Abstract
Water extractable crude polysaccharides from three sweet cherry varieties, raspberries, and ginseng berry pulp were evaluated for their chemical composition, structural features, molecular weight, and bioactive properties. The yields of the crude polysaccharides isolated from cherries, raspberries, and ginseng berry pulp ranged from 0.79 to 0.18% on a dry weight fruit basis. All of the crude polysaccharides contained protein, phenolic compounds, and uronic acid. Each crude polysaccharide contained the sugar monomers: rhamnose, arabinose, xylose, mannose, galactose, and glucose. Of the water extractable polysaccharides obtained from the mature fruits, the crude polysaccharides from the raspberries had the highest molecular weight while the crude polysaccharides from the ginseng berry pulp had the smallest molecular weight. Results from Fourier transform infrared spectroscopy suggested that the crude polysaccharides contained a monosaccharide with six carbon atoms in a D-glucopyranose ring and a protein component. The crude polysaccharides from cherries, raspberries, and ginseng berry pulp were shown to possess antioxidant activity as determined with the ferric reducing antioxidant power and the 2,2-azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) radical scavenging assays. The effect of crude polysaccharides on: (1) caspase 3 activation, which was determined using a hypoxia/reoxygenation model, and (2) immunostimulation, which was determined by evaluating the inflammatory mediator response, were examined. Only crude polysaccharides obtained from certain varieties of sweet cherries inhibited caspase 3 activation in a dose-dependent manner, while only the crude polysaccharides obtained from ginseng berry pulp stimulated immune function. Crude polysaccharides present in small fruits do possess bioactivities that may enhance human health.
INTRODUCTION
Phenolic compounds are a major group of phytochemicals in fruits, including flavonoids (anthocyanins, flavonols, flavones, flavanols, flavanones, proanthocyanidins, and isoflavonoids), stilbenes, hyrdrolyzable tannins, and simple phenolic acids. Studies have implicated phenolic compounds as important bioactive compounds due to their antioxidant properties.[Citation1] High consumption of fruits has been linked to the prevention of degenerative diseases, such as cardiovascular diseases, diabetes, cancer, and arthritis.[Citation2] As such, it is well established that the phenolic component of fruits may be important components of health promoting diets.[Citation1] However, in recent years, water extractable polysaccharides, which may be pure or conjugated with proteins, lipids, or phenolics, have emerged as an important class of compounds present in plant material that possess biological activities.[Citation3,Citation4] The majority of information regarding the bioactivity of water-extracted polysaccharides has been documented for non-fruit plant materials, such as tea, herbs, and fungi.[Citation5−Citation7] Polysaccharides isolated from hot water extract of ginseng root have been shown to possess strong antioxidant activities as well.[Citation8] Both traditional ginseng formulations, which are high in polysaccharides, as well as extracts of ginseng show cardiac protective effects by reducing myocardial injury in ischemia/reperfusion.[Citation9] Compared to ginseng root polysaccharides, little work has been performed with respect to examining bioactive properties of ginseng berry polysaccharides, although research has been performed demonstrating the potential anti-hyperglycemic properties of ginseng berry polysaccharides.[Citation10]
To a limited extent, work characterizing the bioactivity of polysaccharides present in fruits and vegetables has been performed. Celery stalks[Citation11] and sweet bell peppers[Citation12] have been shown to contain pectic polysaccharides with anti-inflammatory activity. Lui and Lin[Citation13] demonstrated the anti-inflammatory and anti-apoptotic effect of water extractable strawberry and mulberry fruit polysaccharides. Work by Fan et al.[Citation14] has demonstrated the antioxidant activity of water extractable polysaccharides from cherry, cranberry, and kiwi fruits; however, the relationship between their antioxidant activity and chemical composition, as well as the structure of their active components, was not established. Water extractable polysaccharides from wolfberry/goji berry (Lycium barbarum) have been reported to possess important bioactive functions, including hypoglycemic and hypolipidemic activities,[Citation15] immuno-modulating action,[Citation16] and antioxidant activity.[Citation14,Citation17] Wolfberry/goji berry polysaccharides have also been shown to be effective in reducing myocardial injury in ischemia/reperfusion of rat heart.[Citation18]
There is strong evidence that a diet rich in naturally occurring phytochemicals present in fruits and vegetables is more effective than the same phytochemicals consumed as purified products or extracts.[Citation19] Based on these facts, water extractable polysaccharides may also contribute to the health effects of fruits, such as cherries, raspberries, and ginseng berries. Water extractable polysaccharides have not been explored as bioactive molecules in fruits even though there is evidence that this is likely. Thus, as part of the ongoing study to test the hypothesis that water extractable polysaccharides may play a role in the demonstrated health benefits of a diet high in fruits and vegetables, we used a hot water extraction method to obtain water extractable crude polysaccharides from cherries, raspberries, and ginseng berries. This work provides information on the chemical composition, molecular weight, and structural features from FTIR spectra along with the in vitro antioxidant activities of water extractable crude polysaccharides isolated from these fruits. Further, the effect of water extractable crude polysaccharides isolated from sweet cherries, ginseng berry, and raspberries on caspase 3 activation was examined by performing a simulated ischemia/reperfusion oxidative stress assessment. The effect of water soluble crude polysaccharides isolated from sweet cherries, ginseng berry, and raspberries on immunostimulatory response was also examined. This work examined the relationships between chemical composition and bioactivities of water extractable crude polysaccharides present in sweet cherries, raspberries, and ginseng berry pulp, which is not well established and is an area of research that requires continued study. This work presents evidence supporting that water extractable crude may act as potential health promoting compounds in small fruits and serves as an impetus for increased work in this area.
METHODS AND MATERIALS
Samples
Sweet cherries (Prunus avium L.), including three varieties (Lapins, Skeena, and Sweetheart) were harvested at commercial maturity. The Lapins cherries were also harvested at two additional maturity levels, immature edible (IM) and overmature (OM). All cherries were picked from the orchard of the Agriculture and Agri-Food Canada, Pacific Agri-Food Research Centre, Summerland, BC. The fresh cherries were de-stemmed and de-pitted by hand and then frozen and stored at −20°C until use. Ginseng (Panax quinquefolius) berry pulp was obtained from Agriculture and Agri-Food Canada (London, ON) and collected from a cooperating southern Ontario commercial producer. The samples were frozen as soon as possible (about 1 h) after field collection and kept at −20°C until use. The raspberries (Rubus idaeus) were purchased from Triple Crown Packers (Langley, BC) and were received in a frozen condition and kept at −20°C. All of the frozen fruit samples were freeze dried (Virtis Freeze Dryer, Model 50-SRC-5, Gardiner, NY, USA), placed in polyethylene bags, and stored −20°C until being subjected to extraction.
Hot Water Extraction of Crude Polysaccharides from Fruits
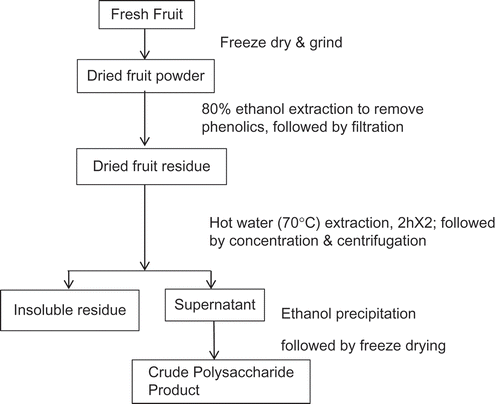
Crude polysaccharides were extracted from the fruits samples using a method adapted from the works of Fan et al.,[Citation14] Luo et al.,[Citation15] and Li et al.[Citation17] The extraction of these crude polysaccharides is an important aspect of their characterization and, therefore, the method is given in detail. shows the extraction scheme for obtaining crude polysaccharides from sweet cherry, raspberry, and ginseng berry pulp. The ground and dried fruit samples were first subjected to an 80% ethanol pre-extraction step to remove simple sugars, phenolics, and other low molecular weight compounds. Briefly, the freeze-dried samples were removed from −20°C freezer and brought to room temperature. Upon reaching room temperature, the samples were ground to a fine powder with a hand blender. Dry ground material (500 g) and 2500 mL of 80% ethanol were added to a 4-L beaker and stirred at 500 rpm for 60 min using a Heidolph MR Hei-Standard Stirrer/Hotplate (Heidolph Instruments GmbH & Co., Schwabach, Germany) at room temperature. As such, a 1:5 solid to solvent ratio was used for the first ethanol extraction. The solids were separated from the ethanol solution by centrifuging for 10 min at 7000 rpm (˜5200× g). After centrifugation, the supernatant was collected and filtered through Whatman #4 paper on an 18.5 cm Buchner funnel under vacuum. The filtered supernatant was transferred to a 20-L pail. The solids/precipitate were added back to the 4-L beaker and subjected to re-extraction with 1250 ml of 80% ethanol as a 1:5 solid to solvent ratio was used for the second ethanol extraction. The second ethanol extraction was performed as noted for the first extraction. The solids were separated as noted for the first ethanol extraction. A third ethanol extraction was performed as noted for the second ethanol extraction. The solids were separated as noted for the first and second ethanol extractions. The solids/precipitate was transferred to an aluminium weighing dish and dried overnight in a vacuum oven (Isotemp Vacuum Oven 280A, Fisher Scientific, Ottawa, ON) at 60°C. The ethanol extracted fruit residue was added to water in a 1:20 solid to solvent ratio and subjected to constant stirring with Heidolph MR heater/stirrer plate with EKT temperature controller (Heidolph Instruments GmbH & Co., Schwabach, Germany) for 2h at 70°C for the water extraction step. Centrifugation at 8627× g for 15 min was used to separate the solid residue from the first hot water extraction step. Both the supernatant and solid residue from the first warm water extraction was collected. The collected solid residue was subjected to another 70°C water extraction for 2h using a 1:20 sample to solvent ratio (i.e., the first extraction step was repeated). Centrifugation at 8627× g for 15 min was again used to separate the solids from the water extraction solvent. Both the supernatant and solid residue from the second warm water extraction was collected. The supernatants of the first and second warm water extractions were pooled. The pooled supernatant was concentrated to about 1/4 volume (less than 500 ml) of the original using a rotary evaporator at 60–70°C. Four volumes of anhydrous ethanol were added to the solution to give an 80% final concentration of ethanol, and the mixture was kept overnight at 4°C to precipitate the polysaccharides. Centrifugation at 8627× g for 15 min was again used to separate the precipitated solids from the ethanol solvent. The precipitate was collected as water extractable crude polysaccharide and dried in a vacuum oven (Isotemp Vacuum Oven 280A, Fisher Scientific, Ottawa, ON) overnight at 60°C. The weights were recorded to determine yields. Moisture content of the water extractable crude polysaccharides was determined according to the AOAC method.[Citation20]
Chemical Analyses
Carbohydrate Content and Uronic Acid Determination
Total carbohydrates were assayed by phenol-sulfuric acid[Citation21] using glucose (Sigma-Aldrich Co., St. Louis, MO, USA) as a standard. Uronic acid content of the water extractable crude polysaccharides was quantified by the Scott method.[Citation22] The uronic acid content was determined by averaging the absorbance at 400 and 450 nm and comparing it to a standard curve of galacturonic acid (Sigma-Aldrich Co.). Experiments were performed in duplicate and results are expressed on a dry weight basis.
Neutral Sugar Monomer Content Determination
The neutral sugar monomer content was determined using the methods of Fan et al.[Citation14] and Blakeney et al.[Citation23] The polysaccharides were first hydrolyzed with 1 M H2SO4 at 100°C for 3 h in a convection laboratory oven into monosaccharides before derivitization into alditol acetates. The alditol acetate derivatives were analyzed via gas-liquid chromatography on an Agilent DB-225 glass-capillary column (30 m × 0.32 mm, i.d., film: 0.25 μm) in an Agilent 6890N Network GC System (Agilent Technologies, Mississauga, ON), equipped with a flame ionization detector (FID). Different concentrations of neutral sugar standard mixtures (rhamnose, fucose, arabinose, xylose, mannose, galactose, and glucose) were prepared, converted to their derivatives, and analyzed. Sugar concentrations were calculated as the anhydro sugars relative to myo-inositol (used as an internal standard). Experiments were performed in duplicate and results are expressed on a dry weight basis.
Protein Content Determination
Protein content was estimated from the nitrogen content of the samples using the method of Tamaki and Mazza.[Citation24] Briefly, nitrogen content was determined by combusting the dried samples at 850°C using a Leco FP-528 nitrogen analyzer (Leco Corporation, St. Joseph, MI, USA). Approximately 100 mg of a sample was analyzed. A standard curve for nitrogen was produced using ethylenediaminetetraacetic acid (EDTA) and corn flour (Leco Corporation). Protein content was estimated by multiplying the nitrogen content (%) by a factor of 6.25. Experiments were performed in duplicate and results are expressed on a dry weight basis.
FTIR Analysis
FTIR analysis was performed using the methods of Tamaki and Mazza,[Citation25] Briefly, all of the Fourier transform mid-infrared (FTIR) spectra were measured using a Nicolet 380 spectrometer (Thermo Fisher Scientific Inc., Madison, WI, USA) with SMART iTR diamond attenuated total reflectance (ATR) with a 45° incident angle generating one bounce. The spectrometer was equipped with a deuterated triglycine sulfate detector scanning over the wavenumber range of 4000–650 cm−1 at a resolution of 4 cm−1. For each spectrum, a total of 32 repetitive scans was accumulated using OMNIC 8.0 software (Thermo Fisher Scientific Inc.). For analysis, approximately 3 mg of sample was placed on the head of the ATR crystal (mm in diameter) and pressed using a pressure tower. Spectra were collected in triplicate for each sample and then averaged to one spectrum.
Total Phenolics
The total phenolics content of the crude polysaccharide samples was determined by the Folin-Ciocalteu colorimetric method based on the procedure described by Singleton and Rossi.[Citation26] Quantification was determined based on a standard curve for gallic acid by measuring absorbance at 765 nm on a spectrophotometer (Cary 50, Agilent Technologies, Mississauga, ON). The total amount of phenolic content was expressed at mg gallic acid equivalent per gram crude polysaccharide sample (mg GAE g−1 crude polysaccharide sample).
Molecular Weight Determination
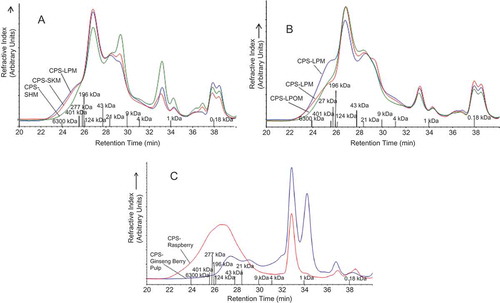
The molecular weight of the water extractable crude polysaccharides was determined with high performance size exclusion chromatography (HPSEC). The HPSEC system consisted of a guard column (Ultrahydrogel, 6 × 40 mm, Waters, Milford, MA, USA) in combination with two Ultrahydrogel 120 and linear (7.8 × 300 mm, Waters) columns in series to maximize the resolution. The elution was carried out with 0.1 M NaNO3 as the mobile phase at a flow rate of 0.5 mL/min. The injection volume of standards and samples was 50 μL, and the running time was 90 min. The columns and RID were kept at 40°C and the autosampler temperature was set at 15°C. The eluant was monitored using an Agilent 1200 series refractive index detector (Agilent, Santa Clara, CA, USA). Retention times from HPSEC were recorded and time variations were dependent on the average molecular weight of the dextran standards. Dextran T-series standards (Waters; American Polymer Standards, Mentor, OH, USA) with molecular weights of 1, 4.4, 9.9, 21.4, 43.5, 277, and 6300 kDa were used for calibration. Glucose (Sigma Aldrich, ON, Canada), which has a molecular weight of 180 Da (0.18 kDa), was also used as a standard for calibration. The molecular weight standards and polysaccharides were prepared using the same solvent as the mobile phase. All sample solutions were filtered through a 0.45-μm membrane filter (Chromatographic Specialties Inc., Brockville, ON, Canada) prior to analysis. The molecular weight of the standards at their respective retention times are noted on the HPSEC chromatograms presented in –. In accordance with the method of Carnachan et al.,[Citation27] molecular weights of polysaccharides were estimated by comparison of the retention/elution times with those of the dextran and glucose standards by use of a standard curve. The standard curve represented the linear relationship of retention time and the logarithm of the respective molecular weights (Log MW) of the standards. In the linear equations, y = Log MW and x = retention time. Initially a standard curve containing the entire molecular weight range (6300–0.18 kDa) with respective retention time ranging from 23.97–37.87 min, was used to obtain the linear relationship y = --0.3003x + 10.168 with an R2 value of 0.9212. However, in order to improve the strength of the linear relationship (i.e., R2 value), the two standard curves were used to determine molecular weight of the polysaccharide extracts. A standard curve containing the larger molecular weights ranging from 6300–43 kDa, with respective retention times ranging from 23.97–27.75 min was used to obtain the linear relationship y = --0.7244x + 21.141 with an R2 value of 0.9952. Also, a standard curve containing the smaller molecular weights ranging from 43–0.18 kDa with respective retention times ranging from 27.75–37.87 min was used to obtain the linear relationship y = --0.2249x + 7.6829 with an R2 value of 0.9787.
FIGURE 2 A: Molecular weight profiles of water extractable crude polysaccharides from Lapins, Skeena, and Sweetheart cherries, B: molecular weight profiles of water extractable crude polysaccharides from Lapins cherries at different maturity levels (immature edible, mature, overmature), C: molecular weight profiles of water extractable crude polysaccharides from ginseng berry pulp and raspberries. CPS--LPM: water extractable crude polysaccharide isolated from Lapins cherry variety; CPS--SHM: water extractable crude polysaccharide isolated from Sweetheart cherry variety; CPS-Cherry-SKM: water extractable crude polysaccharide isolated from Skeena cherry variety, all harvested at commercial maturity; CPS--LPIM: crude polysaccharide isolated from Lapins cherry variety harvested prior to commercial maturity; CPS--LPOM: crude polysaccharide isolated from Lapins cherry variety harvested after commercial maturity; CPS--Ginseng Berry Pulp: crude polysaccharide isolated from ginseng berry pulp; CPS--Raspberry: crude polysaccharide isolated from raspberries.
Bioactivity Tests
Antioxidant Activity-Chemical In Vitro Assays
The antioxidant activity of the crude polysaccharide samples in terms of reducing power towards Fe(III) and the ability to act as a radical scavenging compound was investigated by the ferric reducing antioxidant power (FRAP) assay and the ABTS•+ radical scavenging assay, respectively. For both of these assays, aqueous solutions of the crude polysaccharide were prepared as follows: an aliquot (5 mg) of each crude polysaccharide sample was placed in a -ml polypropylene centrifuge tube; 1 mL of water containing ethanol (10%, v/v) was added, and the tube was vortexed at room temperature to ensure adequate mixing. The samples were then centrifuged at 13400 rpm for 5 min in an Eppendorf Minispin (Eppendorf, Mississauga, ON) and the supernatant was recovered for testing antioxidant activity. The supernatant of each aqueous crude polysaccharide supernatant was serially diluted from 1 to 30 times for use in the antioxidant assays.
ABTS•+ radical scavenging activity
The radical scavenging activity of the crude polysaccharides were determined using the ABTS (2,2ʹ-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) decolorization assay as described by Mateos-Aparicio et al.[Citation28] For analysis, 50 μL of the aqueous crude polysaccharide supernatent was added to a well on a 96-well plate. To each crude polysaccharide sample in each well, 250 μL of the diluted ABTS•+ solution was added. After incubating for 5 min at room temperature, the absorbance at 734 nm was measured. A plate reader (SpectraMax M2, Molecular Devices, Sunnyvale, CA, USA) was used to measure spectrophotometric absorbance. Absorbance was measured for each sample in triplicate for each dilution. The results are expressed as Trolox equivalents (TE)/g crude polysaccharide on a dry weight basis.
Ferric reducing antioxidant power (FRAP)
The antioxidant activity, in terms of ferric reducing antioxidant (FRAP), of each crude polysaccharide sample was evaluated using the method of Mateos-Aparico et al.[Citation28] For analysis, 50 μL of the aqueous crude polysaccharide supernatant was added to a well on a 96-well plate. To each crude polysaccharide sample in each well, 250 μL of FRAP was added. After incubating for 5 min at room temperature, the absorbance at 593 nm was measured. A plate reader (SpectraMax M2, Molecular Devices, Sunnyvale, CA, USA) was used to measure spectrophotometric absorbance. Absorbance was measured for each sample in triplicate for each dilution. The results are expressed as Trolox equivalent (TE)/g crude polysaccharide on a dry weight basis.
Caspase 3 Assay and Immunostimulatory Response
To examine whether water extractable crude polysaccharides possess cardiac protective properties, a simulated ischemia/reperfusion oxidative stress assessment and subsequent determination of caspase 3 activation was performed as adapted from the works of Mockridge et al.[Citation29] and Li and Cohen.[Citation30] Also, the effect of water extractable crude polysaccharides on immunostimulatory response was determined using the methods adapted from Lui et al.[Citation31]
Statistical Analysis
Statistical analysis was conducted using SAS Institute Inc. software, version 9.1 (SAS Institute Inc., Cary, NC, USA). Data were subjected to analysis of variance (ANOVA) with replication using the SAS PROC GLM procedure. Least square (LS) means and least significant difference (LSD) at 5% significance level were generated using the SAS PROC GLM procedure.
RESULTS AND DISCUSSION
Extraction and Characterization of Water Extractable Polysaccharides
shows the yield of the crude polysaccharides extracted with hot water extraction from the different cherry varieties and maturity levels along with ginseng berry pulp and raspberries. The highest water extractable crude polysaccharide yields were obtained from the raspberries at levels of .18% (dry matter basis). The ginseng berry pulp and overmature Lapins cherries exhibited crude polysaccharide yields of 1.47 and 1.1% (dry matter basis) while the undermature edible Lapins, Sweetheart, and Skeena cherries showed lower yields of the water extractable crude polysaccharides at 0.83, 0.79, and 0.91% (dry matter basis), respectively. These values are much smaller than the yields of water extractable crude polysaccharides obtained from wolfberries, kiwi, cranberries, and sweet cherries, which as reported by Fan et al.,[Citation14] ranged from 4.7 to11.0%. The work of Fan et al.[Citation14] did not use an initial ethanol pre-extraction step, which may account for the different yields.
TABLE 1 Yield, total carbohydrates, protein, total phenolics contents, and molecular weights of crude polysaccharides from cherry, raspberry, and ginseng fruits
The total carbohydrate content of the water extractable crude polysaccharides from all of the fruit samples ranged from ˜20 to 42%. The water extractable polysaccharides obtained from the mature, immature edible, and overmature Lapins cherries demonstrated the highest total carbohydrate contents of 39.3, 41.5, and 41.3%, while the water extractable crude polysaccharides obtained from the ginseng berry pulp demonstrated the lowest total carbohydrate content of 19.7%. The water extractable crude polysaccharides obtained from the mature Sweetheart cherries, mature Skeena cherries, and raspberries possessed intermediate carbohydrate contents ranging from 28.9–34.8%. The work of Fan et al.,[Citation14] which extracted and characterized water soluble polysaccharides obtained from cherry, kiwi, cranberry, and wolfberry fruit samples, did not report the total carbohydrate content of these polysaccharides. To our knowledge no work has been performed characterizing the carbohydrate of crude polysaccharides isolated from ginseng berry pulp and raspberries.
The protein contents of the water extractable crude polysaccharides obtained from the various fruit samples in our work ranged from ˜6 to 17%, also indicating that these crude polysaccharides were conjugated with protein moieties. The water extractable crude polysaccharides obtained from the ginseng berry pulp and mature Sweetheart cherries contained the highest protein contents, 16.81 and 11.94%, respectively. The water extractable crude polysaccharides obtained from the mature Skeena cherry and raspberry samples contained moderate levels of protein, 9.12 and 7.78%, respectively. Lower levels of protein were measured in the water extractable crude polysaccharides obtained from the mature Lapins, immature edible, and overmature Lapins cherries samples, 6.68, 6.07, and 7.06%, respectively. Barbier and Thibault[Citation32] reported the protein content of water soluble polysaccharides obtained from cherries (cv. Bigarreaux Napoleon) to be 12.4%, which is comparable to our results.
The water extractable crude polysaccharides were analyzed for total phenolics content and the results indicated () that the crude polysaccharides obtained from all of the fruit samples contained phenolics with total phenolics contents ranging from ˜13 to 49 mg GAE/g crude polysaccharide. The total phenolics content of the water extractable crude polysaccharides obtained from the ginseng berry pulp and the raspberry samples were the highest at 49.07 and 40.70 mg GAE/g crude polysaccharides, respectively, while the water extractable crude polysaccharides obtained from the immature edible Lapins and mature Sweetheart cherry samples presented the lowest levels of total phenolics, 12.7 and 13.19 mg GAE/g crude polysaccharide, respectively. To our knowledge, this work is the first to characterize the phenolic content of crude polysaccharides isolated from cherries, raspberries, and ginseng berry pulp. The concept of non-extractable phenolics associated with polysaccharides is growing in importance in filling the gap in the field of dietary phenolics in terms of fully defining the physiological and health related properties of phenolics.[Citation33] Although attributing potential bioactivity of these crude polysaccharides to the phenolic fraction may potentially detract from promoting the bioactivity of the polysaccharide component, it is necessary for fully understanding the food components responsible for the demonstrated health benefits fruits. Based on the literature it is likely that both the polysaccharide[Citation4] and phenolic componentd[Citation34] contribute to bioactivity and should be further examined.
TABLE 2 Neutral sugars, uronic acid, and sugar monomer composition of crude polysaccharides obtained from cherry, raspberry, and ginseng fruits
The molecular weight data contained in the high performance size exclusion (HPSEC) chromatograms of the water extractable polysaccharides are presented in –. The molecular weight profiles of the water extractable crude polysaccharides obtained from the: Lapins, Skeena, and Sweetheart cherry varieties (), Lapins cherries at the different maturity levels (), and ginseng berry pulp and raspberries () all show the appearance of a multimodal distribution spanning a 22 to 39.5-min retention time, which encompasses the entire molecular weight range of the standards, 6300 to 0.18 kDa. This indicates that the water extractable crude polysaccharides from all the fruits examined were heterogeneous and contained a range of molecular weight populations. The HPSEC chromatograms of the water extractable crude polysaccharides for all cherry varieties exhibited a main peak at a retention time of 26.8 min, which corresponded to a molecular weight of 52 kDa. This implies that the main molecular weight population resided in this size. There was no substantial difference in the molecular weights of the water extractable crude polysaccharides from the different cherry varieties. The effect of maturity on the molecular weights of the water extractable polysaccharides was also examined (). For all of these crude polysaccharides, the main peak occurred at the 26.8-min retention time, which corresponded to a molecular weight of 52 kDa. This implies that the main molecular weight population resided in this size. Notably the HPSEC chromatogram associated with the crude polysaccharides obtained from the immature edible Lapins cherries showed a shoulder at the 25.4-min retention time, which corresponds to a molecular weight of 551 kDa, indicating that the polysaccharides from the immature edible cherries possessed a higher molecular weight fraction. Our results are in agreement with those present in the literature, as the work of Fan et al.[Citation14] reported that the water extracted cherry polysaccharides contained heterogeneous molecular weight populations ranging from ˜18 to 1400 kDa depending on cherry variety. For the ginseng berry pulp, the water extractable crude polysaccharides exhibited two main peaks at retention times of 27.9 and 29. min (), which corresponded to molecular weights of 26 and 13 kDa. This implies that the main molecular weight population resided in these sizes. The water extractable crude polysaccharides obtained from the raspberries () showed a large peak at a retention time of 26.3 min, which corresponds to a molecular weight of 123 kDa. This implies that the main molecular weight population resided in this size. Another strong peak was observed at a retention time of 33 min, which corresponds to a molecular weight of 1.82 kDa, respectively. Of all the water extractable polysaccharides the crude polysaccharides obtained from ginseng berry pulp exhibited a main molecular weight population with the smallest molecular weight. Alternatively, the crude polysaccharides obtained from the raspberry samples demonstrated a main molecular weight population with the highest molecular weight. To our knowledge, no work has been performed characterizing the molecular weights of crude polysaccharides isolated from ginseng berry pulp or raspberries. also includes a summary of the molecular weights associated with the multimodal distributions contained in the HPSEC chromatograms of the water extractable polysaccharides.
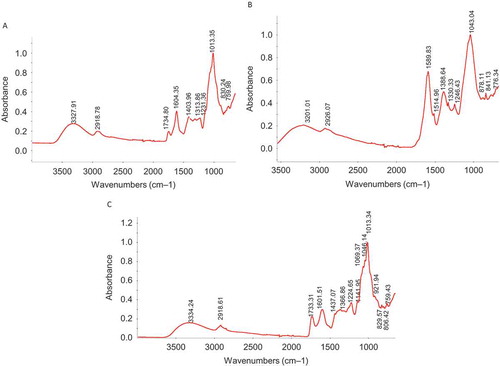
FIGURE 3 A: Fourier transform infrared (FTIR) spectra of crude polysaccharides: cherries-mature Lapins. B: FTIR spectra of crude polysaccharides: Ginseng Berry Pulp, C: FTIR spectra of crude polysaccharides: raspberries.
shows results for the neutral sugars content, uronic acid content, and sugar monomer profiles of the water extractable crude polysaccharides. The neutral sugars content of the crude polysaccharides ranged from ˜18 to 44% for all of the fruit samples, which as expected, were comparable to the values for total carbohydrates. The crude polysaccharides obtained from the mature Skeena, mature and overmature Lapins, and mature Sweetheart cherries demonstrated the highest neutral sugars contents of 43.96, 43.46, 40.88, and 41.37%, respectively, while the crude polysaccharides obtained from the ginseng berry pulp presented the lowest neutral sugars content, 18.04%. The uronic acid content of the crude polysaccharides ranged from 4.62 to 31.08% for all of the fruit crude polysaccharide samples. The crude polysaccharides obtained from the raspberries and immature edible Lapins cherries showed the highest uronic acids contents of 31.08, and 21.02%, respectively, while the crude polysaccharides obtained from the ginseng berry pulp demonstrated the lowest uronic acid content, 4.62%.
The sugar monomer content of the water extractable crude polysaccharides obtained from all the fruit was determined. All fruits contained rhamnose, arabinose, xylose, mannose, galactose, and glucose. Generally, the main sugars found in the all of the crude polysaccharides from each fruit sample were arabinose, galactose, and glucose. Our results are mostly in agreement with those of the literature as Fan et al.[Citation14] reported that the key sugar monomers present in the crude polysaccharides isolated from cherry were arabinose, galactose, and glucose. To our knowledge, the sugar monomer content of ginseng berry pulp crude polysaccharides has not been characterized. Hwang and Shin[Citation35] isolated bioactive polysaccharide fraction from black raspberry wine and characterized this fraction as containing appreciable amounts of rhamnose, galactose, and arabinose. The crude polysaccharides obtained from the raspberry samples exhibited the highest amounts of arabinose, which accounted for 45.76% of the neutral sugars present. The crude polysaccharides obtained from the ginseng berry pulp demonstrated the lowest amounts of arabinose, which accounted for 20.68% of the neutral sugars present. Galactose accounted for 43.74% of the neutral sugars present in the crude polysaccharides obtained from the ginseng berry pulp samples while galactose was present in the lowest amount (22.97%) in the crude polysaccharides obtained from the raspberry samples. Mannose and xylose were present in all of the fruit polysaccharides in relatively lowers amounts ranging from 4.57–7.36% and 2.93–5.39%, respectively. Fucose was present in all of the cherry and raspberry polysaccharides in low amounts ranging from 0.31–0.62%, yet absent in the ginseng berry pulp polysaccharides.
FTIR Spectra of Water Extractable Crude Polysaccharides
The FTIR spectra of the crude polysaccharides obtained from representative fruit samples are presented in –. The absorptions, functional groups, and structural characteristics are summarized in . The crude polysaccharides obtained from the fruit samples all showed bands of absorption around 3200 cm−1, which can be attributed to –OH stretching in hydrogen bonds and is indicative of strong inter- and intramolecular interaction between the polysaccharide chains.[Citation36] Stretching vibrations of the N-H bonds of an amino may also indicate the presence of proteins,[Citation37] which is in agreement with the chemical composition data presented in . The crude polysaccharides from all the fruit samples exhibited absorption peaks around 2900 cm−1, which could be attributed to the C–H stretching vibrations.[Citation35−Citation37] The crude polysaccharides obtained from the cherry and raspberry samples showed absorption bands at: 1700 cm−1, which corresponded to a carboxylic ester band; 1600 and 1388–1400 cm−1, which corresponded to carboxylate groups as an asymmetrical stretching band occurs around 1650 cm−1; and a weaker symmetric stretching band occurs around 1400 cm−1, all suggesting the presence of uronic acid.[Citation36−Citation38] The crude polysaccharides obtained from ginseng berry pulp did not show absorption at 1700 cm−1 but showed absorption at 1500 and 1600 cm−1, which may be indicative of feathering of a protein, which corresponds to the carbonyl group of an amide bond and bending vibration of the N–H bond, respectively.[Citation37,Citation39] This is in agreement with the chemical characterization data in which the ginseng berry pulp polysaccharides presented a lower uronic acid and higher protein content, 4.62 and 19.7%, respectively. All of the crude polysaccharide samples all showed absorptions around 1600 cm−1, which can also be attributed to an aromatic C=C skeletal stretching due to the aromatic ring of a phenolic compound.[Citation40] Each particular crude polysaccharide had a specific band in the 1200–1000 cm−1 region. This region is characterized by pyranose ring vibrations overlapped with stretching vibrations of (C–OH) side groups and (C–O–C) glycosidic band vibrations.[Citation37,Citation39] Differences in absorption for the different crude polysaccharides were also observed in the 960–740 cm−1 region of the FTIR spectra. Only the crude polysaccharides obtained from the raspberry samples demonstrated an absorption peak at about 920 cm−1. The crude polysaccharides obtained from the mature Sweetheart cherries, mature Skeena cherries, and ginseng berry pulp exhibited an absorption peak around 890 cm−1. All of the crude polysaccharide samples except those obtained from the mature Sweetheart cherries showed an absorption peak around 830 cm−1 while only the crude polysaccharides obtained from the raspberry samples exhibited an absorption peak around 806 cm−1. Yi et al.[Citation37] reported that absorption peaks at about 920 and 850 cm−1 might be characteristic of (1→4)-α-glucans; absorptions around 920 cm−1 may be attributed to the antisymmetrical ring vibration of a D-glucopyranose ring; and α-pyranoses may demonstrate band absorption at about 850 cm−1. Thetsrimuang et al.[Citation39] noted that the C–H bond in the α configuration has an absorption peak around 844 cm−1 while the C–H bond in the β configuration has an absorption peak nearby 891 cm−1. All of the crude polysaccharides obtained from the fruit samples exhibited absorption peaks around 760 cm−1. Absorptions at about 770 cm−1 have been noted to suggest symmetrical ring vibration of D-glucopyranose rings.[Citation37] Therefore, the crude polysaccharides extracted from the ginseng berry pulp and mature Skeena cherries displayed FTIR spectra possessing attributes of both α and β configurations. The FTIR spectra of the crude polysaccharides obtained from the raspberry and mature Lapins cherry samples indicated qualities of primarily α configurations, while the FTIR spectra of the crude polysaccharide samples obtained from the mature Sweetheart cherry samples indicated qualities of primarily the β configuration. NMR analysis is required for elucidation of chemical structure of the crude polysaccharides in order to clearly identify the configurations as α or β.
TABLE 3 Fourier transform infrared (FTIR) spectrum analysis of functional groups present in crude polysaccharides of cherry, raspberry, and ginseng fruits
Antioxidant Activity of Water Extractable Crude Polysaccharides
The results of the antioxidant activity of the crude polysaccharides in terms of (1) the reducing power towards Fe(III), and (2) the ability to act as a radical scavenging compound was investigated by the ferric reducing antioxidant power (FRAP) assay and the ABTS•+ radical scavenging assay, respectively, as presented in . For comparison purposes, the reducing power and radical scavenging activity of a phenolic antioxidant, gallic acid, is also presented in . Although the absolute values obtained for the antioxidant activities using these assays are different, in general, the trends of antioxidant activity are the same for both assays. The antioxidant activities determined for the crude polysaccharides obtained from the fruit samples using both the ABTS•+ radical scavenging and FRAP assays were at least 41 and 54 times lower than the values obtained for the gallic acid standard, respectively. The crude polysaccharides obtained from the raspberry and ginseng berry pulp samples demonstrated the highest antioxidant activities, 438.0 and 184.49 μmole Trolox eq/g CPS, respectively, as determined with the ABTS•+ radical scavenging assay, and 263.77 and 95.99 μmole Trolox eq/g CPS, respectively, as determined using the FRAP assay. The crude polysaccharides obtained from the mature Sweetheart, Skeena, Lapins, and immature edible and overmature Lapins cherries demonstrated relatively lower antioxidant activities ranging from 57.06 to 107.54 μmole Trolox eq/g CPS, respectively, as determined with the ABTS•+ radical scavenging assay, and 31.10 to 57.17 μmole Trolox eq/g CPS, respectively, as determined using the FRAP assay. To our knowledge, no work has been performed characterizing the antioxidant activity of crude polysaccharides isolated from ginseng berry pulp and raspberry. Fan et al.[Citation14] examined the antioxidant activity of crude polysaccharides from cherries using the ABTS•+ radical scavenging assay and ORAC (oxygen radical absorbance capacity) antioxidant activity assay, and reported a value of 159.3 μmole Trolox eq/g crude cherry polysaccharide, which is in agreement with the values reported in our work.
TABLE 4 Antioxidant activity of crude polysaccharides from cherry, raspberry, and ginseng fruits
Inhibition of Caspase 3 Activation by Water Extractable Crude Polysaccharides
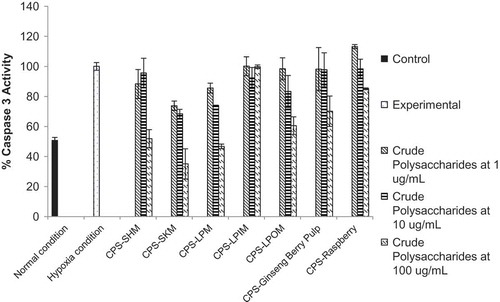
Myocardial damage induced by ischemia reperfusion has been shown to be associated with apoptosis, and caspase 3 activation is an important marker of apoptosis.[Citation29,Citation30] shows data from our model of hypoxia/reoxygenation, which induced a two-fold increase in caspase 3 activity in H9c2 cells. The crude polysaccharides obtained from the immature edible Lapins cherries did not provide any protection against caspase 3 activation as nearly 100% hypoxia was measured. The crude polysaccharides obtained from the ginseng berry pulp and raspberry samples gave minimal protection at the highest concentration (100 ug/mL), as ˜70 and 85% levels of caspase 3 activity were measured, respectively. The crude polysaccharides obtained from the mature Sweetheart cherries showed protection only at the highest concentration (100 ug/mL), as ˜52% caspase 3 activity was measured but a concentration-dependent trend was not observed. The crude polysaccharides obtained from the mature Lapins, overmature Lapins, and mature Skeena cherries provided protection against caspase-3 activation and a concentration-dependent trend was evident. At the highest concentration tested (100 ug/mL), ˜47, 61, and 35% caspase 3 activity was measured in the cells treated with polysaccharides obtained from the mature Lapins, overmature Lapins, and mature Skeena cherries, respectively. This work is in agreement with the work of Lu and Zhao,[Citation18] which showed that crude goji berry polysaccharides decreased myocardial cell apoptosis and concluded that administration of goji berry polysaccharides can help in the prevention of cardiovascular disease. In our work, crude polysaccharides from certain cherry varieties and certain maturity levels may aid in protecting the heart from ischemia-reperfusion injury, which has not yet been reported in the literature. Future work should include further purification of the crude polysaccharide fractions into neutral and acidic fractions with column chromatography and subsequent characterization as it is possible that purification may enhance the bioactive properties of these polysaccharides.[Citation41]
FIGURE 4 Effect of crude polysaccharides from cherries, raspberries, and ginseng berry pulp on caspase 3 activation. CPS--SHM: crude polysaccharide isolated from Sweetheart cherry variety; CPS--SKM: crude polysaccharide isolated from Skeena cherry variety; CPS--LPM: crude polysaccharide isolated from Lapins cherry variety, all harvested at commercial maturity; CPS--LPIM: crude polysaccharide isolated from Lapins cherry variety harvested prior to commercial maturity; CPS--LPOM: crude polysaccharide isolated from Lapins cherry variety harvested after commercial maturity; CPS--Ginseng Berry Pulp: crude polysaccharide isolated from ginseng berry pulp; CPS--Raspberry: crude polysaccharide isolated from raspberries.
Immunostimulatory Response of Water Extractable Crude Polysaccharides
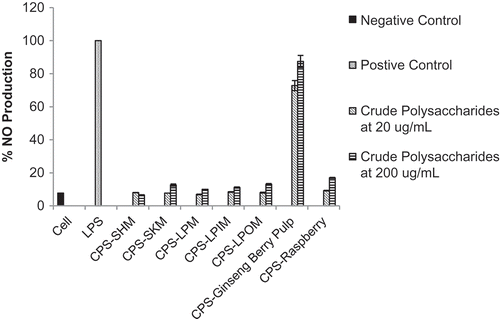
shows data from our model of immunostimulatory response, which evaluated a dose-related stimulation of inflammatory mediator response in vitro. The results show that only the crude polysaccharides obtained from the ginseng berry pulp provided a dose dependent immunostimulatory response as nitric oxide production was measured to range from 72.8–87.4%, which is comparable to the lipopolysaccharide (LPS) positive control (100%), which has not yet been reported. The crude polysaccharides obtained from the cherry and raspberry samples did not stimulate a LPS-induced response as nitric oxide production was measured to range from 6.5–16.9%. Based on these results, future work should investigate the direct inhibitory effect of polysaccharides on LPS-stimulated immune function. Also, future work should include further purification of the crude polysaccharide fractions into neutral and acidic fractions with column chromatography and subsequent characterization. It is possible that purification may affect the immunomodulating properties of these polysaccharides.[Citation31]
FIGURE 5 Effect of crude polysaccharides from cherries, raspberries, and ginseng berry pulp on Immunostimulatory response. CPS--SHM: crude polysaccharide isolated from Sweetheart cherry variety; CPS--SKM: crude polysaccharide isolated from Skeena cherry variety; CPS--LPM: crude polysaccharide isolated from Lapins cherry variety, all harvested at commercial maturity; CPS--LPIM: crude polysaccharide isolated from Lapins cherry variety harvested prior to commercial maturity; CPS--LPOM: crude polysaccharide isolated from Lapins cherry variety harvested after commercial maturity; CPS--Ginseng Berry Pulp: crude polysaccharide isolated from ginseng berry pulp; CPS--Raspberry: crude polysaccharide isolated from raspberries; LPS is lipopolysaccharide.
Relating Chemical Characteristics and Bioactivity
The bioactivities of polysaccharides can be affected by many factors, including chemical components, molecular mass, structure, conformation, extraction, and isolation methods.[Citation28,Citation38] In our work, the crude polysaccharides obtained from the various fruit samples exhibited different antioxidant activities, caspase 3 activation responses, and immunostimulating properties and different chemical characteristics. With respect to antioxidant activity, the crude polysaccharides obtained from the raspberry, ginseng berry pulp, mature Skeena cherries, and mature and overmature Lapin cherries all demonstrated the highest antioxidant activities. Antioxidant activity of fruits and vegetables often correlates to its total phenolic content,[Citation39] and the crude polysaccharides did contain phenolic compounds (). The crude polysaccharides obtained from the ginseng berry pulp and raspberries contained both the highest antioxidant activity and phenolic content, while the crude polysaccharides obtained from the mature Sweetheart and immature edible Lapins cherries possessed the lowest antioxidant activity and phenolic content. This does suggest that phenolic compounds may be contributing to the antioxidant activity. Molecular weight of polysaccharides has been shown to affect antioxidant activity.[Citation5] Li et al.[Citation38] noted that low molecular weight polysaccharides extracted from pumpkin demonstrated greater antioxidant activity. The crude polysaccharides obtained from the ginseng berry pulp and raspberries contained comparable phenolic contents yet the antioxidant activity of the ginseng berry pulp crude polysaccharides were nearly two times lower than the raspberry crude polysaccharides. The molecular weights of the ginseng berry crude polysaccharides were lower than the molecular weights of the crude raspberry polysaccharides. Both the crude polysaccharides obtained from the immature edible Lapin cherries and mature Sweetheart cherries had comparably low phenolic contents and comparable antioxidant activities, yet the crude polysaccharides obtained from the immature edible Lapins cherries contained a higher molecular weight fraction. No clear relationship between antioxidant activity and molecular weight could be defined.
The work of Chen et al.[Citation42] reported a direct relationship between the uronic acid contents and the antioxidant activity of tea polysaccharide conjugates, which is in agreement with our work when comparing the antioxidant activity of the raspberry and ginseng berry polysaccharides. This relationship was not observed when comparing the uronic acid content and antioxidant activity of the cherry crude polysaccharides. Also, a correlation between antioxidant ability and the amount of total glucans content in polysaccharides has been reported,[Citation43] and this relationship was observed for comparisons between the antioxidant activity and neutral sugars/total carbohydrate contents of the raspberry and ginseng berry pulp crude polysaccharides; however, this relationship was not observed for the crude polysaccharides obtained from cherries. Monosaccharide composition has been related to antioxidant ability.[Citation44] When comparing the antioxidant activity of the raspberry and ginseng berry crude polysaccharides, arabinose was found to be a sugar positively correlated to antioxidant ability. Comparing the antioxidant activity of the cherry crude polysaccharides, fucose, although present in low levels, was noted to be a sugar monomer positively correlated to antioxidant ability.
The crude polysaccharide obtained from the ginseng berry pulp was the only sample that demonstrated an immunostimulatory response. The chemical characteristics of the ginseng berry pulp crude polysaccharides included low uronic acid content, higher protein content, lower neutral sugars/carbohydrate content, and of the neutral sugars present high levels of galactose, and lower levels of arabinose. The work of Lo et al.[Citation45] reported that galactose played an important role in immunostimulation. Also, the molecular weight of the ginseng berry pulp polysaccharide was the low as the main molecular weight populations were 81 and 25 kDa.
Only the crude polysaccharides obtained from the mature Skeena cherries, and mature and overmature Lapins cherries inhibited caspase-3 activation in a dose dependent manner. Notably of the cherry crude polysaccharides, these samples did contain the highest phenolics content. There was no clear relationship between inhibition of capase 3 activation and protein content, uronic acid content, molecular weight, and neutral sugars/total sugars content. However, of the neutral sugars present, the samples that inhibited caspase 3 activation contained higher levels of the monosaccharide fucose. For all of the crude polysaccharides obtained from the different fruits examined in this work, it is difficult to state a general relationship between chemical characteristics and bioactivity. Many factors of chemical characteristics influence bioactive properties and more study is required to define the relationship between polysaccharides and bioactivity.
CONCLUSION
This work evaluated and compared the chemical, structural, and molecular characteristics along with the bioactive properties of water extractable crude polysaccharides from sweet cherries, raspberries, and ginseng berry pulp. The crude polysaccharides from cherries, raspberries, and ginseng berry pulp all contain between ˜20–41% carbohydrate content and an uronic acid component; however, they all also contain protein and phenolic compounds. All of the crude polysaccharides fractions demonstrated antioxidant activity while only the crude polysaccharides fractions obtained from certain varieties of sweet cherries inhibited caspase 3 activation, an indicator for a cardio protective property, and only the crude polysaccharides fraction obtained from ginseng berry pulp stimulated immune function. A clear correlation between chemical characteristics of the crude polysaccharides and bioactivity could not be established based on the present results as many factors of the chemical characteristics of the studied fractions may influence bioactive properties. In order to define the relationship between specific polysaccharides and bioactivity, more study is required. However, this work does provide novel information supporting the hypothesis that polysaccharides present in small fruits possess bioactivities that may play a role in enhancing human health.
REFERENCES
- Herken, E.N.; Guzel, S. Total antioxidant capacity and total phenol contents of selected commercial fruit juices. International Journal of Food Properties 2010, 13, 1373–1379.
- Adnan, L.; Osman, A.; Hamid, A.A. Antioxidant activity of different extracts of red pitaya (Hylocereus polyrhizus) seed. International Journal of Food Properties 2011, 14, 1171–1181.
- Ross, K.A.; Mazza, G. Conjugated polysaccharides as potential health promoting compounds in berries and cherries. In: Berries: Properties, Consumption and Health Benefits; Tuberoso, C.; Ed.; Nova Science Publishers Inc.: New York, 2012; 55–81.
- Pawlaczyk, I.; Czerchawki, L.; Kuliczkowski, W.; Karolko, B.; Pilecki, W.; Witkiewicz, W.; Gancarz, R. Anticoagulant and anti-platelet activity of polyphenolic-polysaccharide preparation isolated from the medicinal plant Erigeron canadensis L. Thrombosis Research 2011, 127, 328–340.
- Chen, H.; Zhang, M.; Zhishuang, Q.; Bijun, X. Antioxidant activities of different fractions of polysaccharide conjugates from green tea (Camellia sinensis). Food Chemistry 2008, 106, 559–563.
- Chen, Y.; Xie, M.Y.; Nie, S.P.; Li, C.; Wang, Y.X. Purification, composition analysis and antioxidant activity from the fruiting bodies of Ganoderma atrum. Food Chemistry 2008, 107, 231–241.
- Inngjerdingen, K.T.; Debes, S.C.; Inngjerdingen, M.; Hokputsa, S.; Harding, S.E.; Rolstad, B.; Michaelsen, T.E.; Diallo, D.; Paulsen, B.S. Bioactive pectic polysaccharides from Glinus oppositifolius (L.) Aug. DC., a malian medicinal plant, isolation and partial characterization. Journal of Ethnopharmacology 2005, 101, 204–214.
- Luo, D.; Fang, B. Structural identification of ginseng polysaccharides and testing of their antixoxidant activities. Carbohydrate Polymers 2008, 72, 376–381.
- Zheng, S.Y.; Sun, J.; Zhao, X.; Xu, J.G. Protective effect of shen-fu on myocardial ischemia-reperfusion injury in rats. American Journal of Chinese Medicine 2004, 32 (20), 209–220.
- Xie, J.T.; Wu, J.A.; Mehendale, S.; Aung, H.H.; Yuan, C.S. Anti-hyperglycemic effect of the polysaccharides fraction from American ginseng berry extract in Ob/Ob mice. Phytomedicine 2004, 11, 182–187.
- Ovodova, R.G.; Golovchenko, V.V.; Popov, S.V.; Popova, G.Y.; Paderin, N.M.; Shashkov, A.S.; Ovodov, Y.S. Chemical composition and anti-inflammatory activity of pectic polysaccharide isolated from celery stalks. Food Chemistry 2009, 114, 610–615.
- Popov, S.V.; Ovodova, R.G.; Golovchenko, V.V.; Popova, G.Y.; Viatyasev, F.V.; Shashkov, A.S.; and Ovodov, Y.S. Chemical composition and anti-inflammatory activity of a pectic polysaccharide isolated from sweet pepper using a simulated gastric medium. Food Chemistry 2011, 124, 309–315.
- Lui, C.J.; Lin, J.Y. Anti-inflammatory and anti-apoptotic effects of strawberry and mulberry fruit polysaccharides on lipopolysaccharide-stimulated macrophages through modulating pro-/anti-inflammatory cytokines secretion and Bcl-/Bak protein ratio. Food Chemistry and Toxicology 2012, 50 (9), 3032–3039.
- Fan, H.; Mazza, G.; Liao, X. Purification, composition and antioxidant activity of polysaccharides from wolfberry, cherry, kiwi, and cranberry fruits. Croatian Journal of Food Science and Technology 2010, (1), 9–17.
- Luo, Q.; Cai, Y.; Yan, J.; Sun, M.; Corke, H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sciences 2004, 76, 137–149.
- Gan, L.; Zhang, S.H.; Yang, X.L.; Xu, H.B. Immunomodulation and antitumor activity by a polysaccharide-protein complex grom Lycium barbarum. Immunopharmacology. 2004, 4, 563–569.
- Li, X.M.; Li, X.L.; Zhou, A.G. Evaluation of antioxidant activity of the polysaccharides extracted from Lycium barbarum fruits in vitro. European Polymer Journal 2007, 43, 488–497.
- Lu, S.P.; Zhao, P.T. Chemical characterization of Lycim barbarum polysaccharides and their reducing myocardial injury in ischemia/repurfusion. International Journal of Biological Macromolecules 2010, 47, 681–684.
- Boivin, D.; Lamy, S.; Lord-Dufour, S.; Jackson, J.; Beaulieu, E.; Cote, M.; Moghrabi, A.; Barrette, S.; Gingras, D.; Beliveau, R. Antiproliferative and antioxidant activities of common vegetables: A comparative study. Food Chemistry 2009, 112, 374–380.
- AOAC. Moisture in dried fruits. Method 934.06. In: Official Methods of Analysis; Association of Official Analytical Chemists International: Arlington, VA, 1990; 911–912.
- Dubios, M.; Gilles, K.A.; Hamilton, J.K.; Robers, P.A.; Smith, F. Colormetric method for determination of sugars and related substances. Analytical Chemistry 1956, 28 (3), 350–356.
- Scott, R.W. Colorimetric determination of hexuronic acids in plant materials. Analytical Chemistry 1979, 51 (7), 936–941.
- Blakeney, A.B.; Harris, P.J.; Henry, R.J.; Stone, B.A. A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydrate Research 1983, 113, 291–299.
- Tamaki, Y.; Mazza, G. Measurement of lignin from flax shives as affected by extraction structural carbohydrates, lignins and micro-components of straw and shives: Effects of extractives, particle size and crop species. Industrial Crops and Products 2010, 31, 534–541.
- Tamaki, Y.; Mazza, G. Rapid determination of carbohydrates, ash, and extractives contents of straw using attenuated total reflectance Fourier transform. mid-infrared spectroscopy. Journal of Agricultural and Food Chemistry 2011, 59 (12), 6346–6352.
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. American Journal of Enology and Viticulture 1965, 16, 144–158.
- Carnachan, S.M.; Bootten, S.M.; Mishra, S.; Monro, J.A.; Sims, I.M. Effects of simulated digestion in vitro on cell wall polysaccharides from kiwifruit (Actinidia spp.). Food Chemistry 2012, 133, 132–139.
- Mateos-Aparicio, I.; Mateos-Peinado, C.; Jimenez-Escrig, A.; Ruperez, P. Multifunctional antioxidant activity of polysaccharide fractions from the soybean by-product okara. Carbohydrate Polymers 2010, 82, 245–250.
- Mockridge, J.W.; Marber, M.S.; Heads, R.J. Activation of Akt during simulated ischemia/reperfusion in cardiac myocytes. Biochemical and Biophysical Research Communications 2000, 270, 947–952.
- Li, Y.; Cohen, R. Caspase inhibitors and myocardial apoptosis. International Anesthesiology Clinics 2005, 43 (2), 77–89.
- Lui, E.M.K.; Azike, C.G.; Guerrero-Analco, J.A.; Romeh, A.A.; Pei, H.; Kaldas, S.J.; Arnason, J.T.; Charpentier, P.A. Bioactive polysaccharides of American ginseng Panax quinquefolius L. in modulation of immune function: Phytochemical and pharmacological characterization. In: The Complex World of Polysaccharides; Karunaratne, D.N.; Ed.; InTech: Rijeka, Croatia, 2012; 513–534.
- Barbier, M., Thibault, J.F. Petic substances of cherry fruits. Phytochemistry 1982, 21 (1), 111–115.
- Saura-Calixto, F. Dietary fiber as a carrier of dietary antioxidants: An essential physiological function. Journal of Agricultural and Food Chemistry 2011, 59, 43–49.
- Saura-Calixto, F. Concept and health-related properties of nonextractable polyphenols: The missing dietary polyphenols. Journal of Agricultural and Food Chemistry 2012, 60, 11195–11200.
- Hwang, Y.C.; Shin, K.S. Isolation and characterization of immuno-stimulating polysaccharide in Korean black raspberry wine. Journal of the Korean Society for Applied Biological Chemistry 2011, 54 (4), 591–599.
- He, J.Z.; Ru, Q.M.; Dong, D.D.; Sun, P.L. Chemical characteristics and antioxidant properties of crude water soluble polysaccharides from four common edible mushrooms. Molecules 2012, 17, 4373–4387.
- Yi, Y.; Liao, S.T.; Zhang, M.W.; Shi, J.; Zhang, R.F.; Deng, Y.Y.; and Wei, Z.C. Physicochemical characteristics and immunomodulatory activities of three polysaccharide-protein complexes of Longan pulp. Molecules 2011, 16, 6148–6164.
- Li, J.; Wang, Y.; Zhang, D.; Hu, X.; Zhang, Z.; Xiang, C. Characterization and bioactivity of water-soluble polysaccharides from the fruit of pumpkin. Journal of Food Agriculture and Environment 2010, 8 (2), 237–241.
- Thetsrimuang, C.; Khammuang, S.; Sarnthima, R. Antioxidant activity of crude polysaccharides from edible fresh and dry mushroom fruiting bodies of Lentinus sp. strain RJ-2. International Journal of Pharmacology 2011, 7 (1), 58–65.
- Sene, C.F.B.; McCann, M.C.; Wilson, R.H.; Grinter, R. Fourier transform Raman and Fourier transform infrared spectroscopy: An investigation of five higher plant cell walls and their components. Plant Physiology 1994, 106 (4), 1623–1631.
- Sun, Y.X.; Kennedy, J.F. Antioxidant activities of different polysaccharide conjugates isolated from the fruiting bodies of Chroogomphis rutilus (Schaeff.: Fr.) O.K. Miller. Carbohydrate. Polymers 2010, 82, 510–514.
- Chen, H.X.; Zhang, M.; Xie, B.J. Quantification of uronic acids in tea polysaccharide conjugates and its antioxidant properties. Journal of Agricultural and Food Chemistry 2004, 52, 3333–3336.
- Kozarski, M.; Klaus, A.; Niksic, M.; Jakovljevic, D.; Helsper, J.P.F.G.; van Griensven, L.J.L.D. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chemistry 2011, 129, 1667–1675.
- Tsiapali, E.; Whaley, S.; Kalbfleisch, J.; Ensley, H.E.; Browder, I.W.; Williams, D.L. Glucans exhibit weak antioxidant activity, but stimulate macrophage free radical activity. Free Radical Biology & Medicine 2001, 30, 393–402.
- Lo, T.C.T.; Jiang, Y.H.; Chao, A.L.J.; Chang, C.A. Use of statistical methods to find the polysaccharide structural characteristics and the relationships between monosaccharide composition ratio and macrophage stimulatory activity of regionally different strains of Lentinula edodes. Analytica Chimica Acta 2007, 584, 50–56.