Abstract
Aflatoxins are toxic metabolites produced by fungi. The objective of this study was to develop an HPLC method to detect the presence of aflatoxins in Pu-erh tea. The developed method had recovery rates of 81.02–90.69%. The results revealed that Pu-erh teas from Yunnan, China, contained aflatoxins, especially AFB1 (8.333 μg/kg in raw and 20.149 μg/kg in ripe tea). All tea samples had aflatoxin levels below the national maximum permissible limits; however, the tea samples had higher aflatoxin levels than those allowed in the European Union, the USA, and Japan. The presence of aflatoxins in Pu-erh tea hinders its international marketability.
Keywords:
INTRODUCTION
China is the main producer and consumer of tea (Camellia sinensis) worldwide. At least three types of tea (green, black, and oolong) are produced there. Pu-erh tea, which results from the fermentation of black tea, is produced in the province of Yunnan. The name Pu-erh refers to the name of the county that served as a trading post for black tea during imperial China. Pu-erh has been consumed in China for centuries and has recently become popular all over the world. As a functional beverage, Pu-erh tea, like other types of teas, has a several biological effects including antioxidant, hypocholesterolemic, anti-tumor, and anti-obesity properties.[Citation1−Citation3] Furthermore, a recent study has reported that nanofibrous membranes in Pu-erh tea have antibacterial activities against Eschericia coli.[Citation4]
Pu-erh tea is available as loose leaf and in several compressed forms, i.e., brick-, cake-, or bowl-shaped. There are ripe (shou) and raw (sheng) Pu-erh tea varieties. Ripe and raw Pu-erh tea share similar production methods; however, ripe tea production involves wet piling following the fixation step and prior to the drying step; this process is similar to composting. Wet piling is a key process during tea production; the microorganisms and number of processes in wet piling affect Pu-erh tea flavor, quality, and biological properties. In wet piling, several microorganisms (mold, bacteria, and fungi) are used including Aspergillus niger and Blastobotrys adeninivorans.[Citation5]
Aflatoxins are mycotoxins produced by several fungi; the most common ones are A. flavus and A. parasiticus. At least 14 different types of aflatoxins are naturally produced (B1, B2, G1, G2, M1, M2, Q1).[Citation6] Aflatoxin B1 (AFB1), produced by A. flavus and A. parasiticus, is the most potent liver carcinogen.[Citation7] Aflaxotins have mutagenic, teratogenic, and immunosuppressing activities. Prolonged consumption of aflatoxin-containing foods is associated with increased risk for liver, stomach, and colon cancers. Additionally, aflatoxins are associated with depressed immune function.[Citation8]
Different methods including thin layer chromatography (TLC), high-performance liquid chromatography (HPLC), and enzyme-linked immunosorbent assay (ELISA) have been used to detect the presence of aflatoxins in food and feed. Among these methods, HPLC is ideal because of its specificity and sensitivity (0–320 μg/kg).[Citation9] HPLC is more sensitive than ELISA in the detection of aflatoxins in milk and feed.[Citation10] However, few methods have been validated for the determination of aflatoxins in tea. The objective of this study was to develop an HPLC method for the detection of aflatoxins in Pu-erh tea. Additionally, the prevalence of aflatoxins in 30 representative tea samples from Yunnan was determined.
MATERIALS AND METHODS
Sampling
Five prefectures of Yunnan, i.e., Xishuangbana, Lincang, Dali, Baoshan, and Pu-erh, which are the main producers of Pu-erh tea, were selected for this study. Three tea companies were selected per county; one raw and one ripe Pu-erh tea sample were obtained per company, amounting to 30 Pu-erh tea samples.
An aflatoxin-free Pu-erh tea sample was used for the development of the HPLC method. Sampling and processing were performed according to Chinese guidelines (GB/T8302-2002 and GB/T8303-2002).
Development of the HPLC-Based Method
Processing of Pu-erh tea samples
In this experiment, 25 g of aflatoxin-free Pu-erh tea was transferred to a 250-ml flat-bottom flask and mixed with 100 ml of 84% acetonitrile. The mixture was placed in an electric oscillator for 30 min and filtered through a qualitative filter paper; 4 ml was removed for purification. A TC-M160 column (Xinnong Technology & Trade Co., Ltd., Beijing, China) was used for the purification step; 2 ml of purified solution was transferred to a derivatization bottle, which was placed in a water bath at 60°C and dried under a stream of nitrogen gas. Hexane (200 μl) and trifluoroacetic acid (TFA; 100 μl) were added, mixed for 30 s, and heated at 40°C for 15 min. The mixture was dried under a stream of nitrogen gas; 1 ml of 85% acetonitrile was added, mixed for 30 s, and centrifuged at 1000 rpm for 15 min. The resulting supernatant was subjected to HPLC.
Preparation of aflatoxin standard solutions
Standard solutions of aflatoxin (0.5, 1, 5, 25, and 50 μg/L) were prepared in acetonitrile and subjected to the derivatization step described above. The aflatoxin concentrations in the tea samples were quantified by comparing the peak areas of the samples with those of aflatoxin standards.
Optimization of HPLC conditions
The column type, temperature, emission wavelength, and flow rate of the fluorescence detector used in the HPLC system were optimized.
Precision and recovery rate
The precision (i.e., reproducibility) of the HPLC method was assessed by measuring five times the aflatoxin concentrations in the standard solutions. Recovery rate was determined by spiking aflatoxin-free Pu-erh tea with different concentrations of aflatoxins (1, 3, and 9 μg/kg). The recovery rate was calculated by the following formula,
Prevalence of Aflatoxin in Pu-erh Tea
The presence of aflatoxin in the 30 Pu-erh tea samples was assessed by the developed HPLC method.
RESULTS
Development of the HPLC Method
The HPLC system consisted of a C18 column (Xinnong Technology & Trade Co., Ltd., Beijing, China; 4.6 mm × 250 mm, 5-μm particle size) and a fluorescence detector. The column temperature was 30°C, the flow rate was 1.2 ml/min, and the injection amount was 25 μl. The emission wavelength was 440 nm and the excitation wavelength was 360 nm. HPLC was performed on a Waters 2695 Alliance Separations (Milford, MA, USA).
Chromatogram of Standard Solution
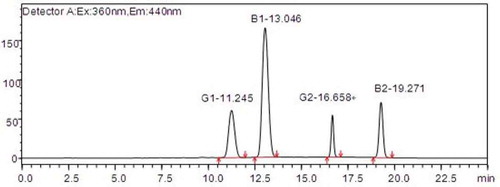
AFB1, AFG1 (both at 0.5, 1, 5, 25, and 50 μg/L), AFB2, and AFG2 (both at 0.125, 0.25, 1.25, 6.25, and 12.5 μg/L) were injected (25 μl) into the HPLC system. Linear regression equations were established based on the chromatogram of the standard solutions: AFG1, Y = 27,537.61X-438.70, R2 = 0.9997; AFB1, Y = 75,160.80X+5,752.38, R2 = 0.9996; AFG2 Y = 10,221.50X+1,583.90, R2 = 0.9999; and AFB2 Y = 20,500.40X+3,247.93, R2 = 0.9997 (). The retention times of AFG1, AFB1, AFG2, and AFB2 were 11.245, 13.046, 16.658, and 19.271 min, respectively.
Precision
The relative standard deviations of AFG1, AFB1, AFG2, and AFB2 by the HPLC method were 1.954, 1.750, 4.408, and 1.974%, respectively ().
TABLE 1 HPLC Precision
Recovery Rate
Aflatoxin-free Pu-erh tea was spiked with aflatoxin (1, 3, and 9 μg/kg). The results revealed that the recovery rates of AFG1, AFB1, AFG2, and AFB2 were 82.28–90.87, 81.64–90.49, 82.91–91.16, and 79.56–90.73%, respectively ().
TABLE 2 Recovery Rates of Aflatoxin Standards (n = 5)
Presence of Aflatoxin in Pu-erh Tea
AFG1 and AFB1 were detected in raw and ripe Pu-erh tea samples; however, the concentrations of aflatoxin differed among the samples. Compared to AFG1 and AFB1, AFB2, and AFG2 concentrations were lower in the samples. AFB1 reached 20.149 μg/kg in the Pu-erh tea samples ().
TABLE 3 Presence of Aflatoxin in Pu-erh Tea from Yunnan, China (Mg/Kg)
DISCUSSION
Wet piling, which involves piling and dampening, is similar to composting. Tea leaves are subjected to microbial fermentation in a warm and humid environment under controlled conditions. Microorganisms play direct and indirect roles on the quality of Pu-erh tea. The main microorganisms during wet piling/fermentation include A. niger, Penicillium, Rhizopus, A. gloucus, Saccharomyces, A. terreus, A. candidus, and bacteria. A. niger is the most predominant microorganism followed by Saccharomyces.[Citation11] The control of the fermentation process (e.g., humidity and microbial growth) is essential for Pu-erh tea quality.[Citation12] Also aspergillus species have been isolated in herbal teas obtained from the Swiss market.[Citation13]
Aflatoxins are naturally occurring mycotoxins that may appear during different production steps of both raw and ripe Pu-erh tea. The concentration of aflatoxin varies within batches as a result of differences in storage conditions and environmental factors. It has been reported that 34 fungal species were isolated in the air of tea-processing facilities, especially toxin-producing strains such as A. flavus and A. parasiticus.[Citation14]
HPLC has been used to detect the presence of aflatoxins in peanuts, maize, medicinal herbs, milk, cheese, tree nuts, and traditional medicines.[Citation15−Citation18] Forty-one aflatoxin detection methods have been published by the Association of Official Analytical Chemists (AOAC); the European Committee for Standardization (CES) has reported six aflatoxin detection methods.[Citation8] Currently, the methods for aflatoxin detection include TLC, ELISA, and HPLC. TLC is the most commonly used method;[Citation19] however, it is not specific or sensitive for AFB1.[Citation20] ELISA is rapid, simple, and inexpensive;[Citation21] however, ELISA is subject to interference by mycotoxins, fat, tea polysaccharides, and tea pigments. As a result, aflatoxin concentration by the direct dilution method is considerably higher. Even though these interferences can be resolved with an organic phase extraction, this pre-treatment and purification process is very complex and results in AFB1 losses and poor recovery rates. Compared with ELISA, HPLC has a high resolution, good accuracy, high sensitivity, and low detection limits. In this study, the HPLC method resulted in high recovery rates (81.02–90.69%) and in accurate determinations of AFB1, AFB2, AFG1, and AFG2. Therefore, as reported in a previous study, ELISA results need to be confirmed by HPLC.[Citation8]
The results of this study revealed that raw and ripe Pu-erh tea contained AFB1 (8.333 and 20.149 μg/kg, respectively), raising concerns about the effects of aflatoxins on human health. Aflatoxins are readily soluble in solvents (e.g., chloroform, methanol, and dimethyl sulfoxide) and dissolve in water at 10–20 mg/L. Further studies are needed to assess the potential effects of aflatoxins in Pu-erh tea on human health. To our knowledge, few countries have set maximum permissible limits for aflatoxins in tea. In India, the maximum permissible limit is 30 μg/kg; in China, the maximum permissible limit is 5–20 μg/kg depending on the food matrix (GB 2761-2011). The results of this study revealed that the aflatoxin level in Pu-erh tea from Yunnan was within national permissible levels, but considerable higher than those allowed in the European Union, the USA, and Japan.[Citation22]
Chinese food exports tend to have high aflatoxin levels. Several strategies, including biological control and development of resistant cultivars, have been implemented to control aflatoxin in crops. Among them, biological control appears to be the most promising approach.[Citation23] Pu-erh tea is becoming a popular beverage around the world; the presence of aflatoxins may hinder its marketability. Prevention and control of aflatoxins in Pu-erh tea, at the pre- and post-harvest levels, are required to improve its quality and safeguard the Pu-erh tea industry. In summary, an HPLC method was developed for the detection of aflatoxins in Pu-erh tea. Aflatoxin was detected in Pu-erh tea originating from the province of Yunnan, China.
FUNDING
This work was supported by the National Key Technology Research (Grant No. 2007BAD58B05) and the Department of Finance of Yunnan Province (Grant No. A3003063).
REFERENCES
- Cao Z.H.; Gu, D.H.; Lin, Q.Y.; Xu, Z.Q.; Huang, Q.C.; Rao, H.; Liu, E.W.; Jia, J.J.; Ge, C.R. Effect of pu-erh tea on body fat and lipid profiles in rats with diet-induced obesity. Phytotherapy Research 2011, 25 (2), 234–238.
- Zhao, L.; Jia, S.; Tang, W.; Sheng, J.; Luo, Y. Pu-erh tea inhibits tumor cell growth by down-regulating mutant p. 53. International Journal of Molecular Sciences 2011, 12 (11), 7581–7593.
- Hou, Y.; Shao, W.; Xiao, R.; Xu, K.; Ma, Z.; Johnstone, B.H.; Du, Y. Pu-erh tea aqueous extracts lower atherosclerotic risk factors in a rat hyperlipidemia model. Experimental Gerontology 2009, 44 (6–7), 434–439.
- Su, Y.; Zhang, C.; Wang, Y.; Li, P. Antibacterial property and mechanism of a novel Pu-erh tea nanofibrous membrane. Applied Microbiology and Biotechnology 2012, 93 (4), 1663–1671.
- Abe, M.; Takaoka, N.; Idemoto, Y.; Takagi, C.; Imai, T.; Nakasaki, K. Characteristic fungi observed in the fermentation process for Pu-erh tea. International Journal of Food Microbiology 2008, 124 (2), 199–203.
- Murphy, P.A.; Hendrich, S.; Landgren, C.; Bryant, C.M. Food mycotoxins: An update. Journal of Food Science 2006, 71 (5), R51–R65.
- Lee, D.J.; Wales, J.H.; Sinnhuber, R.O. Promotion of aflatoxin-induced hepatoma growth in trout by methyl malvalate and sterculate. Cancer Research 1971, 31 (7), 960–963.
- Reiter, E.; Zentek, J.; Razzazi, E. Review on sample preparation strategies and methods used for the analysis of aflatoxins in food and feed. Molecular Nutrition & Food Research 2009, 53 (4), 508–524.
- Braga, S.M.; De Medeiros, F.D.; De Oliveira, E.J; Macedo, R.O. Development and validation of a method for the quantitative determination of aflatoxin contaminants in Maytenus ilicifolia by HPLC with fluorescence detection. Phytochemical Analysis 2005, 16 (4), 267–271.
- Pirestani, A.; Tabatabaei, S.N.; Fazeli, M.H. Comparison of HPLC and Elisa for determination of aflatoxin concentration in the milk and feeds of dairy cattle. Journal of Research in Agricultural Science 2011, 7 (1), 71–78.
- Zhou, H.; Li, J.; Zhao, L.; Han, J.; Yang, X.; Yang, W.; Wu, X. Study on main microbes on quality formation of yunnan pu-erh tea during pile-fermentation Process. Journal of Tea Science 2004, 24 (3), 212–218.
- Chen, K.; Zhu, H.; Wang, D.; Zhang, Y.; Yang, C. Isolation and identification of aspergillus 13 species from the post fermentative process of pu-er ripe tea. ACTA Botanica Yunnanica 2006, 28 (2), 123–126.
- Storari, M.; Dennert, F.G.; Bigler, L.; Gessler, C.; Broggini, G.A.L. Isolation of mycotoxins producing black aspergilli in herbal teas available on the Swiss market. Food Control 2012, 26 (1), 157–161.
- Dutta, B.K.; Dutta, S.; Nath, P.K. Mycotoxin production potential of mycoflora in tea. Economic Crisis in Tea Industry. Studium Press, LLC: Houston, TX, 2008; 221–232.
- Viswanath, P.; Nanjegowda, D.K.; Govindegowda, H.; Dattatreya, A.M.; Siddappa, V. Aflatoxin determination in black tea (Camellia Sinensis)-status and development of a protocol. Journal of Food Safety 2012, 32 (1), 13–21.
- Zareen, S.; Ismail, I.S.; Shaari, K.; Yusof, K.N.; Akhtar, M.N. Quantitative HPLC analysis of benzene derivatives of melicope ptelefolia leaves. International Journal of Food Properties 2013, 16 (8), 1830–1838.
- Ahmad, R.S.; Butt, M.S.; Huma, N.; Sultan, M.T.; Arshad, M.U.; Mushtaq, Z.; Saeed, F. Quantitative and qualitative portray of green tea catechins (GTC) through HPLC. International Journal of Food Properties 2014, 17 (7), 1626–1636.
- Gul, O.; Dervisoglu, M. Occurrence of aflatoxin M1 in vacuum packed kashar cheeses in Turkey. International Journal of Food Properties 2013, 17 (2), 273–282.
- Dugan, E. Detection of aflatoxins by TLC. Biotechnology Lab 1989, 7 (5), 46–48.
- Liang, Z.; Huang, J.; Chen, W.; Liao, H. Methods for detection of aflatoxin B1. Occupation and Health 2003, 19 (3), 41–42.
- Liu, D. Determination of aflatoxin B1 by enzyme-linked immunosorbent assay in food. Packaging and Mechanical 2002, 23 (10), 78–80.
- Farombi, E.O. Aflatoxin contamination of foods in developing countries: Implications for hepatocellular carcinoma and chemopreventive strategies. African Journal of Biotechnology 2006, 5 (1), 1–14.
- Yin, Y.N.; Yan, L.Y.; Jiang, J.H.; Ma, Z.H. Biological control of aflatoxin contamination of crops. Journal of Zhejiang University Science B (Biomedicine & Biotechnology) 2008, 9 (10), 787–792.