Abstract
Phenolic profile, antioxidant activity, and color parameters of Polish wines, produced from the multispecies hybrid and Vitis vinifera L. grapes were analyzed. A principal component analysis was applied, in order to differentiate the investigated wines in terms of content of phenolic compounds. The white wines turned out to be similar to each other in terms of color parameters and the results of principal component analysis, while the red wines strongly differed in this respect. However, the white wine produced from the multispecies hybrid grapes contained a higher level of phenolic acids and flavonoids, as compared to the white wines obtained from the hybrid cultivars. Out of the red wines, Rondo wine, produced from the multispecies hybrid grapevine was the richest in total phenolic and phenolic acids content. Caffeic acid and quercetin were the predominant phenolics in majority of the wines tested.
INTRODUCTION
The wines produced from the noble grape varieties (e.g., Vitis vinifera L.) are known for their high quality. However, these cultivars do not possess a high resistance against fungal infections and winter frost.[Citation1] Therefore, an examination of adaptability of the new grape varieties to cold climate conditions, as well as investigation of the wines produced from these cultivars, seem to be useful tools in choosing the appropriate grape varieties for cold climates. These new grape varieties (known as multispecies hybrid grapes) are originating from a crossing of two or more species of Vitis, and are introduced as a solution to the many viticultural problems of cool climate, and more humid wine regions. These cultivars possess an excellent tolerance to cold climate and fungal infections. In Poland, the most commonly cultivated grape varieties are the multispecies hybrid grapes, in particular those being the products of a crossing European grapevine (Vitis vinifera L.) with North American grapevine (e.g., Vitis rupestis, riparia, or abrusca) or even Asiatic grapevine (e.g., Vitis amurensis). Among most popular grape varieties, cultivated in Poland, the leading ones are Cascade, Leon Millot, Marechal Foch, Regent, Rondo, and Seyval Blanc. In the experimental Vineyard “Golesz,” located in south-eastern Poland (Podkarpacie region), mainly the new hybrid grape varieties are cultivated, including extremely cold-hardy grape cultivars able to withstand temperatures down below −30°C (e.g., Frontenac, Regent, Rondo, Sabbrevois, and St. Croix in case of red grapevines). Rondo and Regend are the dark-skinned grape varieties that possess a significantly higher resistance against most fungal diseases in comparison to Vitis vinifera L. grapes, despite their moderate resistance against winter frost. On the other hand, the cultivars such as Sabbrevois, St Croix, and Frontenac are extremely cold-hardy (below –30°C) and also possessing a very high resistance against fungal infections. However, red wines produced from the last cultivars are of lower quality in comparison to those obtained from the noble cultivars. Among the varieties of grape, used for production of white wines in Vineyard “Golesz,” the leading ones are the new hybrid grapevines (e.g., St. Pepin, Praire Star, and Adalmiina), possessing a very high resistance against winter frost (below –30°C) and fungal infections. However, they are also producing the wines of a significant lower quality in comparison to those obtained from the noble cultivars.
Phenolic compounds are plant secondary metabolites being one of the most important quality parameters of wine, since they are responsible for red wine color,[Citation2] and other sensorial characteristics of wine such as: Astringency, bitterness, and aroma. Despite a number of studies on phenolic profile and antioxidant activity (AA) of wines, literature lacks data reporting attempts to determine phenolic profile of Polish wines using a HPLC method, as well as to differentiate wines produced from the Vitis vinifera L. and multispecies hybrid grapes in conditions of cool climate. This information also seems to be of key importance for consumers due to the health-promoting and antioxidant properties of phenolic constituents in wines.
Several studies have reported a principle component analysis (PCA) in order to show grouping of the wines and interrelationship of the variables, including analytical and sensorial parameters of wines. As shown by Boselli,[Citation3] the PCA enabled to show which was the impact of phenolic fraction on sensorial attributes of Italian white wines, originating from Marche region. A PCA was undertaken by Tinttunen[Citation4] to determine whether it is possible to distinguish organic wines from normal ones, on the basis of chemical composition and spectral data. This type of analysis was successfully applied by Kallithraka,[Citation5] in order to classify Greek red wines, produced from Vitis vinifera L. species, on the basis of phenolic and anthocyanin content. Again, the last authors used a PCA to the data of commercial Greek wines, in order to study which analytical and sensorial parameters are most important for Greek wine authentication.[Citation6] Regarding Croatian wines investigated by Rastija,[Citation7] their classification according to the type and geographical origin was performed by a PCA, based on analytical parameters of wines (i.e., flavonols and trans-resveratrol content). However, scarce information about classification of Polish wines, produced from Vitis and multispecies hybrid cultivars.
The aim of the reported study was to analyse the phenolic profile (i.e., total phenolic, phenolic acids, and flavonoids content), AA, and color parameters of Polish wines, produced from the multispecies hybrid and Vitis (grape) species, cultivated in Vineyard “Golesz” (Podkarpacie region) in cool climate conditions, as well as to classify the investigated wines by a PCA using analytical parameters of the wines.
MATERIALS AND METHODS
Chemicals
Acetic acid, ethyl acetate, methanol, sodium carbonate, sodium chloride, and acetonitrile were of analytical grade and purchased from POCh (Gliwice, Poland). Caffeic acid (98%), chlorogenic acid (95%), p-coumaric acid (98%), ferulic acid (99%), gallic acid (98%), syringic acid (95%), catechin (98%), epicatechin (98%), DPPH (2,2-diphenyl-1-picrylhyrazyl radical), Folin-Ciocalteu reagent, and quercetin (95%) were purchased from Sigma-Aldrich (Steinheim, Germany). Kaempferol (96%) and ABTS (2,2ʹ-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) (99%) were purchased from Fluka Chemie AG (Buchs, Switzerland).
Wine Samples
Seven red and five white wines of the 2007 vintage (), including single- and multi-variety wines were purchased from Vineyard “Golesz,” located in Podkarpacie region. The wines under study were produced from the multispecies hybrid grapevines, including the new cold-hardy varieties, apart from the red and white multi-variety wines from a kind of Couveé vinifera (R-5 and W-5), produced from the Vitis vinifera L. species. The grapes were harvested at the optimal stage of ripening, during the 2007 season. Winemaking conditions were as follows: Following destalking and crushing the grapes, the process of maceration was conducted. The maceration lasted two hours for white wines and two weeks for red wines, respectively. Subsequently, residual solid parts were separated, and the must was run straight into a fermentation tank. The must was supplied with potassium metabisulphite, in the amount of 0.1 g per dm3 of must, and then inoculated with the separate yeast starters for red and white grapes, respectively, and alcoholic fermentation was conducted at temperature of 25°C. At the end of fermentation, the wine was racked and bottled. Wine samples were stored in the dark at 4°C, no longer than one week after their purchase from the vineyard, and each one was opened immediately before the analysis.
TABLE 1 The grape cultivars used for production of the wines studied
Determination of Total Phenolic Content (TPC)
The TPC in the investigated wines was determined using the Folin-Ciocalteu reagent following the reported procedure.[Citation8] In summary, 1 cm3 of the appropriate diluted wine sample (red wine to distilled water ratio of 1:5, v/v) was diluted with 70 cm3 of distilled water, then the obtained solution was mixed with 5 cm3 of 0.2 M Folin-Ciocalteu reagent. After 2 min of incubation, 15 ml of 20% (m/v) sodium carbonate solution was added to the sample, and the obtained mixture was diluted with distilled water to volume of 100 cm3. After 2 h of incubation, the absorbance was measured at λ = 756 nm against a blank sample using an UV/Vis V-530 spectrophotometer (Jasco, Japan). The measurements were performed in triplicate. The TPC was expressed in mg of gallic acid per 1 dm3 of wine, based on a calibration curve. The latter was plotted for standard solutions of gallic acid used in concentration range of 0–200 mg per 1 dm3 according to the above described procedure, using gallic acid solution instead wine sample.
Determination of AA Against DPPH Radical
The AA of the wines was assayed using a DPPH radical method.[Citation9] In summary, 100 μl of wine (diluted with water at the ratio of 1:5 (v/v), in the case of red wine) was mixed with 3.9 cm3 of methanolic solution of DPPH (25 mg per dm3) and left for 15 min. Then an absorbance of the mixture was measured, using an UV/Vis V-530 spectrophotometer (Jasco, Japan) at λ = 515 against methanol. The measurements were performed in triplicate. The AA was expressed as Trolox equivalent antioxidant capacity (TEAC) in mM of Trolox per 1 dm3 of wines, using a calibration curve plotted for Trolox solutions in the concentration range of 0.1–1.0 mM, according to the above described procedure, using Trolox solution in methanol instead wine sample.
Determination of AA against ABTS Cation Radical
The AA of the wines was assayed using an ABTS radical method.[Citation10] In summary, 100 μl of the wine (diluted with distilled water at the ratio of 1:2 (v/v), or 1:10 (v/v) in the case of red or white wine, respectively) was mixed with 6 cm3 of ABTS cation radical solution and after 30 min an absorbance of the mixture was measured using an UV/Vis V-530 spectrophotometer (Jasco, Japan) at λ = 734 nm against phosphate buffer. The measurements were performed in triplicate. The AA was expressed as TEAC in mM of Trolox per 1 dm3 of wine, using a calibration curve plotted for Trolox solutions in the concentration range of 0.1–1.0 mM, according to the above described procedure, using Trolox solution instead wine sample.
Extraction and Chromatographic Analysis of Phenolic Compounds
Phenolic compounds were extracted from the investigated wine samples with using ethyl acetate, as described by Woodring with a minor modification.[Citation11] Unlike the method employed by the latter authors, in the present study, the wine samples were extracted four times using a separating funnel. The values of recovery of phenolic compounds for such extraction, are collected in . In summary, 75 cm3 of the wine sample was concentrated under reduced pressure at temperature of 35°C, in a rotary evaporator to the volume of approximately 65 cm3 in order to remove the alcohol without destroying phenolic compounds. The dealcoholized solution was diluted to the primary volume of 75 cm3 by distilled water. The resulting sample was adjusted to pH = 2 with HCl solution, and then saturated with sodium chloride in the amount of 3 g per 10 cm3 of sample to prevent formation of an emulsion. The resulting solution was extracted with ethyl acetate using 25 cm3 of the solvent. Fractions of ethyl acetate were combined and evaporated to dryness in a rotary evaporator. The dry residue was dissolved in 5 cm3 of methanol. The samples prepared in such way were subjected to a HPLC analysis prior to the chromatographic analysis, the extracts were filtered through a Millex-LCR (Millipore) filter with a pore diameter of 0.45 μm. For each sample, the extraction procedure was conducted in two replications.
TABLE 2 Retention times and recovery of phenolic compounds determined in the wines
The qualitative and quantitative analysis of phenolic compounds was conducted with the use of a HPLC system (LaChrom; Merck-Hitachi, Japan) based on the reported procedure.[Citation12] Hydroxybenzoic acids (i.e., gallic and syringic acid) and flavanols (i.e., (+)-catechin and(–)-epicatechin) were identified at λ = 280 nm, whereas, hydroxycinnamic acids (i.e., caffeic, chlorogenic, p-coumaric, and ferulic acid) and flavonols (i.e., quercetin and kaempferol) were detected at λ = 340 nm. The analysis was carried out on an octadecylsilane column (Thermo scientific, USA: 250 × 4.6 μm, particle size 5 μm) at a temperature of 30°C in a gradient mode with two phases: Phase A: aqueous solution of acetic acid (2.5 g per 100 cm3), and phase B: acetonitrile. The eluent flow rate was of 1 cm3 per min. The chromatographic analysis was conducted as follows: For the first 10 min–linear gradient with phase B contribution increasing in the mobile phase from 3 to 8%, followed by increase in phase B contribution to 15, 20, 30, and 40% at 20, 30, 40, and 50 min, respectively. Finally, the column was eluted isocratically for 10 min with acetonitrile before the next injection. The qualitative analysis of phenolic compounds was carried out by comparing their retention times with those of standards (). The calibration curves of the analyzed phenolics were made in triplicate for each individual standard and were plotted separately for each standard at four different concentrations (0.02–0.2 mg cm−3). The determination coefficients for the calibration curves were higher than 0.997 and 0.988 for phenolic acids and flavonoids, respectively. The analyses of particular phenolic compounds in wine samples were carried out in duplicate.
Determination of Color Parameters
Color parameters (L*, a*, and b*) of the wines were established in the CIELAB system by transmission method (illuminant D65, range 400–700 nm, observer 10°) using a CM 3500D spectrophotometer (Konica-Minolta). Measurements of color parameters were carried out in triplicate.
Statistical Analysis
In order to determine the significant differences between the means, the data were treated by a one-way analysis of variance, and the test of least significant difference (LSD) using Tukey test at a significance level α = 0.05 was calculated. The Pearson’s linear correlation coefficients between selected parameters were also calculated. Additionally, a PCA was applied in order to differentiate the wines under study. Calculations were performed with statistical software package Statistica 9.0 (StatSoft Inc. Tulsa, USA).
RESULTS AND DISCUSSION
TPC
The values of TPC, expressed as gallic acid equivalents, are collected in . The obtained results demonstrated a high variability around the TPC value among the wines tested. For the red wines under study, the TPC ranged from 996 to 1668 mg per dm3 for Regent and Rondo (averaging 1292 mg per dm3), respectively, whereas for the white wines, it ranged from 187 to 242 mg per dm3 for Adalmiina and Swenson Red (averaging 210 mg per dm3), respectively. The red wines were characterized by a significantly higher level of TPC than that of the analyzed white wines. This observation was in agreement with the studies reported by previous authors.[Citation13,Citation14] A significantly higher level of TPC in red wines, as compared to white ones might be attributed to differences between red and white wine making process, (including maceration for the former), as well as to differences in phenolic composition between red and white grapes. The TPC in the wines produced from Vitis vinifera L. species (R-5 and W-5) () fell within the range observed in those obtained from the hybrid grapevines in our study (). At this point, it should be stated that selectivity of Folin-Ciocalteu method is low due to other non-phenolic compounds capable of reacting with Folin-Ciocalteu reagent. The TPC in the red wines tested was similar to that found in Croatian red wines, originating from the regions with continental climate (i.e., Istria, Slavonia, and Podunavlje),[Citation7] however, a significantly higher level of TPC was found in Croatian red wines, originating from the regions with Mediterranean climate (i.e., Central and Southern Dalmatia)[Citation7] as compared to these results. The level of TPC in the red wines in the present study was found to be a lower as compared to that observed in majority of wines originating from the countries or regions with warm climate, i.e., Greece,[Citation5] Italy,[Citation13] Portugal,[Citation15] Slovakia,[Citation16] Austria,[Citation16] and Languedoc.[Citation17] On contrary, the level of TPC in the white wines under study showed a great similarity to that observed in the wines originating from the regions with warm climate.[Citation18−Citation20]
TABLE 3 Total phenolic content (TPC) and antioxidant activity (AA) of the wines
AA
The values of AA of the investigated Polish wines, determined in the reaction with DPPH and ABTS free radicals are collected in . The AA, determined using both assays was strongly diversified among the wines tested. The high and significant (α = 0.05) linear correlation (r = 0.989) between these two assays was observed. As with TPC, the AA of wines produced from Vitis vinifera L. cultivars fell within the range observed in those produced from the hybrid cultivars. Out of the red wines, the multi-variety wine (R-4) produced from the mixture of three multihybrid grape varieties (i.e., Cascade, Leon Millot, and Marechal Foch) () was characterized by the highest AA determined against DPPH and ABTS radicals (). With respect to the white wines, Swenson Red (W-4) produced from Vitis labrusca grapevine () presented the highest AA for both DPPH and ABTS assays (). On contrary, the lowest AA for DPPH assay was observed in Heridan (R-1) and Adalmiina (W-2), red and white wine, respectively, while in the case of ABTS assay it was Heridan (R-1) and Seyval Blanc (W-3), respectively. The high and significant linear correlations (α = 0.05) were found between the TPC and AA of the wines, evaluated using DPPH (r = 0.969) and ABTS (r = 0.989) assays, respectively. Similarly, a high correlation between the TPC and AA (r = 0.9737) was noted by Radovanovic[Citation21] when investigated Serbian red wines from different cultivars of Vitis vinifera. The red wines under study were characterized by a lower AA, as compared to that of red wines originating from warm regions of Europe.[Citation20,Citation22] It should be taken into account, that a lower level of AA of the red wines in this study in comparison with that of the wines originating from the regions with warm climate is also reflected in a lower level of phenolic content in red wines determined spectrophotometrically. Unlike the red wines, level of AA of the white wines under study was in close agreement with that observed in wines, originating from countries and regions of warm climates.[Citation19,Citation20]
TABLE 4 Phenolic acids content in the wines
Profile of Phenolic Acids
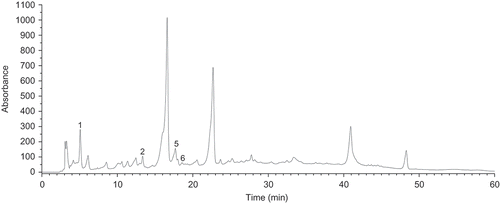
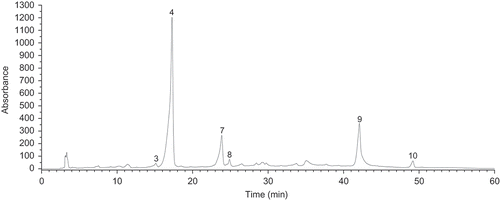
The levels of individual phenolic acids in wines, are collected in . Among the white wines tested, the multi-variety Couveé vinifera (W-5) produced from Vitis vinifera L. grapevines () was characterized by the highest level of total phenolic acids (TPA) On contrary, in the case of Swenson Red wine (W-4) obtained from Vitis labrusca grapevine, sum of phenolic acids was the lowest. When it comes to the red wines, R-6 wine produced from multispecies hybrid Rondo grapevine was found to be richest source of phenolic acids, followed by Couveé vinifera (R-5) produced from the noble grapevines, whilst R-1 (Heridan) was the poorest in this respect (). The representative chromatograms for polyphenol standards mixture and red wine Rondo (R-6) are shown in --.
Two groups of phenolic acids [i.e., hydroxybenzoic () and hydroxycinnamic acids ()] were separated and quantified by the HPLC method employed. With respect to hydroxycinnamates, four compounds were quantified: Caffeic, chlorogenic, p-coumaric, and ferulic acid. This group of phenolic acids seems to be a characteristic for Polish wines under study, unlike the majority of wines, originating from warm-climate regions. In the latter ones, hydroxybenzoic acids are thought to be the predominant phenolics.[Citation5,Citation13,Citation23] Caffeic acid was by far the most predominant compound in all the red wines and in the majority of white wines. Both the red and white multi-variety wine (R-5 and W-5), produced from the noble grape varieties were the richest in caffeic acid among all the wines studied. The level of caffeic acid in the investigated Polish wines was found to be a significantly higher than that observed in majority of red and white wines, originating from the warm climate regions such as: Greece,[Citation5] Italy,[Citation13] and Sicilia[Citation23] for the former, and Italy,[Citation13] Slovakia,[Citation16] Austria,[Citation16] and Croatia[Citation24] for the latter. On the contrary, the level of caffeic acid in the red wines tested was similar to that observed in Croatian red wines,[Citation7] originating from the regions with Mediterranean climate (i.e., Istria and Dalmatia). p-Coumaric acid was the second most abundant phenolic acid in the red wines tested, and its level was the highest in Rondo (R-6) wine produced from the multispecies hybrid grapevine. With respect to red wines in the present study, level of p-coumaric acid was similar to that that found in Austrian,[Citation16] Slovakian,[Citation16] and Croatian[Citation7] wines, whereas, its level in the white wines studied was in close agreement with that observed in Croatian[Citation24] and Spanish table wines.[Citation25]
Among the hydroxycinnamates quantified by the HPLC method employed, chlorogenic and ferulic acid turned out to be the minor constituents in the investigated Polish wines. The level of ferulic acid in the red wines tested was in accordance with that found in wines originating from the regions with warm climates, as reported by Kallithraka[Citation5] in Greek wine, La Torre[Citation23] in Sicilian wines, and Rastija[Citation7] in Croatian wines. The last authors reported that level of ferulic acid was independent on the type and geographical origin of the wine. With respect to hydroxybenzoic acids, two phenolics were quantified: Gallic and syringic acid. Out of them, gallic acid turned out to be the predominant compound in majority of the wines tested (), and its level was the highest in (R-6) wine Rondo, produced from the multispecies hybrid grapevine. However, the level of hydroxybenzoic acids, in particular gallic acid in the investigated Polish wines was a significantly lower when compared to that observed in wines originating from the regions with warm climate.[Citation5,Citation13,Citation23] Namely, a substantially higher level of gallic acid was observed in the wines originating from Austria,[Citation16] Slovakia,[Citation16] some regions of Croatia,[Citation7] (i.e., Central and Southern Dalmatia, Slavonia, and Podunavlje), Languedoc,[Citation17] Greece,[Citation5] Sicilia,[Citation23] and Thailand.[Citation26] On the other hand, some Croatian red wines, originating from region with continental climate (Istria)[Citation7]were found to be the similar in gallic acid content to the red wines studied. The level of gallic acid in grapes is, to a great extent, determined by the degree of biosynthesis of flavanols (in particular, (+)-gallo(catechins) and (–)-epi(gallo)catechins), which in turn are being decomposed to gallic acid, under the action of tannase enzymes,[Citation27] and thus the variety of grape using for a winemaking process, as well as the climatic conditions during cultivation, seem to have a significant influence on level of this phenolic in both grapes and wines. Syringic acid was present in all wines studied (), and its level was similar to that observed in Greek,[Citation5] Italian,[Citation13] Slovakian,[Citation16] and Spanish[Citation28] red wines.
Profile of Flavonoids
Levels of the individual flavonoids are collected in . The wines under study, produced from the Vitis vinifera L. grapevines (R-5 and W-5) was characterized by the highest level of total flavonoids (TF), among all the wines tested. On the contrary, in the case of Swenson Red wine (W-4) produced from the Vitis labrusca grapevine, the sum of flavonoids was the lowest. Two groups of flavonoids [i.e., flavanols () and flavonols ()] were separated and quantified by the HPLC method employed. With respect to flavonols, two phenolics were identified: Quercetin and kaempferol. Quercetin was by far the most abundant flavonoid in all the red wines and majority of the white ones (), and its level was the highest in both red and white Couveé vinifera (R-5 and W-5) produced from Vitis vinifera L. grapevines. Furthemore, this fact differentiated Polish wines under study from the majority of wines originating from the regions with warm climates. In the latter ones, flavanols, including (+)-catechin and (–)-epicatechin, are thought to be a predominant group of flavonoids.[Citation5,Citation13,Citation17] A similar level of quercetin was observed in Sicilian red wines,[Citation23] as compared to that in this study. Kaempferol was identified in all the red wines under study () with R-6 (Rondo) being the richest in its content, while it occurred only in one white wine sample (W-2) (Adalmiina). The level of kaempferol in Polish wines studied was similar to that found in Croatian red wines, originating from Istria as well as from Central and Southern Dalmatia.[Citation7]
TABLE 5 Flavonoids content in the wines
With respect to flavanols, (+)-catechin was identified in all the red wine samples, whereas, (–)-epicatechin was found only in two red wines: Frontenac (R-2) and Rondo (R-6) (), both produced from the multispecies hybrid cultivars. A higher level of flavanols was observed in Italian wines,[Citation13] as well as in the wines originated from Languedoc,[Citation17] Greece,[Citation5] and from Sicilia[Citation23] when compared with the present results. However, level of (+)-catechin in Croatian red wines[Citation7] originating from regions with continental or Mediterranean climate was very close to these results. The fact that amount of flavanols in Polish wines under study was lower than in majority of those originating from regions of warm climates might be attributed not only to the climatic conditions during cultivation but to the vinification technique employed, in particular a maceration process. It should be taken into account that considerable changes in flavanols content that take place during enzymatic maceration.[Citation29] The monomeric flavanols (i.e., catechin and epicatechin) easily undergo oxidation and condensation in the presence of anthocyanins and proanthocyanidins, and thus decrease of their amount may be observed during the crushing of grapes, as well as before the beginning of the fermentation process.
Color Parameters
The color parameters (i.e., L*, a*, and b*) of the studied wines are summarized in . The white wines were similar to each other in terms of the color parameters, while the values of these parameters were strongly diversified among the red wines studied. All the red wines were characterized by the significantly lower values of parameter L*, reflecting lightness, in comparison to the white wines. Values of L* parameter of the red wines correlated negatively (r = –0.720) and significantly (α = 0.05) with the TPC values. These data would suggest that across all the red varieties, the level of TPC was also correlated with amount of anthocyanins, of which lower content was most likely contributed to the higher value of parameter L*. Out of the red wines, Rondo (R-6) was characterized by lowest value of L* parameter. The obtained results showed that this wine was found to be richest source of anthocyanins. Rondo (R-6) was also characterized by the highest value of TPC (). With respect to the single-variety red wines, Rondo (R-6) and Regent (R-7), both produced from the multispecies hybrid grapes were the most diversified in terms of all the determined color parameters (i.e., L*, a*, b*) (). The red wines under study showed positive values of a* parameter (degree of redness), and b* parameter (degree of yellowness). R-6 wine (Rondo) was characterized by the lowest values of both a* and b* parameters of all the red wines tested, while R-7 wine (Regent) was characterized by the highest values of these parameters. When it comes to the white wines, the values of L* parameter were more than 90 (CIELAB units), whereas, a* parameter assumed negative values, indicating larger proportion of green color. This finding was in agreement with what was reported[Citation30] for Spanish white wine. On the contrary, the last authors[Citation31] noted positive values of a* parameter when investigating white Amontilado wines, aged in oak barrels. In the present study, all the white wines assumed positive values of b* parameter, that was in accordance with previous literature data reported for the white wines.
TABLE 6 Color parameters of the wines
PCA
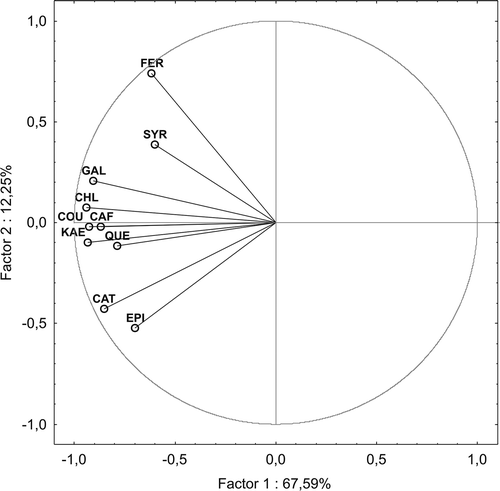
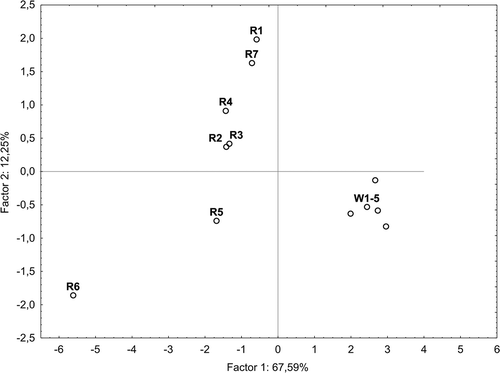
The analytical data of wines were processed statistically using a PCA in order to show grouping of the wines. The PCA explained 79.84% of total variation of the chemical variables, with 67.59 and 12.25% explained by the first and second factor, respectively. Most separations occur among the second factor (). The first factor is dependent on mostly chemical variables, in particular flavonols (i.e., quercetin and kaempferol), gallic acid, and hydroxycinnamic acids with the exception of ferulic acid. Grouping of the wines was performed visually using the PCA plot (),where the wines are represented by function of the first two principal components. As it can be seen (), white wines (W 1-5) produced from the both noble and multispecies hybrid grapevines are categorized as similar to each other in terms of the first and second factor. With respect to the red wines, the PCA strongly differentiated the single-variety wine (R-6) produced from the crossbreeding hybrid Rondo grapevine from the others in terms of the first factor. On the other hand, some grouping could be observed in the PCA plot in terms of the second factor. R-6 wine (Rondo) located in left lower part of the plot seems to be most similar to R-5 wine produced from the Vitis vinifera L. grapevines. These wines were also the richest in both phenolic acids and flavonoids content determined chromatographically, and strongly differed from the single-variety red wines such as Heridan (R-1) and Regent (R-7), located in the left upper part of the plot. The last wines turned out to be most similar to each other in terms of the first and second factor, and contained insignificant amounts of both phenolic acids and flavonoids. Additionally, some similarities among the multi-variety red wines (R 2-4) could be observed in terms of the first and second factor of analyzed parameters.
CONCLUSIONS
Analysis of the obtained results allowed stating that the level of phenolic content and AA of Polish wines produced from the noble varieties of grape (i.e., red and white Couveé vinifera) fell within the range observed in wines obtained from the multispecies hybrid cultivars. However, white Couveé vinifera (W-5) turned out to be richest in both phenolic acids and flavonoids content determined chromatographically, among all the white wines tested. With respect to the red wines tested, Couveé vinifera (R-5) and Rondo wine (R-6) were the richest source of both phenolic acids and flavonoids, whereas, the varieties such as Heridan and Regent were the poorest in this respect. The white wines under study seem to be the similar to each other in the terms of color parameters (i.e., L*, a*, and b*) and the results the PCA, whereas, the red wines strongly differed among each other in this respect. Rondo (R-6) wine, produced from the multispecies hybrid grapes was clearly differentiated from the other red wines in terms of first factor of the PCA plot. It was also the darkest, which indicated the highest content of anthocyanins. Rondo (R-6) might have the potential to be used in conditions of cool climates (e.g., in Polish wine industry), in order to enrich the phenolic content, AA and improve the color of other varieties. Therefore, this cold-hardy grape variety would be worthy of the further investigations, as well as to be used for the production of quality wines due to its better adaptability to cold climate in comparison to the noble cultivars (e.g., Vitis vinifera). Moreover, Rondo (R-6) wine seems to be most similar to red Couveé vinifera (R-5), produced from the Vitis vinifera L. species, in terms of content of phenolic compounds. The investigated Polish red wines were characterized by a significant lower value of both TPC and AA, as compared with that observed in majority of wines, originating from the regions with warm climates. On the contrary, some Croatian red wines originating from specific regions, in particular those with continental climates, exhibited a great similarity in TPC, as well as in level of (+)-catechin, caffeic and gallic acid with Polish wines studied. The hydroxycinnamic acids, in particular caffeic acid, as well as quercetin, turned out to be the predominant phenolics in majority of wines under study. This fact differentiates investigated Polish wines from those originating from the warmer climate regions.
REFERENCES
- Alleweldt, G.; Possingham, J.V. Progress in grapevine breeding. Theoretical and Applied Genetics 1988, 75, 669–673.
- Martí, N.; Lizama, V.; Verdú, J.A.; Muñoz. N.; Aleixandre, J.L.; Saura, D. Prediction of phenolic composition of Monastrell and Tempranillo wines: Correlation between phenolic content and traditional variables of fruit maturity. International Journal of Food Properties DOI:10.1080/10942912.2011.570467.
- Boselli, E.; Minardi, M.; Giomo, A.; Frega, N.G. Phenolic composition and quality of white d.o.c. wines from Marche (Italy). Analytica Chimica Acta 2006, 563, 93–100.
- Tinttunen, S.; Lehtonen, P. Distinguishing organic wines from normal wines on the basis of concentrations of phenolic compounds and spectral data. European Food Research and Technology 2001, 212, 390–394.
- Kallithraka, S.; Tsoutsouras, E.; Tzourou, E.; Lanaridis, P. Principal phenolic compounds in Greek red wines. Food Chemistry 2006, 99, 784–793.
- Kallithraka, S.; Kefalas, P.; Arvanitoyannis, I.; El-Zajouli, E.; Soufleros, E.; Psarra, E. Instrumental and sensory analysis of Greek wines: Implementation of principal component analysis (PCA) for classification according to geographical origin. Food Chemistry 2001, 73, 501–514.
- Rastija, V.; Srečnik, G.; Medić-Šarić, M. Polyphenolic composition of Croatian wines with different geographical origins. Food Chemistry 2009, 115, 54–60.
- Waterhouse, A.L. The phenolic wine antioxidants. In: Handbook of Antioxidants; Cadenas, E.; Packer, L.; Eds.; Marcel Dekker, Inc.: New York, 2002; 401–416.
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. Free radical scavenging capacity of selected red, rosé, and white wines. Journal of the Science of Food and Agriculture 1999, 79, 1301–1304.
- Baltrušaitytė, V.; Venskutonis, P.R.; Čekserytė, V. Radical scavenging activity of different floral origin honey and beebread phenolic extracts. Food Chemistry 2007, 101, 502–514.
- Woodring, P.J.; Edwards, P.A.; Chisholm, M.G. HPLC determination of non-flavonoid phenols in vidal blac wine using electrochemical detection. Journal of Agricultural and Food Chemistry 1990, 38, 729–732.
- Socha, R.; Juszczak, L.; Pietrzyk, S; Fortuna, T. Antioxidant activity and phenolic composition of herbhoneys. Food Chemistry 2009, 113, 568–574.
- Minussi, R.C.; Rossi, M.; Bologna, L.; Cordi, L.; Rotilio, D.; Pastore, M.G.; Durán, N. Phenolic compounds and total antioxidant potential of commercial wines. Food Chemistry 2003, 82, 409–416.
- Soleas, G.J.; Goldberg, D.M. Analysis of antioxidant wine polyphenols by gas chromatography-mass spectrometry. Methods in Enzymology 1999, 299, 137–151.
- Paixão, N.; Perestrelo, R.; Marques, J.C.; Câmara, J.S. Relationship between antioxidant capacity and total phenolic content of red, rosé, and white wines. Food Chemistry 2007, 105, 207–214.
- Staško, A.; Brezová, V.; Mazúr, M.; Čertík, M.; Kaliňak, M.; Gescheidt, G. A comparative study on the antioxidant properties of Slovakian and Austrian wines. Food Science and Technology 2008, 41, 2126–2135.
- Teissedre, P.; Landrault, N. Wine phenolics: Contribution to dietary intake and bioavailability. Food Research International 2000, 33, 461–467.
- Frankel, E.N.; Waterhouse, A.L.; Teissedre, P.L. Principal phenolic phytochemicals in selected California wines and their antioxidant activity in inhibiting oxidation of human low-density lipoproteins. Journal of Agricultural and Food Chemistry 1995, 43, 890–894.
- Li, H.; Wang, X.; Li, Y.; Li, P.; Wang, H. Polyphenolic compounds and antioxidant properties of selected China wines. Food Chemistry 2009, 112, 454–460.
- Fernandéz-Pachón, M.S.; Villanõ, D.; García-Parrilla, M.C.; Troncoso, A.M. Antioxidant activity of wines and relation with their polyphenolic composition. Analytica Chimica Acta 2004, 513, 113–118.
- Radovanovic, B.; Radovanovic, A; Tomic, V. Relations between the phenolic composition and free radical scavenging, and antibacterial activities of red wines from different cultivars of Vitis vinifera L. International Journal of Food Properties 2012, 15, 725–735.
- Rivero-Pérez, M.D.; Mũniz, P.; Gonzáles-Sanjosé, M.L. Antioxidant profile of red wines evaluated by total antioxidant capacity, scavenger activity, and biomarkers of oxidative stress methodologies. Journal of Agricultural and Food Chemistry 2007, 55, 5476–5483.
- La Torre, G.L.; Saitta, M.; Vilasi, F.; Pellicanò, T.; Dugo, G. Direct determination of phenolic compounds in Sicilian wines by liquid chromatography with PDA and MS detection. Food Chemistry 2006, 94, 640–650.
- Herjavec, S.; Jeromel, A.; Da Silva, A.; Orlic, S.; Redzepovic. S. The quality of white wines fermented in Croatian oak barrels. Food Chemistry 2007, 100, 124–128.
- Fernández-Pachón, M.S.; Villãno, D.; Trousoco, A.M.; García-Parrilla, M.C. Determination of the phenolic composition of sherry and table white wines by liquid chromatography and their relation with antioxidant activity. Analytica Chimica Acta 2006, 563, 101–108.
- Wororatphoka, J.; Intarapichet, K.; Indrapichate, K. Phenolic compounds and antioxidative properties of selected wines from the northeast of Thailand. Food Chemistry 2007, 104, 1485–1490.
- Alén-Riuz, F.; García-Falcón, M.C.; Pérez-Lamela, M.C.; Martínez-Carballo, E.; Simal-Gándara, J. Influence of major polyphenols on antioxidant activity in Mencía and Brancellao red wines. Food Chemistry 2009, 113, 53–60.
- Peña-Neira, A.; Hernández, T.; García-Vallejo, C.; Estrella, I.; Suarez, J.A. A survey of phenolic compounds in Spanish wines of different geographical origin. Eur. Food Res. Technol. 2000, 210, 445–448.
- Czyżowska, A.; Pogorzelski, E. Changes to polyphenols in the process of production of must and wines from blackcurrants and cherries. Part II. Anthocyanins and flavanols. Eur. Food Res. Technol. 2004, 218, 355–359.
- Recamales, Á.F.; Sayago, A.; González-Miret, M.L.; Hernanz, D. The effect of time and storage conditions on the phenolic composition and colour of white wine. Food Res. Int. 2006, 39, 220–229.
- Recamales, Á.F.; Hernanz, D.; Álvarez, C.; González-Miret, M.L.; Heredia, F.J. Colour of Amontillado wines aged in two oak barrels types. Eur. Food Res. Techol. 2007, 224, 321–327.