Abstract
Cadaverine and tyramine are biogenic amines that are commenly found in fermented foods. This study focused on the reduction of cadaverine and tyramine formation by a new method using proteolytic enzymes (trypsin and chymotrypsin). Cadaverine and tyramine were synthesized from lysine and tyrosine in the presence of their decarboxylase enzymes, respectively. In this study, trypsin and chymotrypsin were used separately or in combination to reduce formation of cadaverine and tyramine in model system. Tyramine synthesis was reduced by trypsin and chymotrypsin as 57.2 and 52.1%, respectively. Moreover, the reduction of cadaverine was 80.4 and 83.0% by trypsin and chymotrypsin, respectively. Maximum reduction values were obtained when trypsin and chymotrypsin were used together for the reduction of tyramine (59.1%) and cadaverine formation (84.8%). In model system, cadaverine and tyramine formation were (p < 0.05) significantly reduced. It was concluded that trypsin and chymotrpsin could be used to reduce tyramine and cadaverine formation.
INTRODUCTION
Biogenic amines (BA) are low molecular weight, organic basic nitrogenous compounds. They are synthesized in the presence of decarboxylases by removal of α-carboxyl group from a precursor amino acid. Enzymes in raw material or produced by microorganisms lead to formation of BA during ripening and storage of food.[Citation1,Citation2] The presence of BA has been used as an indicator of quality and/or acceptability in some foods. Intake of foods containing high concentrations of certain BA causes a health hazard through the direct toxic effect of these compounds and their interaction with some medicaments.[Citation3] In the human body there is a detoxification system which degrades BA to physiologically less active forms. Diamine oxidase and monoamine oxidase are two enzymes that are responsible for detoxification. However, intake of high concentrations of BA causes deactivation of this detoxification system.[Citation4] In addition, some drugs reduce the activity of monoamine oxidase. In that case, this enzyme cannot act, leading to the absorption of BA by the human body and occurrence of toxic effects. Also, BA are considered as precursors of carcinogenic amines such as N-nitrosamines that are produced from nitrites and secondary amines.[Citation4,Citation5]
BA are the most commonly studied quality and safety parameters in fermented products.[Citation6,Citation7] Tyramine is mainly related to the activity of fermentative lactic acid bacteria while cadaverine is usually resulted of the action of non-fermentative strains.[Citation8] The presence of high cadaverine concentrations has been associated with poor quality meat used in the manufacturing of meat products and mainly, with high levels of microbial contamination.[Citation9] Among the microbial groups producing BA, Enterobacteriaceae seem to have an important role in cadaverine production, as high Enterobacteriaceae counts are directly related to high cadaverine concentrations.[Citation10]
In fact, presence of cadaverine indicates low quality of raw materials in which high proliferation of microorganisms occurs.[Citation10] Cadaverine is not considered toxic individually; however, it can enhance the effect of histamine and tyramine by interacting with the amino oxidases and interfering with the detoxifying mechanism.[Citation11] Numerous efforts have been made to reduce or to prevent formation of BA in food. The prevention of BA formation can be achieved by using temperature control,[Citation12] high-quality raw material,[Citation13] good manufacturing practice,[Citation14] use of enzymes to oxidize amine,[Citation15] use of microbial modeling to assess favorable conditions,[Citation16,Citation17] packaging techniques,[Citation18] high hydrostatic pressure (HHP),[Citation19] irradiation,[Citation20] and food additives.[Citation21] HHP and irradiation processes are used easily in laboratory conditions, however, could not be used extensively in the food industry due to the high cost of the processes.[Citation22] Additives could show inhibitory effect on BA formation. Further investigation is needed for reducing of BA formation in variety of foods. Controlled temperature is a way to control BA formation except for some bacteria produce BA at temperatures below 5○C.[Citation23,Citation24]
The amount of BA will be decreased by limiting the activity of enzymes which converts free amino acids to BA. It was reported that trypsin and chymotrypsin were effective on the reduction of decarboxylase activity in rats.[Citation25] They studied the effect of proteolytic enzymes (trypsin and chymotrypsin) on the histamine formation in rats. Use of these enzymes reduced the formation of histamine in rats. In the literature, there is no information about the reduction of BA formation in foods or model systems by use of proteolytic enzymes (trypsin and chymotrypsin). Therefore, the aim of this study was to develop a new method to reduce cadaverine and tyramine formation by proteolytic enzymes trypsin and chymotrypsin in model system.
MATERIALS AND METHODS
Chemicals
Cadaverine dihydrochloride, tyramine hydrochloride, trypsin (one Nα-Benzoyl-L-arginine ethyl ester (BAEE) unit will produce a A253 of 0.001 per min at pH 7.6 at 25°C using BAEE as a substrate), chymotrypsin (one unit will hydrolyze 1.0 μmole of N-Benzoyl-L-Tyrosine Ethyl Ester (BTEE) per min at pH 7.8 at 25°C), tyrosine, lysine decarboylase (LDC), tyrosine decarboxylase (TDC), and lysine were obtained from Sigma (St. Louis, MO). Cadaverine dihydrochloride and tyramine hydrochloride were used as BA standards, sodium hydroxide, 25% ammonium and sodium bicarbonate were obtained from Merck (Darmstadt, Germany), acetone from Reidel De Haen (Germany), dansyl chloride from Sigma Co. (St. Louis, MO), ammonium acetate from Merck (Darmstadt, Germany), and perchloric acid from JT Baker (Holland). All chemicals except acetonitrile were of analytical grade (extra pure) and acetonitrile was high-performance liquid chromatography (HPLC) grade.
Preparation of Standards
Standard solutions of BA (cadaverine and tyramine) and amino acids (lysine and tyrosine) were prepared and diluted to 1 mL with 0.4 M perchloric acid to give concentrations from 0.5 to 10 μg/mL.
Determination of BA and Amino Acids
Liquid chromatography (LC) mobile phases
The chromatographic method was used for the determination of the BA and amino acids.[Citation26] The HPLC consisted of a Shimadzu gradient pump (Shimadzu LC 20AB, Shimadzu Solvent Delivery Module, Kyoto, Japan), a Shimadzu auto injection unit (Shimadzu SIL20AHT, Kyoto, Japan), a Shimadzu ultra violet (UV) detector (Shimadzu SPD 20A, Kyoto, Japan) and a RP-18 guard column. The wavelength of UV detector was 254 nm. The HPLC column was Spherisorb ODS2, 200 μm and 4.6 × 200 mm (Inertsil, ODS-2). Ammonium formate solution (0.4 M) prepared by ultra-pure water (Millipore Elix 10UV and Milli-Q, Millipore S.A.S. 67120 Molsheim, France) and acetonitrile were filtered through a 0.45 μm Millipore filter (Billerica, MA). Ammonium formate and acetonitrile were used as the LC mobile phases. A gradient elution program was used with mobile phases of acetonitrile (solvent A) and 0.4 M ammonium formate (solvent B), starting with 50% solvent A and 50% solvent B and finishing with 90% solvent A and 10% solvent B after 35 min. The flow rate was 1.0 mL/min.
Derivatization method
One mililiter of the each sample (lysine, tyrosine, cadaverine, and tyramine) was made alkaline by adding 200 μL of 2 N NaOH solution; 300 μL of saturated sodium bicarbonate was also added as buffer. Two milliliters of dansyl chloride solution (10 mg/mL in acetone) was added to each sample and incubated for 45 min at 40°C. Residual dansyl chloride was removed by adding 100 μL of 25% ammonia. After 30 min, the solution was adjusted to 5 mL with acetonitrile, centrifuged for 5 min at 1790xg. The supernatant was filtered (0.45 μm), and 20 μL was then injected onto the HPLC. Lysine, tyrosine, cadaverine, and tyramine content was found from calibration curves.
Synthesis and determination of BA in model system
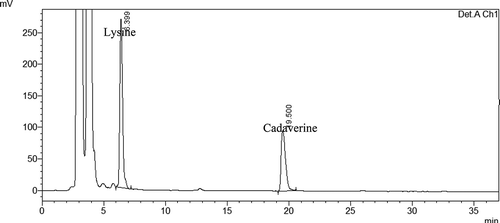
One mililiter of standard solutions of lysine were mixed with 1 mL of its decarboxylase enzyme (2 mg/mL lysine decarboxylase). Lysine concentration in solutions was adjusted to 25, 50, 100, 150, and 200 ppm. The solutions were incubated in orbital shaker (Innova 40) at 30°C with 70 rpm for 30 min. After 30 min, derivization procedure was applied and then analyzed by HPLC. shows the peaks of residual lysine and formed cadaverine. The same procedure was applied for tyrosine by using 2 mg/mL of TDC enzyme. Tyrosine concentration in solutions was adjusted to 25, 40, 75, 100, and 125 ppm.
Reduction of BA in Model System
Each model systems were prepared as two replicates and dublicate analyses were carried out in each replicate.
Application of trypsin
One mililiter of lysine solution, 1 mL of trypsin (2 mg/mL), and 1 mL of lysine decarboxylase enzyme (2 mg/mL) were mixed and incubated with incubation shaker at 30°C with 70 rpm for 30 min. Lysine concentration in solutions was adjusted to 25, 50, 100, 150, and 200 ppm. After 30 min, derivization procedure was applied to find formation and reduction in cadaverine and then analyzed by HPLC. The same procedure was applied for standard solution of tyrosine by using 2 mg/mL of TDC enzyme and 1 mg/mL of trypsin to detect tyramine formation. Tyrosine concentration in solutions was adjusted to 25, 40, 75, 100, and 125 ppm.
Application of chymotrypsin
One mililiter of lysine solution, 1 mL of chymotrypsin (2 mg/mL), and 1 mL of its decarboxylase enzyme (2 mg/mL) were mixed and incubated with incubation shaker at 30°C with 70 rpm for 30 min. Lysine concentration in solutions was adjusted to 25, 50, 100, 150, and 200 ppm. After 30 min, derivization procedure was applied to find cadaverine formation and then analyzed by HPLC. The same procedure was applied for standard solution of tyrosine by using 2 mg/mL of TDC enzyme and 1 mg/mL of chymotrypsin enzyme to detect tyramine formation. Tyrosine concentration in solutions was adjusted to 25, 40, 75, 100, and 125 ppm.
Application of trypsin and chymotrypsin
One mililiter of standard solution of lysine (25, 50, 100, 150, and 200 ppm), 1 mL of chymotrypsin (2 mg/mL), 1 mL of trypsin (2 mg/mL), and 1 mL of its decarboxylase enzyme (2 mg/mL) were mixed and incubated with incubation shaker at 30°C with 70 rpm for 30 min. Lysine concentration in solutions was adjusted to 25, 50, 100, 150, and 200 ppm. After 30 min, derivization procedure was applied to find cadaverine formation and then analyzed by HPLC. The same procedure was applied for standard solution of tyrosine by using 1 mg/mL of TDC enzyme, trypsin enzyme (1 mg/mL), and chymotrypsin enzyme (1 mg/mL) to detect tyramine formation. Tyrosine concentration in solutions was adjusted to 25, 40, 75, 100, and 125 ppm.
Determination of Percent Reduction of Cadaverine and Tyramine Formation
Percent reduction of cadaverine and tyramine was calculated as:
A is the amount of cadaverine or tyramine produced with their decarboxylase enzyme, B is the amount of cadaverine or tyramine produced with their decarboxylase enzyme in the presence of proteolytic enzymes (trypsin and chymotrypsin).
Statistical Analysis
The results were analyzed statistically using the statistical packages SPSS 16.0 for Windows (SPSS Inc., Chicago, IL, USA). The one-way analysis of variance (ANOVA test) and Duncan’s multiple range test was performed. Values of p < 0.05 were used to indicate significant differences.
RESULTS AND DISCUSSION
Synthesis and Reduction of Tyramine
Tyramine is one of the BA that can cause some health disorders in sensitive individuals.[Citation27] shows the tyramine synthesis in model system in the presence of TDC enzyme. Concentrations of synthesized tyramine increased (p < 0.05) when the concentrations of standard solution of tyrosine were increased (). Conversion of tyrosine to tyramine was achieved by a range from 78.4 to 96.7%. At 100 ppm, the percentage of tyrosine converted to tyramine is at its maximum value, and then that value decreases (). This may be due to the maximal reaction rate is obtained at 100 ppm tyrosine concentration and increasing tyrosine concentration did not increase the tyramine concentration afterall in which enzyme is saturated with substrate.
TABLE 1 Synthesis of tyramine and cadaverine
Trypsin and chymotrypsin were applied to reduce tyramine formation in model system. Constant concentration of trypsin, chymotrypsin enzymes, and decarboxylase enzymes were used for different concentrations of tyrosine and it was observed that trypsin and chymotrypsin reduce efficiently (p < 0.05) tyramine synthesis (). Tyrosine (100 ppm) was converted to 96.75 ppm tyramine in the presence of TDC enzyme (). It was reduced to 82.2 ppm (15.0%) by chymotrypsin, to 69.8 ppm (27.8%) by trypsin, and to 63.4 ppm (34.5%) by a mixture of trypsin and chymotrypsin (). A maximum of 57.2 and 52.1% tyramine reduction was observed when trypsin and chymotrypsin enzymes were used, respectively. Also 59.1% reduction was observed by use of trypsin and chymotrypsin together ().
TABLE 2 Effect of trypsin and chymotrypsin on tyramine synthesis
As the concentration of tyrosine was increased, the percentage of the tyramine synthesis decreased. This could be explained by the insufficient concentrations of trypsin and chymotrypsin to reduce tyramine formation. Results show that 1 mg/mL of trypsin and chymotrypsin concentrations were not enough to reduce tyramine synthesis so that higher trypsin and chymotrypsin concentrations could be needed. It was concluded that use of a mixture of proteolytic enzymes results in highest reduction of tyramine. Trypsin shows better inhibitory effect on tyramine formation compared to chymotrypsin. Inhibition mechanism of trypsin and chymotrypsin did not obey any of reversible inhibition types. The inhibition mechanism was probably irreversible and proteolytic enzymes could alter the active site of the decarboxylase enzymes of amino acid residues. Proteolytic enzymes have been already used in the food industry to accelerate the fermentation but their actions on reduction of BA was first investigated by this study.
In the literature there is no study about reduction of formation of BA by proteolytic enzymes in model systems; however, there are different methods, such as controlled temperature, starter culture, HPP, irradiation, and using preservatives, that were studied to reduce formation of BA in foods (). Ruiz-Capillas et al.[Citation28] studied the reduction of formation of BA with high hyrostatic pressure at 350 MPa for 15 min. They observed a 17.0% reduction of tyramine formation compared to sausage not treated with HHP. Also, 17.7% tyramine reduction was observed by application of irradiation.[Citation29] Results found by other researches in foods are quite lower than that found in this study.
TABLE 3 Reduction of formation of biogenic amines by different methods
Novella-Rodriguez et al.[Citation19] studied the effect of HHP on BA reduction and they obtained higher (84%) reduction of tyramine during the ripening of goat cheese compared to the results obtained in this study. In addition, tyramine concentration dropped approximately from 200 to <100 ppm (about >50% reduction) in Indian mackerel (whole) by use of turmeric (curcumin), red pepper (capsaicin), and black pepper (piperine),[Citation30] and tyramine was reduced by 31.2% in Myeolhi-Jeot (fermented anchovies) by preservatives.[Citation21] Tyramine concentration dropped from 24.7 to 9.3 (62.3%) ppm in beef by the application of irradiation.[Citation31] Although many studies show the inhibitory effect of irradiation, there is an unfavorable point that is public acceptance.[Citation32–Citation34] Results showed that using trypsin and chymotrypsin together had acceptable reduction of synthesis of tyramine compared to the other reduction methods.
Synthesis and Reduction of Cadaverine
Cadaverine has less pharmacological activity than the aromatic amines but it is probably potentiators of their toxicity.[Citation35] shows the synthesis of cadaverine from lysine in the presence of lysine decarboxylase enzyme. According to the results, as the concentrations of lysine increased up to 100 ppm, concentrations of synthesized cadaverine increased (p < 0.05) as well (). Synthesized cadaverine changed between 54.7–74.4% (). A significant drop (p < 0.05) was observed in cadaverine formation when trypsin (maximum 80.4% reduction) and chymotrypsin (maximum 83% reduction) were used (). Also, the reduction of cadaverine formation was significant (p < 0.05), maximum 84.8%, when trypsin and chymotrypsin were used together ().
TABLE 4 Effect of trypsin and chymotrypsin on cadaverine synthesis
According to the results of this study, when 100 ppm lysine was treated with lysine decarboxylase enzyme, 74.4 ppm cadaverine was detected. It was reduced to 31.0 ppm (58.3%) by trypsin, reduced to 15.4 ppm (79.3%) in the presence of chymotrypsin and to 20.2 ppm (72.8%) when chymotrypsin and trypsin were used together (). Synthesized cadaverine concentration increased as the initial concentration of lysine was increased. Cadaverine formation reduced significantly (p < 0.05) by use of trypsin, chymotrypsin, and the mixture of trypsin and chymotrypsin. The use of trypsin separately had less inhibitory effect than the other formulation of trypsin and chymotrypsin enzymes. Chymotrypsin showed better reduction of cadaverine formation compared to trypsin. However, the use of both trypsin and chymotrypsin enzymes together inhibited cadaverine formation more than the use of trypsin and chymotrypsin enzymes alone, as about 85%.
Ruiz-Capillas et al.[Citation28] were observed lower reduction of cadaverine (12.5%) by HHP compared to our results. In another study, 98% cadaverine reduction was observed by use of HHP.[Citation36] HHP provides significant reduction or control of BA; however, it has some limitations. Reduction of BA formation depends on the level of pressure applied.[Citation22] Low-pressure treatment of 50 MPa for 72 h increased BA content, while a high-pressure treatment of 400 MPa for 5 min plus 50 MPa for 72 h showed a slight decrease.[Citation19] Also, it was observed that cadaverine concentration was approximately dropped from 200 to 100 ppm (50.0%) in Indian mackerel (whole) by use of preservatives,[Citation30] and dropped from 18.9 to 10.1 ppm (53.4%) by Mbarki et al.[Citation32] Although there has been many studies that show inhibitory effects of food preservatives and additives on BA,[Citation21,Citation37,Citation38] some researches show promoting effects on BA formation.[Citation39,Citation40] On the other hand, sufficient knowledge about effectiveness of additives on different BA is not present and needs to be tested in variety of food systems.
CONCLUSION
Researches have been trying to reduce BA formation due to their toxic risks, especially on people suffering from allergic reactions, and have a role as indicators of quality and/or acceptability in some foods. In this study a new method, the use of proteolytic enzymes (trypsin and chymotrypsin), was applied to reduce formation of BA in model system. Significant reduction of cadaverine and tyramine synthesis were detected (p < 0.05) in model system. Compared to the separate use of trypsin and chymotrypsin, in most cases, using trypsin and chymotrypsin mixture gave better results for cadaverine and tyramine reduction. Although use of trypsin separately showed better inhibitory effect on tyramine reduction compared to chymotrypsin, the use of trypsin had no similar effect on cadaverine inactivation. In cadaverine inactivation the use of chymotrypsin resulted in higher reduction compared to that of trypsin. It was found that the use of trypsin and chymotrypsin could be used to reduce selected BA formation in model system. However, further studies are required to determine the effect of trypsin and chymotrypsin in food systems on different BA.
REFERENCES
- Moret, S.; Smela, D.; Populin, T.; Conte, L.S. A Survey on Free Biogenic Amine Content of Fresh and Preserved Vegetables. Food Chemistry 2005, 89, 355–361.
- Stadnik, J.; Dolatowski, Z.J. Biogenic Amines in Meat and Fermented Meat Products. ACTA Scientiarum Polonorum Technologia Alimentaria 2010, 9, 251–263.
- Ruiz-Capillas, C.; Moral, A. Production of Biogenic Amines and Their Potential Use As Quality Control Indices for Hake (Merluccius Merluccius, L.) Stored in Ice. Journal of Food Science 2001, 66, 1030–1032.
- Anlı, R.E.; Bayram, M. Biogenic Amines in Wines. Food Reviews International 2009, 25, 86–102.
- Şahin-Ercan, S.; Bozkurt, H.; Soysal, Ç. Significance of Biogenic Amines in Foods and Their Reduction Methods. Journal of Food Science and Engineering 2013, 3, 395–410.
- Özdestan, Ö.; Alpözen, E.; Güven, G.; Üren, A. Monitoring of Biogenic Amines in Kumru: A Traditional Fermented Cereal Food. International Journal of Food Properties 2012, 15, 972–981.
- Özdestan, Ö.; Üren, A. Biogenic Amine Content of Tarhana: A Traditional Fermented Food. International Journal of Food Properties 2013, 16, 416–428.
- Simon-Sarkadi, L.; Pasztor-Huszar, K.; Dalmadi, I.; Kisko, G. Effect of High Hydrostatic Pressure Processing on Biogenic Amine Content of Sausage During Storage. Food Research and International 2012, 47, 380–384.
- Bover-Cid, S.; Izquierdo-Pulido, M.; Vidal-Carou, M.C. Effect of the Interaction Between a Low Tyramine Producing Lactobacillus and Proteolytic Staphylococci on Biogenic Amine Production During Ripening and Storage of Dry Sausage. International Journal of Food Microbiology 2001, 65, 113–125.
- Suzzi, G.; Gardini, F. Biogenic Amines in Dry Fermented Sausages: A Review. International Journal of Food Microbiology 2003, 88, 41–54.
- Ruiz-Capillas, C.; Colmenero-Jimenez, F. Biogenic Amines in Meat and Meat Products. Critical Reviews in Food Science and Nutrition 2004, 44, 489–499.
- Du, W.X.; Lin, C.M.; Phu, A.T.; Cornell, J.A.; Marshall, M.R.; Wei, C.I. Development of Biogenic Amines in Yellowfin Tona (Thunnus Albacares): Effect of Storage and Correlation with Decarboxylase Positive Bacterial Flora. Journal of Food Science 2002, 67, 292–301.
- Maijala, R.; Nurmi, E.; Fischer, A. Influence of Processing Temperature on the Formation of Biogenic Amines in Dry Sausages. Meat Science 1995, 39, 9–22.
- Komprda, T.; Neznalova, J.; Standara, S.; Bover-Cid, S. Effect of Starter Culture and Storage Temperature on the Content of Biogenic Amines in Dry Fermented Sausage Polian. Meat Science 2001, 59, 267–276.
- Dapkevicius, M.L.N.E.; Nout, M.J.R.; Rombouts, F.M.; Houben, J.H.; Wymenga, W.; Biogenic Amine Formation and Degradation by Potential Fish Silage Starter Microorganisms. International Journal of Food Microbiology 2000, 57, 7–14.
- Emborg, J.; Dalgard, P. Growth, Inactivation, and Histamine Formation of Morganella Psychrotolerans and Morganella Morganii—Development and Evaluation of Predictive Models. International Journal of Food Microbiology 2008, 128, 234–243.
- Emborg, J.; Dalgaard, P. Modelling the Effect of Temperature, Carbon Dioxide, Water Activity, and pH on Growth and Histamine Formation by Morganella Psychrotolerans. International Journal of Food Microbiology 2008, 128, 226–233.
- Mohan, C.O.; Ravishankar, C.N.; Gopal, T.K.S.; Kumar, K.A.; Lalitha, K.V. Biogenic Amines Formation in Seer Fish (Scomberomorus Commerson) Steaks Packed with O2 Scavenger During Chilled Storage. Food Research International 2009, 42, 411–416.
- Novella-Rodriguez, S.; Veciana-Nogues, M.T.; Saldo, J.; Vidal-Carou, M.C. Effects of High Hydrostatic Pressure Treatments on Biogenic Amine Contents in Goat Cheeses During Ripening. Journal of Agriculture and Food Chemistry, 2002, 50, 7288–7292.
- Kim, J.H.; Ahn, H.J.; Kim, D.H.; Jo, C.; Yook, H.S.; Park, H.J.; Byun, M.W. Irradiation Effects on Biogenic Amines in Korean Fermented Soybean Paste During Fermentation. Journal of Food Science 2003, 68, 80–84.
- Mah, J.H.; Hwang, H.J. Effects of Food Additives on Biogenic Amine Formation in Myeolchijeot, a Salted and Fermented Anchovy (Engraulis Japonicus). Food Chemistry 2009, 114, 168–173.
- Naila, A.; Flint, S.; Fletcher, G.; Bremer, P.; Meerdink, G. Control of Biogenic Amines in Food—Existing and Emerging Approaches. Journal of Food Science 2010, 75, 139–150.
- Emborg, J.; Laursen, B.G.; Dalgaard, P. Significant Histamine Formation in Tuna (Thunnus Albacares) at 2°C Effect of Vacuum and Modified Atmosphere Packaging on Psychrotolerant Bacteria. International Journal of Food Microbiology 2005, 101, 263–279.
- Emborg, J.; Dalgaard, P. Formation of Histamine and Biogenic Amines in Cold-Smoked Tuna: An Investigation of Psychrotolerant Bacteria from Samples Implicated in Cases of Histamine Fish Poisoning. Journal of Food Protection 2006, 69, 897–906.
- Yamada, M.; Watanabe, T.; Harino, S.; Fukui, H.; Wada, H. The Effects of Protease Inhibitors on Histidine Decarboxylase Activities and Assay if Enzyme in Various Rat Tissues. Biochimica et Biophysica Acta (BBA)–Enzyme 1980, 615, 458–463.
- Eerola, S.; Hinkkanen, R.; Lindfors, E.; Hirvi, T. Liquid Chromatographic Determination of Biogenic Amines in Dry Sausage. Journal of AOAC International 1993, 76, 575–577.
- Andiç, S.;Tunçtürk, Y.; Gençcelep, H. The Effect of Different Packaging Methods on the Formation of Biogenic Amines and Organic Acids in Kashar Cheese. Journal of Dairy Science 2011, 94, 1668–1678.
- Ruiz-Capillas, C.; Colmenero, F.J.; Carrascosa, A.V.; Munoz, R. Biogenic Amine Production in Spanish Dry-Cured “Chorizo” Sausage Treated with High-Pressure and Kept in Chilled Storage. Meat Science 2007, 77, 365–371.
- Kim, J.H.; Ahn, H.J.; Lee, J.W.; Park, H.J.; Ryu, G.H.; Kang, I.J.; Byun, M.W. Effects of Gamma İrradiation on the Biogenic Amines in Pepperoni with Different Packaging Conditions. Food Chemistry 2005, 89, 199–205.
- Shakila, R.J.; Vasundhara, T.S.; Rao, D.V. Effect of Spices on the Biogenic Amine Formation and Other Quality Characteristics of Indian Mackerel During Refrigerated Storage. Asian Fisheries Science 2005, 9, 191–199.
- Min, J.S.; Lee, S.; Jang, A.; Jo, C.; Lee, M. Irradiation and Organic Acid Treatment for Microbial Control and the Production of Biogenic Amines in Beef and Pork. Food Chemistry 2007, 104, 791–799.
- Mbarki, R.; Miloud, N.B.; Semli, S.; Dhib, S.; Sadok, S. Effect of Vacuum Packaging and Low-Dose Irradiation on the Microbial, Chemical, and Sensory Characteristics of Chub Mackerel (Scomber japonicus). Food Microbiology 2009, 26, 821–826.
- Kim, J.H.; Ahn, H.J.; Jo, C.; Park, H.J.; Chung, Y.J.; Byun, M.W. Radiolysis of Biogenic Amines in Model System by Gamma Irradiation. Food Control 2004, 15, 405–408.
- Min, J.S.; Lee, S.; Jang, A.; Jo, C.; Lee, M. Control of Microorganisms and Reduction of Biogenic Amines in Chicken Breast and Thigh by Irradiation and Organic Acids. Poultry Science 2007, 86, 2034–2041.
- Joosten, H.M.L.J.; Van Boekel, M.A.J.S.; Conditions Allowing the Formation of Biogenic Amines in Cheese. A Study of the Kinetics of Histamine Formation in An Infected Gouda Cheese. Netherlands Milk and Dairy Journal 1988, 42, 329–357.
- Latorre-Moratalla, M.L.; Bover-Cid, S.; Aymerich, T.; Marcos, B.; Vidal-Carou, M.C.; Garriga, M. Aminogenesis Control in Fermented Sausages Manufactured with Pressurized Meat Batter and Starter Culture. Meat Science 2007, 75, 460–469.
- Shalaby, A.R. Significance of Biogenic Amines to Food Safety and Human Health. Food Research International 1996, 29, 675–690.
- Mah, J.H.; Kim, Y.J.; Hwang, H.J. Inhibitory Effects of Garlic and Other Spices on Biogenic Amine Production in Myeolchi-Jeot, Korean Salted and Fermented Anchovy Product. Food Control 2009, 20, 449–454.
- Komprda, T.; Smela, D.; Pechova, P.; Kalhotka, L.; Stencl, J.; Klejdus, B. Effect of Starter Culture, Spice Mix, and Storage Time and Temperature on Biogenic Amine Content of Dry Fermented Sausages. Meat Science 2004, 67, 607–616.
- Bhutani, M.K.; Bishnoi, M.; Kulkarni, S.K. Anti-Depressant Like Effect of Curcumin and İts Combination with Piperine in Unpredictable Chronic Stress-Induced Behavioral, Biochemical, and Neurochemical Changes. Pharmacology Biochemistry and Behavior 2009, 92, 39–43.
- Mah, J.H.; Hwang, H.J. Inhibition of Biogenic Amine Formation in a Salted and Fermented Anchovy by Staphylococcus xylosus As a Protective Culture. Food Control 2009, 20, 796–801.