245–254.
- Vacha, F.; Cepak, M.; Urbanek, M.; Vejsada, P.; Hartvich, P.; Rost, M. Impact of long-term storage on the instrumental textural properties of frozen common carp (Cyprinus carpio, L.) flesh. International Journal of Food Properties 2013, 16, 241–250.
- Sun, X.D.; Holly, R.A. Factors influencing gel formation by myofibrillar proteins in muscle foods. Comprehensive Reviews in Food Science and Food Safety 2011, 10, 33–51.
- Prabhu, R.M.; Shamasunder, B.A.; Krishnamurthy, B.V.; Chandrasekhar, T.C. Suitability of lesser sardine (Sardinella gibbosa) meat for the preparation of fish sausage. In: The First Indian Fisheries Form; Mohan Joseph, M.; Ed.; Asian Fisheries Society: Indian Brach, Mangalore, 1986; 57–59.
- Ravishankar, C.N.; Setty, T.M.R.; Shetty, T.S. Effect of frozen storage of minced meat from Indian oil sardine (Sardinella longiceps) on sausage quality. Indian Journal of Fisheries 1994, 41, 100–105.
- Natseba, A.; Lwalinda, I.; Kakura, E.; Muyanja, C.K.; Muyonga, J.H. Effect of pre-freezing icing duration on quality changes in frozen Nile perch (Lates niloticus). Food Research International 2005, 38, 469–474.
- Cheng, C.S.; Hamann, D.D.; Webb, N.B.; Sidwell, V. Effect of species and storage time on minced fish gel texture. Journal of Food Science 1979, 44, 1087–1092.
- Smith, D.M. Functional and biochemical changes in deboned turkey due to frozen storage and lipid oxidation. Journal of Food Science 1987, 52, 22–27.
- Vidyasagar Reddy, G.; Srikar, L.N. Effect of ice storage on protein and related changes in pink perch (Nemipterus japonicus). Journal of Food Science and Technology 1991, 28, 101–104.
- Karthikeyan, M.; Shamasundar, B.A.; Mathew, S.; Kumar, R.P.; Prakash, V. Physico-chemical and functional properties of proteins from pelagic fatty fish (Sardinella longiceps) as a function of water washing. International Journal of Food Properties 2004, 7, 353–365.