Abstract
The textural characteristics of gels formed with green gram (Vigna radiata L.) flour at different concentrations (8–14 g/100 g, dry solid basis) along with selected cations were studied. Gels were prepared at different concentrations of monovalent (NaCl) and divalent (CaCl2 and FeSO4) cations. A minimum concentration of 10 g/100 g green gram was needed to obtain a well set gel having adequate integrity, and textural (fracture strain 42–43%) and sensory characteristics (categorized as best gel by panel). The gel forming ability improved with an increase in concentrations of CaCl2 and FeSO4, while a high level (2 g/100 g) of NaCl was required for a well set gel having a fracture strain of about 43%. Acceptable gel with the desirable sensory attributes could be achieved with green gram at a concentration of 10 to 11 g/100 g. The presence of 0.5 g/100 g of CaCl2 or 0.2 g/100 g of FeSO4 could offer a gel with a green gram solid concentration of 9 g/100 g. Protein digestibility of the raw sample (55 g/100 g) improved to 89 g/100 g on gelling.
INTRODUCTION
Green gram (Vigna radiata L.; GG) is one of the commonly used pulses for the production of soups, weaning foods, and extruded snacks in different parts of Asia.[Citation1].It is also being cultivated in other continents like North America, Africa, and Australia. Several traditional foods like cooked dhal (split pulse), sweet and savory snacks, and cooked thick batter comprising GG and rice are also manufactured.[Citation2] The high level of protein[Citation3] and easy digesting characteristics of GG make it popular in several countries particularly for infants and kids.[Citation4]
Functional properties of food components are the properties which can improve the desirable sensory characteristics by changing the rheological behavior of the product. The textural properties of a food refer to the physical characteristics that arises from the structural elements of the food which are sensed by the feeling of touch, are related to the deformation, disintegration, and flow of the food under a force.[Citation5] These properties are measured objectively to correlate sensory assessment in relation to developing new products and for quality control of routine manufacture of products.
Food gels are typical viscoelastic materials containing high level of moisture. Gels maintain their integrity and shape, and provide convenience to consumers. Hydrocolloid based gels are usually characterized by their rheological status. Structural property affects the hardness, elasticity, grittiness, cohesion, and chewiness of the formed gels. The process of gel formation primarily depends on the gel forming ingredients like starch, protein, hydrocolloid, temperature history of sol, pH, and presence of ions and/or co-solutes like sugar.[Citation6,Citation7] For example, for a starch gel, the configuration of the swollen starch granule during heat treatment, the amounts and types of amylose and amylopectin leached out from the granule, the interaction among amylose, amylopectin and the granule, and different heating conditions such as temperature, heating period, and rate, etc. are the important factors for gelling.[Citation8] In several cases, the presence of monovalent and divalent ions affects the formation of gels and attributes to the formed gel.[Citation9,Citation10]
Protein based gels have been studied extensively. The common raw materials used are soy protein,[Citation11] whey protein,[Citation12] etc. However, gels made from other pulses and oilseeds are limited possibly due to lack of commercial demand. Pea protein, in the presence of heat and carrageenan, has been reported to form gels.[Citation13,Citation14] Further, the present authors have not come across any research literature on the gelation of GG though scope exists to develop such a product as a nutritious convenience food.
Application of pulse flour dispersions in gel formation to formulate a variety of confectionery products and new products appears interesting. Textural studies of selected pulse flour based gels with different binding agents and cations will be useful in developing specialty gels to be used as a ready-to-eat food. Thus, the objective of the present work is to study the gel forming ability and characteristics of the gel formed using GG flour at different concentrations along with the effect of different concentrations of selected cations like NaCl, CaCl2, and FeSO4.
MATERIALS AND METHODS
Materials
GG splits (dehusked split halves) were procured from the local supermarket of Mysore, India. They were cleaned and ground in a laboratory model pulverizer. The material was initially cooled to about 10°C such that the grinding process was conducted below 40°C to avoid any thermal changes. The ground powder obtained was passed through a 150 μm aperture British Standard (BS) sieve, and was used for further studies. All solvents/chemicals used were of analytical grade and were obtained from Merck, Mumbai, India.
Physicochemical Properties
The proximate composition of raw GG flour and freeze dried gel (containing 10 g/100 g solids, 5.5% moisture content) was determined following AOAC[Citation15] methods. Trypsin inhibitor activity (TIA) was estimated using the procedure mentioned by.[Citation16] In-vitro protein and carbohydrate digestibility,[Citation17] and water holding capacity (WHC)[Citation18] of GG flour were determined. The results for WHC were expressed as milliliter of water absorbed per gram of dry flour. All determinations were replicated three times.
Preparation of Gel
GG flour based gels at different concentrations of 8, 9, 10, 11, 12, and 14 g/100 g (dry solid basis) were prepared. Different cations such as NaCl (0.5, 1, and 2 g/100 g), CaCl2 (0.1, 0.3, and 0.5 g/100 g), FeSO4 (0.1, 0.2, and 0.3 g/100 g) were used during the preparation of gel having GG concentrations of 8, 9, and 10 g/100 g. Initially, GG flour was hydrated with the required quantity of distilled water for 30 min at room temperature (about 25°C) with constant stirring. Later, it was heated at 90°C for 20 min in a water bath in covered condition followed by cooling for 1 min and then transferred to petri plates (49 mm diameter, 10 mm depth) as detailed earlier.[Citation19] In a preliminary study, the set gels were examined for compression characteristics (discussed in the subsequent section) at an interval of 30 min to understand the gel maturity. It was observed that the instrumental textural values do not change significantly after 4 h. Hence, the 4 h set gels were subjected to compression by employing a texture measuring instrument (Model #TAHD, Texture Analyzer, Stable Microsystems, Surrey, UK) at a crosshead speed of 1 mms−Citation1 using a 100 mm diameter flat compression surface. The maximum strain employed was 0.80 and measurements were performed on 10 samples. The parameters, calculated from the force-deformation curves, were peak stress, energy for compression, firmness, and fracture strain. The maximum, or peak force, was divided by the initial cross-section of gels to obtain the peak stress. The energy for compression was the area under the curve till the strain of 0.80. Firmness was obtained from the slope of the initial linear portion of the force-deformation curve.[Citation20] Fracture strain was the ratio of the deformation at fracture and the initial height of the sample, and expressed as percent basis.
Sensory Assessment
The non-oral sensory assessment of gel samples were performed by 10 trained panelists by using their fingers to determine the sensory attributes such as time of gelling (1- most quickly forming, 9- most slowly forming), hardness (1- most soft, 9- extreme hard), stickiness (1- least sticky, 9- extremely sticky), cohesiveness (1- least cohesive, 9- extremely cohesive), springiness (1- no springiness, 9- extremely springy), and spreadability (1- most difficult to spread, 9- extremely easy to spread) based on the 9-point hedonic scale. Gel forming ability was the time taken to form the gel. The definitions of stickiness and cohesiveness were followed as suggested earlier.[Citation21] Ten judges from the Institute participated in the analysis who had previous experience in descriptive sensory analysis. Each panel members participated in one-hour training session for familiarization of the definitions and the standards used (). For evaluation, coded gel samples were served on a white porcelain dish. The desirable criteria for the gel sample were minimum stickiness and good integrity but soft enough to spread easily. The process of sensory assessment was replicated twice.
Statistical Analysis and Principal Component Analysis (PCA)
Multiple comparisons were made for all gel samples employing Duncan’s multiple range test (DMRT) at p ≤ 0.05 using the statistical software Statistica’99 (StatSoft, Tulsa, OK, USA). Data were analyzed using PCA to explore the underlying relationships between and among different parameters including objective and subjective data[Citation22] using the software Statistica (version 5.5, Stat Soft, Tulsa, USA). The PCA results were presented as biplots.
RESULTS AND DISCUSSION
Physicochemical Properties
Physicochemical properties () of the GG flour (raw and dried gels) showed a drastic reduction in TIA due to thermal treatment during gel preparation, and attained a desirable safe level of 2.2 TIU/mg. Trypsin inhibitor is known to bind with dietary protein and thus inhibits its digestion. Inhibition of TIA was accompanied by a concomitant increase in the nutritive value of protein.[Citation23]
TABLE 1 Method of Evaluation of Different Sensory Attributes
TABLE 2 Properties of Raw Flour and Dried Green Gram Gel Flour
The gelling process comprising the cooking of GG in water increased the starch digestibility from 44 g/100 g to 77 g/100 g due to heat-induced gelatinization. Protein digestibility of the raw sample (55 g/100 g) also improved to 89 g/100 g on cooking (); this result matched with earlier observations.[Citation24] Cooked GG flour showed higher WHC compared to raw flour (significant at p ≤ 0.01) which might be attributed to the gelatinization of starch granules that absorbed more water.
Instrumental Texture
The compression graph apparently possessed four zones, such as, (a) initial short linear zone when the sample deformed elastically, (b) sample exhibited irreversible non-linear deformation, (c) fracture occurred, and (d) post-fracture deformation when the sample offered high resistance toward compression (sample graph not included). However, the extent of these zones varied with GG concentration in addition to concentration of ions.
An increase in the concentration of GG flour increased peak stress () which represented the instrumental hardness, i.e., the highest resistance offered by sample per unit of area. The compression energy (the extent of energy needed to achieve the experimental deformation) and fracture strain (extent of deformation when a fracture appeared in the sample) also increased (). These results indicated that an enhanced level of solid increased the water binding capacity to form a solid-like gel that offered more resistance toward compression. Gels with 8 g/100 g GG solids were too weak samples to offer any fracture characteristics (). On the other hand, gels made out of 14 g/100 g solid were a sticky lumpy mass (not a gel) which exhibited progressive compression without slowing any fracture. The latter phenomenon might be attributed to an insufficient quantity of water to completely hydrate and cook the higher amount of GG solids. The hydration/cooking was decided by both starch and protein present in GG. Further, minor or major cracks were observed at the center of the gels during compression when solid concentrations of GG were 9–12 g/100 g. Presence of fractures indicated the formation of a well-set gel that showed brittleness.
TABLE 3 Instrumental Texture of Green Gram Gel with Cations
The presence of different binding agents like monovalent and divalent cations also showed a similar trend; addition of salt from sodium, calcium, and iron made stronger gels. This was due to ionic interactions which promoted protein-water interactions[Citation25] resulting in increased solubility, and more gelatinization of starch that favored the formation of stronger gels. Starch granules swelled during gelatinization when subjected to hydrothermal treatment. The amylose inside the granules leached out and formed a 3-dimensional network; the swollen granules were embedded in a continuous matrix.[Citation26] Starch granules actively participated in the creation of gel structure by removing water from the transient solution during swelling and acted as particulate fillers in the final gel. Gelation was obtained initially by water entrapment (hydration) and immobilization, and then formed the network when subjected to hydrothermal treatment. Cohesiveness or adhesion of the formed gel was obtained by hydrophobic, ionic, and hydrogen bonding in the dispersion.[Citation27]
The heated/cooked GG sol was difficult to pour into molds when the concentration of GG was more than 10 g/100 g. The formed gels were also hard. In presence of cations, this difficulty increased further. Hence, GG gels were prepared at concentrations of 8–10 g/100 g in the presence of ions (). In the present study, an increase in the concentration of ion has increased peak stress, compression energy, and fracture strain of GG gels (). Nickerson et al.[Citation28] found that the critical overlap concentration for gellan gum dispersions decreased with an increase in calcium ion concentration. A concentration up to 2 g/100 g NaCl hardly affected these parameters while CaCl2 at 0.3–0.5 g/100 g and FeSO4 at 0.2–0.3 g/100 g concentrations were effective (). An increase in the concentration of NaCl (upto 2 g/100 g) generally decreased peak stress and energy for compression (). The failure strain increased with concentration of GG solid and/or NaCl concentration. However, we did not use more than 2 g/100 g NaCl concentration as the gel became too salty for consumption.
The use of CaCl2 (up to 0.5 g/100 g) showed an increase in peak stress/energy for compression/fracture strain (). This means that CaCl2 offered a gel with a harder texture having more elastic behavior rather than a brittle characteristic. The effect of FeSO4 was similar to that of CaCl2 (). The highest level of fracture strain (75%) was obtained from 0.5 g/100 g CaCl2 gel compared to NaCl and FeSO4 samples; the fracture strain values were about 60% for these two latter gels. Hydrogen-bonding and disulphide cross-links in GG protein, and starch gelatinization helped the gelation of GG dispersions along with the addition of different ions.[Citation29]
Sensory Assessment
The non-oral sensory scores for time of gelling, hardness, stickiness, cohesiveness, springiness, and spreadability of GG gels are shown in . Time of gelling i.e., the time taken to form the gel decreased markedly with an increase in concentration of solid content of GG flour solids from 8 to 14 g/100 g. The GG dispersion containing 14 g/100 g solid took a minimum time of 10 min to set the gel. Hardness (representing resistance to compression) and cohesiveness of gel (an indication of the internal binding) increased as the solid content was enhanced. The GG gels at 10 and 11 g/100 g solid contents formed the most acceptable gel with appropriate hardness. The stickiness of the gels, an important quality attribute for consumers, reduced with an increase in concentration of GG flour. According to the sensory panelists, the 10 and 11 g/100 g samples formed the best gel with desirable good integrity, binding, and low stickiness characteristics. Above 11 g/100 g solid concentration, the formed gels were too hard which again had poor spreadability () even though stickiness was least in this sample. The monovalent cation like NaCl up to 1% concentration marginally improved gelling time and spreadability, but at 2 g/100 g NaCl concentration, there was a significant improvement in these two indices. The CaCl2 also showed a similar trend up to 0.3% concentration; above this level, there was a marked improvement in gel characteristics even with 9% GG solid content. Divalent cations improved binding property and thus enhanced structural integrity than that of monovalent ion like NaCl (). The addition of cations induced gelatinization of starch[Citation30] leading to an increase in cohesiveness and helped in uniform spreading. A gel with a stickiness score less than five and spreadabililty above eight was selected to obtain the required integrity and uniform spreading characteristics. Thus, a suitable GG gel was obtained with 9 g/100 g GG solids in the presence of 0.5 g/100 g CaCl2 or 0.2 g/100 g FeSO4.
Inter-Relationship
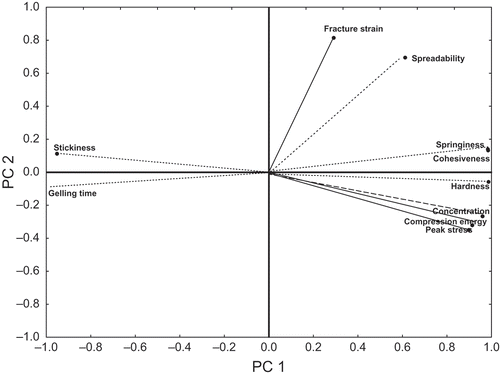
The PCA is a strong tool to determine the inter-relations among the indices (objective and subjective) and their behavior as an individual group/cluster. The PCA plot, in coded forms, for gels prepared without any added cations, is shown in (other PCA plots with cations not reported to avoid repetition). The principal component 1 (PC1) accounted for 78% while principal component 2 (PC2) showed 15% of the total variation. Hence, PC1 and PC2 could explain a total of 93% of the data. Thus, plotting of other principal components was avoided. The springiness and cohesiveness were close to each other in the first quadrant meaning that an increase in cohesiveness automatically increased springiness. Similarly, the instrumental fracture strain and sensory spreadability possessed a good inter-relation. Concentration of solids had a close relationship with hardness, compression energy, and peak stress because all of them lay in the same quadrant and were neighbors. The stickiness and gelling time were inter-related; these two parameters appeared to be inversely related with the cluster consisting of concentration of solids, compression energy, peak stress, hardness, cohesiveness, and springiness.
CONCLUSIONS
Gel formation using GG dispersion was possible and the quality characteristics could be improved further by using CaCl2 and FeSO4 to develop nutritious gels. The use of cation increased the peak stress, energy for compression, and fracture strain in addition to improving sensory attributes like hardness, cohesiveness, and gelling time. Acceptable GG gel (without added cations) was achieved with 10 or 11 g/100 g solid content; GG flour at 10 g/100 g solid content gave an acceptable gel with 1 g/100 g NaCl for spread making. Satisfactory GG gel was also achieved with 9% solid content along with 0.5 g/100 g CaCl2 or 0.2 g/100 g FeSO4. The addition of divalent cations provided nutrition and improved the integrity of gels.
TABLE 4 Sensory Assessment of Green Gram Gel with Cations
ACKNOWLEDGMENT
The first author wishes to thank the Indian Council of Medical Research (ICMR), New Delhi, India for providing the fellowship to conduct the Ph.D. research programme.
REFERENCES
- Balasubramanian, S.; Singh, N.; Ilyas, S. M.; Wanjari, O. D. Effect of selected decorticated legumes protein on rheology of maize extrudate pastes. Journal of Food Science & Technology 2006, 43, 522–525.
- Veena, R.; Bhattacharya, S. Rheological characterization of raw and roasted green gram pastes. LWT-Food Science & Technology 2012, 46, 260–266.
- Dahiya, P.K.; Linnemann, A.R.; van Boekel, M.A.J.S.; Khetarpaul, N.; Grewal, R.B.; Nout, M.J.R. Mung bean: Technological and nutritional potential. International Journal of Food Properties DOI:10.1080/10408398.2012.671202 online: 12 Sep 2013.
- Anon. 2012. http://catalogs.indiamart.com/products/moong-dal.html 4.6.2012.
- Bourne, M.C. Food texture and viscosity: Concept and measurement. New York, NY: Academic Press, 2002.
- Burey, P.; Bhandari, B.R.; Rutgers, R.P.G.; Halley, P.J.; Torley, P.J. Confectionery gels: A review on formulation, rheological, and structural aspects. International Journal of Food Properties 2009, 12 (1), 176–210.
- Lii, C.Y.; Tseng, K.H. Gelation mechanism and rheological properties of rice starch. Cereal Chemistry 1995, 72, 393–400.
- Moritaka, H.; Fukuba, H.; Kumeno, K.; Nakahama, N. Effect of monovalent and divalent cations on the rheological properties of gellan gels. Food Hydrocolloids 1991, 4, 495–507.
- Laplante, S.; Turgeon, S.L.; Paquin, P. Effect of pH, ionic strength, and composition on emulsion stabilizing properties of chitosan in a model system containing whey protein isolate. Food Hydrocolloids 2005, 19, 721–729.
- Bhattacharya, S.; Jena, R. Gelling behavior of defatted soybean flour dispersions due to microwave treatment: Textural, oscillatory, microstructural, and sensory properties. Journal of Food Engineering 2007, 78, 1305–1314.
- Kim, K.-H.; Renkema, J.M.S.; Van Vliet, T. Rheological properties of soybean protein isolate gels containing emulsion droplets. Food Hydrocolloids 2001, 15, 295–302.
- Cavallieri, A.L.F.; Fialho, N.A.V.; Cunha, R.L. Sodium caseinate and κ-carrageenan interactions in acid gels: Effect of polysaccharide dissolution temperature and sucrose addition. International Journal of Food Properties 2011, 14, 251–263.
- Bora, P. S.; Brekke, C.J.; Powers, J.R. Heat induced gelation of pea (Pisum sativum) mixed globulins, vicilin, and legumin. Journal of Food Science 1994, 59, 594–596.
- Nunes, M.C.; Raymundo, A.; Sousa, I. Rheological behaviour and microstructure of pea protein/κ-carrageenan/starch gels with different setting conditions. Food Hydrocolloids 2006, 20, 106–113.
- AOAC. Association of Official Analytical Chemists. Official methods of analysis, 16th Ed. Washington, DC: AOAC, 2002.
- Hamerstrand, G.E.; Black, L.T.; Glover, J.D. Trypsin inhibitors in soy products: Modifications of the standard analytical procedure. Cereal Chemistry 1981, 58, 42–45.
- Walter, R.; Mark, A.S. A pepsin pancreatin digest index of protein quality evaluation. Journal of Nutrition 1964, 83, 257–261.
- Elhardallou, S.B.; Walker, A.F. The water holding capacity of three starchy legumes in the raw, cooked, and fiber-rich fraction forms. Plant Foods for Human Nutrition 1993, 44, 171–179.
- Banerjee, S.; Bhattacharya, S. Compressive textural attributes, opacity, and syneresis of gels prepared from gellan, agar, and three mixtures. Journal of Food Engineering 2011, 102, 287–292.
- Szczesniak, A.S. Physical properties of foods: What they are and their relation to other food properties. In: Physical properties of foods; Peleg, M.; Bagley, E.B.; Eds.; Westport, CT: AVI Publishing Co., 1983; 1–41.
- Kalviainen, N.; Roininen, K.; Tuorila, H. Sensory characterization of high viscosity gels made with different thickeners. Journal of Texture Studies 2000, 31, 407–420.
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices. New York, NY: Chapman & Hall, 1998; 606–608.
- Liener, I.E. Implications of nutritional components in soybean foods. Critical Reviews in Food Science & Nutrition 1994, 34, 31–67.
- Mouliswar, P.; Kurien, S.; Daniel,V.A.; Malleshi, N.G.; Rao, V. In-vitro digestibility of protein and starch energy food and its bulk reduction. Journal of Food Science & Technology 1993, 30, 36–39.
- Damodaran, S. Amino acids, peptides, and proteins. In: Fennema’s Food Chemistry, Damodaran, S.; Parkin, K.L.; Fennema, O.R.; Eds.; 4th Ed. New York, NY: CRC Press, Taylor & Francis, Boca Raton; 2008; 217–330.
- Lii, C.Y.; Tsai, M.L.; Tseng, K.H. Effect of amylose content on the rheological property of rice starch. Cereal Chemistry 1996, 73, 415–420.
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocolloids 2011, 25, 1813–1827.
- Nickerson, M.T.; Paulson, A.T.; Speers, R.A. Rheological properties of gellan solutions: Effect of calcium ions and temperature on pre-gel formation. Food Hydrocolloids 2003, 17, 577–683.
- Shim, J.; Mulvaney, S.J. Effect of heating temperature, pH, concentration, and starch/whey protein ratio on the viscoelastic properties of corn starch/whey protein mixed gels. Journal of the Science of Food & Agriculture 2001, 81, 706–717.
- Hood, L.F.; Oshea, G.K. Calcium binding by hydroxypropyl distarch phosphate and unmodified starches. Cereal Chemistry 1977, 54, 266–271.