Abstract
The effect of the physicochemical characteristics on the moisture diffusion of tobacco was studied. In this research, the physical structure (specific surface area and pore volume) and major chemical components (total nitrogen, protein, pectin, starch, water-soluble sugar, and total sugar) of tobacco were determined. The effective diffusion coefficient of tobacco ranged from 0.5 × 10−14 to 6.5 × 10−14 m2s−1 depending on environment humidity (RH = 33, 43, 75, 83%) at 30°C. This coefficient was significantly affected by the pectin, total sugar, and water-soluble sugar content and specific pore volume of tobacco during desorption.
INTRODUCTION
The moisture content of tobacco can influence its processing characteristics, and “dry” cigarettes can yield harsher and more irritating smoke.[Citation1] Therefore, the water-holding capacity of tobacco leaves is a key factor in cigarette quality. Differences in hygroscopicity may be observed not only between tobacco varieties but also within different grades of the same variety.[Citation2] From the physical structure point of view, tobacco is a porous material, and in terms of chemical composition, it contains some hydrophilic colloid materials and water-soluble crystals, such as sugar, pectin, and organic salts.[Citation3,Citation4] Specific surface area and pore volume are important physical characteristics of tobacco.[Citation4,Citation5] However, most research has emphasized only the effect of curing methods or pretreatments on the porous structure. Little work has examined the relationship between the physical structure of tobacco and its water-holding capacity. In addition, water-holding capacity is dependent on water-soluble substances. Total sugar content significantly influences the water sorption of tobacco. Mutasa et al.[Citation2] suggested that high-sugar tobacco has higher moisture content than low-sugar tobacco at any given water activity. The ratio of fructose as well as glucose, phenylacetic acid, and some aromatic components (e.g., 2-methyl propanoic acid and 2-pyridine formaldehyde) could enhance the water-holding capacity of tobacco.[Citation6,Citation7] However, the influence of other hydrophilic colloid materials, such as pectin and starch, has seldom been investigated.
The moisture content of tobacco is determined not only by its physical structure and chemical components but also by environmental factors.[Citation2,Citation8] Environmental conditions affect the moisture content of tobacco because of moisture sorption or desorption. The exchange of moisture between tobacco and its environment is a response to the difference in vapor pressure between the interior of the tobacco and the environment, with moisture diffusing to the direction of the lower vapor pressure. Therefore, the moisture diffusion is greatly affected by the environment and material characteristics.
To control the moisture content of tobacco, research has focused on the mechanism of moisture transfer in tobacco. Aside from the focus on equilibrium conditions, interest has grown in describing the diffusivity of moisture. A number of studies have determined the moisture diffusivity of foodstuffs, such as greenbeans, black tea, garlic slices, and pears, by using a diffusive model based on Fick’s second law.[Citation9–Citation12] The model considers only the internal resistance to moisture immigration. The diffusion models could also accurately describe the drying of the lamina, midrib, and whole leaves of the burley tobacco.[Citation13,Citation14] Therefore, this work aimed to evaluate the effective diffusion coefficient of tobacco of different water-holding capacities by using the model based on Fick’s second law during desorption and adsorption. The effects of physicochemical characteristics on the moisture diffusion capacity during desorption were also discussed.
MATERIALS AND METHODS
Raw Material
Nine tobacco samples (one air-cured and eight flue-cured tobacco) of different water-holding capacities were obtained from Anhui Cigarette Manufactory (China). These samples are listed in . In the following discussion, B, C, and X represent the upper, middle, and lower locations of tobacco leaves on the plant. The expression “location–leaf position” was used to represent each type of tobacco. For example, Yuxi-C is the middle-part leaves of tobacco grown in Yuxi (Yunnan Province, China). Air-cured and flue-cured tobaccos are generally defined by the curing methods applied to them. All reagents were of analytical grade.
TABLE 1 Varieties of tobacco used in experiment
Physicochemical Analysis
Physical Analysis
In the experiment, the specific surface area and pore volume of the samples were determined through Autosorb-1 (Quantachrome, America). All the samples were ground, and a fraction of 40–60 mesh was used in the physical analysis. The samples were outgassed at 70°C for a minimum of 6 h. The weight of the samples was about 2 g. Helium was used to determine dead space. The specific surface areas of the tobacco samples were calculated by the Brunauer-Emmett-Teller method[Citation15] based on the sorption isotherm of nitrogen.
Chemical Analysis
All the samples were ground, and a fraction of 80 mesh was used in the chemical analysis. Moisture content was determined by the oven method in percent dry basis (d.b.) according to YC/T 31-1996 (Tobacco Industry Standard, China). The samples were dried at 100°C for 2 h. Protein and total nitrogen content was determined by continuous flow analysis on Auto Analyzer 3 (SEAL Analytical GmbH, Germany) according to the methods of YC/T 161-2002 and 249-2008. Total sugar, water-soluble sugar, starch, and pectin content was determined through the salicylic acid method.[Citation16,Citation17]
Sorption Behavior of Tobacco
All the experiments were performed at 30°C in a temperature-controlled cabinet with an accuracy of ± 1°C. The width of cut tobacco samples was 0.8 to 1.0 mm. The moisture migration kinetic was determined by placing the samples in an atmosphere produced from a saturated salt solution with known aw.[Citation18] The relative humidity (RH) was 33 (magnesium chloride), 43 (potassium carbonate), 52 (magnesium nitrate), 75 (sodium chloride), and 83% (potassium chloride). The samples were preconditioned in 52% RH for 2 days to adjust moisture content to about 12% (wet basis), which is the optimum moisture content for tobacco storage. The samples were placed in a culture dish. Thereafter, the dish containing the samples and the salt solution were maintained separately within a desiccator. The moisture content of the samples was then determined every day through the oven method (YC/T 31-1996). All measurements were done in triplicate.
RESULTS AND DISCUSSION
Equilibrium Moisture Content (EMC) of Tobacco in Different Environments
EMC is the point at which the material is neither gaining nor losing moisture, and changes with RH and temperature of the environment. EMC is widely used in the tobacco industry to represent water retention capacity. EMC was determined at 30°C in a controlled atmosphere within a desiccator. The results are listed in . In all tobacco varieties, EMC decreased with decreasing RH. Different water-holding capacities of tobacco could be observed within different varieties. Compared with flue-cured tobacco, air-cured tobacco (Jianshi-C) had the lowest EMC at low RH but moderate EMC at 83% RH. Among the varieties of flue-cured tobacco, Yun85 (grown in Yunnan Province) had higher EMC than K326 at any RH value.
TABLE 2 Equilibrium moisture content in different ambient environment at 30°Ca
Different grades of tobacco could also result in different hygroscopicity. The upper and middle leaves of Yun85 (YuXi-C and Chuxiong-B) exhibited stronger hygroscopicity at low RH. At 33% RH, the EMC of YuXi-C and Chuxiong-B were approximately 0.6 and 1.2% higher than that of lower leaves, respectively. In damp environments, the middle leaves exhibited the strongest moisture adsorption capacity, and EMC was approximately 3.5% higher than that of upper leaves (Chuxiong-B) and 2.7% higher than that of lower leaves (YuXi-X) at 83% RH. Thus, the water-holding capacity of lower leaves was lower than that of the other two leaf types with higher RH. A similar result could be observed in K326. The upper leaves (Bozhou-B and Chenzhou-B) had higher EMC than other samples at low RH. The upper leaves (Bozhou-B) exhibited the strongest moisture adsorption capacity at high RH. The differences in hygroscopicity and moisture retention capacity may have been caused by the chemical composition and physical structure of the leaves, which are discussed below.
Determination of Effective Moisture Diffusivity of Tobacco According to Diffusion Model
EMC represented the equilibrium state of the tobacco with its environment, rather than the moisture transfer mechanism and moisture diffusivity. When the vapor pressure of moisture within a material and the surrounding air are unequal, moisture exchange may be either a drying or wetting process. Moisture migration in tobacco includes a number of transport phenomena, such as Knudsen flow, liquid diffusion, and vapor diffusion, all of which may be present to some extent during the process. With a lumped parameter model concept, all the different types of diffusion are combined in one parameter named as effective moisture diffusivity (Deff). To obtain the values of Deff, a diffusion model derived from Fick’s second law was used.[Citation9–Citation12] It is noteworthy, that the moisture contents used in the diffusion model were in d.b.
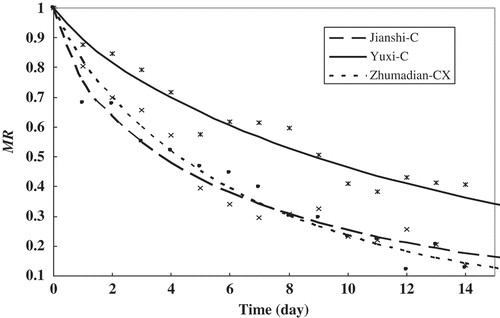
The moisture ratio (MR)[Citation10] is an important parameter involved in the diffusion model, which represents the unaccomplished moisture content under certain condition. Jianshi-C, YuXi-C, and Zhumadian-CX were selected as the representative tobacco samples. MR of the three types of tobacco at 33% RH was plotted as a function of drying time. As shown in , the MR of all tobacco decreased continuously with increasing drying time, and the falling rate of MR decreased with increasing water retention capacity. The sample with higher EMC (YuXi-C) exhibited a higher MR at the same drying duration.
The absence of a period of constant MR indicates that the strongest force governing moisture movement in the tobacco was internal diffusion. Similar results have been obtained from different foodstuff.[Citation19] Therefore, effective moisture diffusivity could be an important parameter in the rate of moisture transfer in tobacco. Experimental data on different kinds of tobacco at different RH (33, 43, 75, and 83%; 30°C) were used in the diffusion model. The results are shown in . Deff varied from 0.5 × 10−14 m2s−1 to 6.5 × 10−14 m2s−1 depending on the type of tobacco and on environment humidity. The results are consisted with Walton et al., who observed that Deff varied from 0.7 × 10−15 m2s−1 to 5.2 × 10−14 m2s−1 during the desorption and adsorption in the tobacco.[Citation14] Samejima and Yano[Citation20] reported that Deff in cured tobacco leaves ranged from 8.80 × 10−11 m2s−1 to 64.2 × 10−11 m2s−1 depending on the type of tobacco and on sorption temperature. Compared with previously reported Deff values for fruit with high water content (3.72 × 10−9 m2s−1 to 12.27 × 10−9 m2s−1 for tomato)[Citation19] and for black tea particles dried at 80 to 120°C (1.141 × 10−11 m2s−1 to 2.985 × 10−11 m2s−1),[Citation10] the water diffusivity of tobacco obtained was low, probably because of its low water content and high hygroscopicity. Another reason may be the external environmental conditions (e.g., air temperature, humidity, and velocity), which could strongly affect Deff.
TABLE 3 Physicochemical characteristics and effective diffusion coefficient at different humidity of tobacco samples
Preconditioned in 52% RH, the tobacco samples would lose moisture in drier environment (33 and 43% RH) and adsorb moisture in more humid environment (75 and 83% RH). During desorption, Deff at 43% RH was slightly higher than at 33%. During moisture adsorption, Deff at 75% RH was higher than at 83%. Adsorption water diffusivity reaches its maximum with intermediate moisture content,[Citation21] possibly because of changes between vapor and liquid diffusion in the diffusion mechanism. As to the variation of Deff with different storage conditions, Deff during desorption was higher than during adsorption, which indicates that tobacco leaves dried considerably faster than they adsorbed moisture. This characteristic may be attributed to the swelling of tobacco during adsorption and its shrinkage during desorption. Swelling requires energy, whereas shrinkage involves a release of energy. Thus, more energy is required for a given moisture content during adsorption than during desorption.[Citation14]
In all tobacco varieties, the Deff of air-cured tobacco (Jianshi-C), which had the lowest EMC at low RH, reached 4 × 10−14m2s−1 during desorption (33% RH). This value is higher than that of flue-cured tobacco. However, consistent with the trend of EMC, the Deff of air-cured tobacco (Jianshi-C) moderated during adsorption. Among the varieties of flue-cured tobacco, the Deff of Yun85, which had higher EMC than K326 at any RH, was lower than that of K326 at low RH but higher at damp environment (83% RH). The lower leaves of both Yun85 and K326 exhibited higher Deff and weaker hygroscopicity during drying, consistent with the above EMC results.
Effect of Physicochemical Characteristics on Effective Moisture Diffusivity
Analysis of Physicochemical Characteristics of Tobacco
Moisture diffusion might include liquid diffusion through the solid pores and vapor diffusion. Moisture diffusivity in materials can significantly be affected by the physical structure of the material, particularly its bulk porosity.[Citation22] Large specific surface area and pore volume may benefit moisture diffusion. Therefore, the specific surface areas and pore volume of the samples were analyzed, the results of which are shown in . The specific surface areas and pore volume of the microspores obtained in this article ranged from 0.1 m2g−1 to 0.3 m2g−1and from 5 × 10−10 m3g−1 to 1.4 × 10−9 m3g−1. The values are in the same range with those reported by Chang and Johnson[Citation23] for tobacco (from 0.19 m2g−1 to 1.70 m2g−1 and from 1.2 × 10−9 m3g−1 to 3.6 × 10−9 m3g−1). Compared with flue-cured tobacco, air-cured tobacco (Jianshi-C), which exhibited the poorest hygroscopicity during desorption, had higher specific surface areas and pore volume. In flue-cured tobaccos, the lower leaves (YuXi-X, Zhumadian-CX, and BoZhou-X), which had weaker hygroscopicity, exhibited higher specific surface areas and pore volume (up to 0.22 m2g−1 and 7.5 × 10−10 m3g−1, respectively) than the leaves grown in other positions.
The sorption capacity of food increased with increasing polar and ionic group content. Protein and some carbohydrates (e.g., sugar and pectin) are the major components of tobacco. Protein with low ionic functional group content is rich in non-polar amino acids. These hydrophobic groups have very weak interactions with water, thereby resulting in lower water-binding capacity.[Citation24] By contrast, hydrophilic carbohydrates result in stronger interactions with water. Thus, the major chemical components in tobacco, including total nitrogen, protein, water-soluble sugar, total sugar, starch, and pectin, were determined in this study.
As shown in , the chemical composition of air-cured tobacco (Jianshi-C) was very different from that of flue-cured tobacco. Air-cured tobacco had higher total nitrogen and protein content, about 2 and 1.5 times, respectively, that of flue-cured tobacco. However, air-cured tobacco exhibited the lowest hydrophilic carbohydrate content. In all types of flue-cured tobacco, the total nitrogen and protein content varied from 1.2 to 1.9% and 3.0 to 4.6%, respectively. The content of these two chemical components had little difference between the varieties of flue-cured tobacco. The water-soluble sugar and total sugar content of Yun85, which had better hygroscopicity, was significantly higher than that of the other kinds of tobacco. The great differences between the physicochemical characteristics could be the main reason for the different effective diffusion coefficients.
Correlation Analysis Between the Physicochemical Characteristics and the Effective Diffusion Coefficient
Simple correlation analysis is a statistical method used to describe the relationship between two variables. Such an analysis was conducted to evaluate the relationship between the effective diffusion coefficient and the physicochemical characteristics. Considering that there was more interest in the moisture loss, the effect of physicochemical characteristics on the effective diffusion coefficient at 33% RH was analyzed. The result is shown in .
TABLE 4 Statistical results of correlation analysis between effective diffusion coefficient and physicochemical characteristics
Samejima et al.[Citation20] reported that the difference in the moisture diffusion coefficient between air-cured and flue-cured tobacco reflects the difference in porosity. The simple correlation analysis results show that the effective diffusion coefficient was significantly influenced by specific pore volume. Large specific pore volume favored moisture diffusion. With constant drying air temperature, the effective diffusivity coefficient depends on the variety and composition of the material.[Citation10] A remarkable negative correlation was found between the effective diffusion coefficient and the pectin, water-soluble sugar, and total sugar content (p < 0.05). Guiral et al.[Citation25] studied the effect of sucrose, pectin, and maltodextrin on the moisture diffusivity during drying of pineapple and found that the effective moisture diffusivity was reduced by the addition of sucrose, pectin, and maltodextin. This finding is also consistent with Saravacos and Maroulls,[Citation26] who observed that sugars such as glucose and sucrose could decrease effective moisture diffusivity in starch materials.
CONCLUSION
In this research, the physical structure (specific surface area and pore volume) and chemical components (total nitrogen, protein, pectin, starch, water-soluble sugar, and total sugar) of tobacco were determined. Air-cured tobacco had higher specific surface area, specific pore volume, total nitrogen, and protein content than flue-cured tobacco but had lower water-soluble sugar, total sugar, starch, and pectin content. The diffusion model based on Fick’s second law was used to describe water migration. The effective diffusion coefficient could be another useful representation of water-holding capacity except for EMC. The effective diffusion coefficient of tobacco ranged from 0.5 × 10−14 m2s−1 to 6.5 × 10−14 m2s−1 at 30°C. This coefficient was significantly affected by the pectin, total sugar, and water-soluble sugar content and specific pore volume of tobacco at desorption. Modeling the entire moisture transfer process facilitates the understanding of the water transfer mechanism. Information on the relationship between the physicochemical characteristics and moisture diffusion of tobacco can facilitate the control of the moisture content of tobacco.
ACKNOWLEDGMENT
The authors thank R&D Center of China Tobacco Anhui Industrial Corporation.
FUNDING
This work was supported by the Scientific Foundation of China National Tobacco Corporation (No. 110200901002).
Additional information
Funding
REFERENCES
- Bevan, P.C. A survey of the water activity-moisture content relationships of tobaccos and reconstituted. B.A.T (U.K. & Export) Ltd., Research & Development Center, Southampton. 1988.
- Mutasa, E.S.; Seal, K.J.; Maga, N. The water content/water activity relationship of cured tobacco and water relations of associated spoilage fungi. International Biodeterioration 1990, 26, 381–396.
- Samejima, T.; Nishikata, Y.; Yano, T. Moisture permeability of cured tobacco epidermis. Agricultural and Biological Chemistry 1984, 48, 439–443.
- Warner, B.R. Surface areas of tobaccos by low temperature nitrogen adsorption. Archives of Biochemistry and Biophysics 1952, 40, 143–152.
- Samejima, T.; Soh, Y.; Yano, T. Specific surface area and specific pore volume distribution of tobacco. Agricultural and Biological Chemistry 1977, 41, 983–988.
- Xu, A.; Wang, C. Study on the relationship between the organic acids/water-soluble sugar and the tobacco’s water-holding capacity. China Tobacco Independent Innovation Forum 2007, 59–69.
- Xu, A.; Wang, C.; Hu, W.; Deng, J. Study on the relationship between the Hongda tobacco’s water-holding capacity and the important aroma content. Yunnan Tobacco 2006, 6, 21–27.
- Ma, L.; Zhou, B. The capacity to absorb and retain moisture of the tobacco itself. Guangdong Chemical Industry 2009, 36, 93–94.
- Doymaz, İ. Drying behaviour of greenbeans. Journal of Food Engineering 2005, 69, 161–165.
- Panchariya, P.C.; Popovic, D.; Sharma, A.L. Thin-layer modelling of black tea drying process. Journal of Food Engineering 2002, 52, 349–357.
- Sacilik, K.; Unal, G. Dehydration characteristics of Kastamonu garlic slices. Biosystems Engineering 2005, 92, 207–215.
- Guine, R.P.F.; Barroca, M.J.; Silva, V. Mass transfer properties of pears for different drying methods. International Journal of Food Properties 2013, 16, 251–262.
- Walton, L.R.; Casada, M.E.; Henson, W.H. Diffusion of moisture from burley tobacco leaves during curing. Transactions of the ASABE 1982, 25, 1099–1102.
- Walton, L.R.; Henry, Z.A.; Henson, W.H. Moisture diffusion in the cured burley tobacco leaf. Transactions of the ASABE 1976, 19, 796–800.
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. Journal of the American Chemical Society 1938, 60 (2), 309–319.
- Ma, D.; Liu, W.; Wei, Q.; Liu, G.; Yang, T. Determination of pectin in tobacco with 3,5-Dinitrosalicylic acid colorimetry. Tobacco Science Technology 2006, 38–41.
- Yin, J.; Lu, H.; Xie, Q.; Ding, J.; Li, N. A study on rapid colorimetric determination of water soluble total sugar, reducing sugar and starch in tobacco with 3,5-dinitrosalicylic acid. Journal of Yunnan Agricultural University 2007, 22, 829–837.
- Wang, C.; Zhang, X.; Dai, Z.; Wu, G. Selection of fitting models on adsorption and desorption isotherms of chrysanthemum. Journal of YANGTZE University (Natural Science Edition) 2008, 5, 24–26, 38.
- Akanbi, C.T.; Adeyemi, R.S.; Ojo, A. Drying characteristics and sorption isotherm of tomato slices. Journal of Food Engineering 2006, 73, 157–163.
- Samejima, T.; Yano, T. Moisture diffusion within shredded tobacco leaves. Agricultural and Biological Chemistry 1985, 49, 1809–1812.
- Yu, X.; Schmidt, A.R.; Bello-Perez, L.A.; Schmidt, S.J. Determination of the bulk moisture diffusion coefficient for corn starch using an automated water sorption instrument. Journal of Agricultural and Food Chemistry 2007, 56, 50–58.
- Fernando, W.J.N.; Low, H.C.; Ahmad, A.L. Dependence of the effective diffusion coefficient of moisture with thickness and temperature in convective drying of sliced materials. A study on slices of banana, cassava, and pumpkin. Journal of Food Engineering 2011, 102, 310–316.
- Chang, C.S.; Johnson, W.H. The relationship of specific surface area and specific pore volume of the tobacco. Tobacco Science 1973, 11, 115–119.
- Xiong, X.; Narsimhan, G.; Okos, M.R. Effect of composition and pore structure on binding energy and effective diffusivity of moisture in porous food. Journal of Food Engineering 1992, 15, 187–208.
- Gujral, H.S.; Oberoi, D.P.S.; Singh, R.; Gera, M. Moisture diffusivity during drying of pineapple and mango leather as affected by sucrose, pectin, and maltodextrin. International Journal of Food Properties 2013, 16 (2), 359–368.
- Saravacos, G.D.; Maroulls, Z.B. Transport Properties of Foods, Marcel Dekker, Inc.: New York, 2001.