Abstract
The morphology and granular properties of oat starch during germination were investigated by scanning electron microscopy and X-ray powder diffractometer. The granule size distribution of oat starch was studied in various concentrations in ethanol using a laser particle-size analyzer. The starch granule shape before germination remained almost the same after germination, as shown by low-magnification (×1000) scanning electron microscopy. However, surface fissures at high-magnification (×5000) were found on the sample at the end of germination. After soaking, the relative crystallinity of the samples significantly reduced, by following increased and then decreased, reaching a maximum crystallinity of 16% at the end of germination. In various ethanol concentrations, the size of starch granules produced in different germination phase slightly varied, but this difference was not easy to detect in the scanning electron microscope ×1000 images. Overall, the particle size of oat starch generally decreased and the specific surface area increased significantly during oat germination.
INTRODUCTION
In 1997, the Food and Drug Administration approved oats as a functional food. Since then, oats have attracted increasing attention from consumers. More and more new snacks and foods based on oats were developed.[Citation1,Citation2] Many studies report that the important functional nutrients of oats are in their bran, such as beta glucan[Citation3] and avenanthramide.[Citation4,Citation5] Oat bran accounts for 35% of the whole oat grain, whereas oat flour accounts for 65%.[Citation6] Therefore, the larger consumer demands for oat bran result in higher production of oat flour. Oat starch utilization has been extensively investigated, except in the case of food applications. Oat starch can also be used as a raw material to manufacture thin film[Citation7] and auxiliary materials for paper making.[Citation6]
Crystal properties of starch have an important effect on enzymatic hydrolysis. Studies on these properties are critical to understanding starch digestion behavior.[Citation8] It is well known that cereal starch presents A-type semi-crystals in its natural state, based on its X-ray diffraction pattern. The granule structure of starch is associated not only with its gelatinization, aging, and crystallization properties, but also with its effects on the melt rheology, mechanical properties, thermal behavior, and biological degradation of thermoplastic starch.[Citation9] Thus, understanding granule morphology and basic properties of starch is important.
Studies on starch granule size always involved extraction of dry starch from a variety of materials, or obtaining samples by wet-heat treatment or other modification methods. The application of starch is often dependent on the solution state: Changes in the solution environment affect the properties of starch. Investigators have realized these problems and have carried out many studies, especially those on the effect of pH, electrolyte, additives, and so forth. However, studies examining the effects of solution polarity changes on the properties of starch are relatively few. As a starting point, this study examined the size of dissolved oat starch granules with various concentrations in ethanol.
MATERIALS AND METHODS
Oat Materials
Oat seeds, cultivar Avena nuda L., were harvested from Shanxi Province, Northern China, in 2008. The naked oat (variety name Jin Yan VIII) was supplied by Dr. Li Gang from the Institute of Alpine Plants of Shanxi Academy of Agricultural Sciences. After removing small and broken kernels, oat seeds were collected and stored at –20°C until further processing. The germination activity of the oat seeds was determined to exceed 99% through the test. All chemicals used in the present study were of analytical grade.
Malting Procedures
The malting procedure based on Beta’s method[Citation10] with some modifications. Oat seed surfaces were sterilized by soaking them in 2.0% aqueous sodium hypochlorite for 15 min at room temperature, and they were then rinsed with tap water for at least 20 min. Before germination, the oat seeds were soaked in distilled water at 16°C, in the dark for 24 h. After rinsing with distilled water, the steeped oat seeds were spread on wet cellulose pads and left to sprout at 16°C in a dark incubator for 0, 24, 48, 72, 96, 124, or 144 h.
Oat Starch Extraction
Raw oat was crushed directly by a plant-splintering machine and then passed through an 80-mesh sieve. A 0.01 M NaOH solution added to the crushed sample at a ratio of 1:10 (w/v). The mixture was soaked and stirred for 1 h at room temperature, and then the oat bran was removed by screening through a 200-mesh bolting cloth. The slurry was centrifuged at 3000 rpm for 10 min, and the supernatant and precipitate from the upper layer of the dark material were removed. The rest of the precipitate was washed repeatedly with 0.01 M NaOH solution and then centrifuged to remove all dark impurities. White starch slurry was obtained by adding water, rinsing with 0.5 M HCl solution, and adjusting the pH until it is neutral after washing it with water three times and centrifuging. Subsequently, the precipitate was dried overnight at 40°C in an oven. The dry starches obtained were defatted by the Soxhlet extraction method. Afterward, the starch samples were dried naturally, and the above processing (initial soaking, centrifugation, washing process) was repeated to remove the protein. Finally, the wet starch was dried again, and then ground into fine powder passable through a 160-mesh sieve. The difference in the starch extraction approaches between the raw and germinated oat was only that the fresh germinated oats were added directly to 0.01 M NaOH solution and crushed in a grinder, and the other follow-up extraction process was the same as that for raw oats.
Scanning Electron Microscopy (SEM)
Starch samples were collected with a toothpick, spread evenly on a loading stage, and then sprayed with gold by conventional methods (gold/palladium, 60:40). The processed samples were placed directly in the sample observation room for SEM (X-650, HITACHI), and then pictures were taken. The accelerating voltage during the scanning process was 20 kV.
X-Ray Powder Diffraction
Crystallographic patterns of oat starches were recorded using an X-ray diffractometer (Rigaku D/max-RA III, Japan) equipped with Ni-filtered Cu Ka radiation and operating at 35 kV and 30 mA. Data were recorded over an angular range of 5 to 50° (2θ) with a step angle of 0.02° under scanning speed of 8°/min. The degree of starch crystallinity was calculated using Jade 5.0 software.[Citation11]
Particle Size Analysis
The particle size distribution of starch was determined using a laser particle-size analyzer (BT-9300H, Bettersize Instruments Ltd.) using different concentrations of ethanol (30, 50, 70, and 90%; v/v) as the dispersion medium. The starch suspension concentration was 0.5% (w/v). Vortex and ultrasonic vibration were used to facilitate uniform distribution of starch granules at the room temperature. Suspension droplets were added to the dispersion slot as soon as possible, and the granule size distribution of the oat starch in a 1 cm sample cell were measured according to the instrument guide.[Citation12]
RESULTS AND DISCUSSION
Oat Starch Micro-Morphology
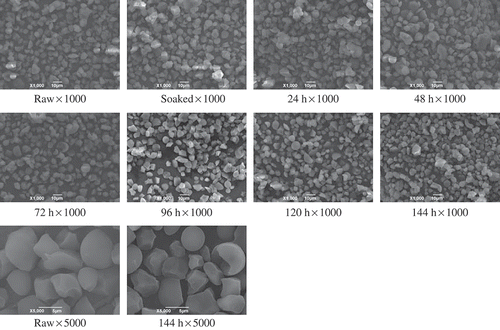
It can be seen in , oat starch granules showed a variety of forms after germination, including polygonal, oval, semi-spherical, and semi-polygonal. Regardless of the granule shape, the surface was almost regular and smooth with no cracks, gaps, or holes. Most of the particles had diameters below 10 μm. In addition, we can also see some small particles aggregating together to form larger clumps. These results agree with the findings of other researchers.[Citation13−Citation15] After 144 h germination of oats, no evidence of changes in the starch micromorphology could be observed with SEM at ×1000 magnification. Clearly, intact samples were found in other germination levels as well. Salmenkallio-Marttila et al. also found that after 3 days of germination, the starch granules were still intact.[Citation16] However, when the SEM magnification was increased to ×5000, it was seen that at the end of germination, the surface of the starch granules became slightly rough compared with that of raw oat starch; the granule size also decreased. From the start of germination to the end, the mean long diameter and short diameter of oat starch granules decreased from 7.48 ± 0.79 to 6.56 ± 0.66 μm and from 5.74 ± 0.52 to 4.92 ± 0.39 μm, respectively, according to software calculations.
X-Ray Powder Diffraction Properties of Oat Starch
Natural starch is a polymer with specific stiffness in the semi-crystalline state. The crystalline nature of starch is affected by factors such as mechanical strength, heat treatment, and enzymatic hydrolysis. Intense mechanical grinding would break natural starch granules, resulting in lattice defects and reduction of the degree of crystallinity. Theoretically, the phase of starch was changed into amorphous structure and the crystallinity was zero when the semi-crystalillinity of starch was totally destroyed by grinding, but the grinding operation is not possible to achieve this ideal state.[Citation9] Heat treatment can cause the starch granules to collapse resulting in crystal disruption; in this case, the peak shapes of the X-ray diffraction patterns become dispersed. In addition, enzymes also affect the crystalline properties of starch. In an experiment,[Citation17] the different hydrolysis procedures and different sources of enzymes were employed to investigate the effect of enzymatic hydrolysis on starch physicochemical properties. The results of this experiment confirmed that enzyme treatment could reduce the degree of crystallinity of all hydrolyzed starch samples of A-type crystals. However, B- and C-types weakened upon enzymatic hydrolysis at 60°C and completely disappeared at 100°C.
shows that regardless of whether germination occurred, oat starch X-ray diffraction patterns were similar. The main crystalline peaks are at 2θ of 15.0°, 17.0°, 18.0°, 20.0°, and 23.0°, which are typical of the A-type pattern. The peak shape is relatively sharp, indicating a high degree of crystallinity. The highest intensity is near 17° and 18°, and the corresponding d values are 5.8, 5.2, and 5.0 nm and around 3.8 nm, respectively. This result agrees with the findings of Hoover and Senanayake.[Citation18] In this study, the difference in the X-ray diffraction patterns between raw and sprouted oats is the change in the shapes and relative intensity of peaks 2θ near 17° and 18°. The oat starch crystallinity calculated by MDI Jade 5.0 software showed in . The degree of crystallinity of all samples ranged from 8.8 to 16.0%, while the minimum corresponded to the soaked sample and the maximum was that of the sample germinated for 144 h. This can explain that endogenous enzyme hydrolysis of amorphous region is a priority comparing to crystallization region, and the hydrolysis process of the later is a transition process of starch structure from orderly to amorphous. So, the part crystalline region could be protected after the amorphous region is formed. Although, the total starch content decreased with the germination, the starch crystallinity not necessarily reduced.
TABLE 1 The crystal properties of starches from raw and germinated oat by X-ray diffraction patterns
The results of this study showed that endogenous amylase of living organisms under growth conditions can have a certain effect on the degree of crystallinity of starch, but not enough to produce obvious changes in the crystal type and the diffraction peak position. The soaking process, compared to the follow-up germination, affected the degree of crystallinity of starch to a maximum extent. These suggested that the effect of endogenous enzyme hydrolysis on the nature of starch crystallinity in vivo is weaker than exogenous enzyme and mechanical grinding.
Oat Starch Particle Size Distribution
Using 30, 50, 70, or 90% ethanol as the dispersing medium, the size distribution of oat starch particles was measured by laser particle-size analyzer. As and show, the volume and area mean diameter of oat starch were signally reduced after germinated for 96 h compare with those of native oat starch in all experimental ethanol concentrations, whereas the specific surface area observably increased. This change was due to the endo-enzymatic activity on the surface of starch granules and at parts of the loose starch granules during oat germination. Therefore, after oat germination, starch granules shrunk and did not swell easily in ethanol. Overall, particle of less than 2 μm diameter present almost only in samples germinated for 120 and 144 h in 30, 50, and 70% ethanol (120 h in 50% ethanol not detected). The particle sizes of the other test samples were no less than 2 μm (). The number of particles with sizes below 10 μm in 70% ethanol accounted for less than 10%, whereas in other tested ethanol concentration, this number exceeds 40% (such as 120 h samples in 30% ethanol).
FIGURE 3 Effects of ethanol concentration on granule size distribution of starches isolated from raw and germinated oat.
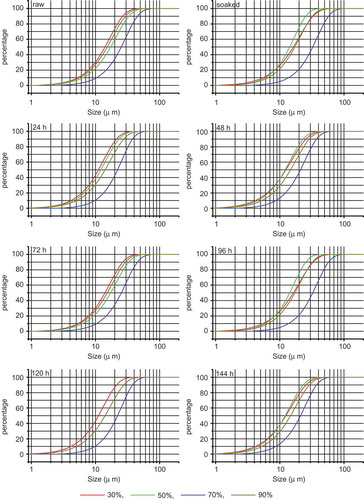
TABLE 2 Granules Characterization of oat starches in different concentration of ethanol*
For native or germinated oat starch samples, the minimum D50 rendered is over 12.42 μm, regardless of ethanol concentration. This is greater than the maximum average particle diameter observed by electron microscopy. One of the possible reasons for this is that the starch granules imbibed the ethanol solvent in water under the experimental conditions. Depending on the changes of ethanol concentration, the starches imbibition capacity demonstrated difference, so the size of the same samples in different ethanol concentration was different (). The average maximum size for each sample responded to 70% ethanol, and the particle size was smaller in other concentration ethanol than in 70% ethanol. For each sample in 30% ethanol, particles of less than 10 μm accounted for about 30% of all particles; if these particles were used as the control, the imbibition became more apparent with the smallest D50 of 13.54 μm when the ethanol concentration was increased to 50% and with the smallest D50 of 23.42 μm at 70% ethanol concentration. With the increased concentration of ethanol, the starch granules reached the largest size, but the particles size was smaller in 70% ethanol than in 90% ethanol. The size order of starch granules in the other ethanol concentrations is uncertain; for example, granules of some samples were larger in 30% ethanol than in 50% ethanol, and the granules of other samples showed an opposite sequence. In addition to the molecular structure of starch, this may have been related to the particle’s own internal gap.
The structure of starch granules in germinated oats was not alike at the different germination times. The starches were dispersed in the ethanol solution, and the size difference between starch particles increased with imbibition. Thus, it was difficult to detect the changes in the size of the germination samples by SEM, but it was relatively easy by using the laser particle-size analyzer. For oat starch in 30, 50, and 90% (v/v) ethanol, the particle size first decreased, then increased, and subsequently decreased again, respectively, with the germination time. However, after germination, sample particle sizes were less than that of the raw sample (except for the soaked sample). In particular, samples at the late germination stage had significantly smaller sizes than those of unmalted samples. The changes in starch particles size were irregular during the first half of germination, suggesting that physiological processes and metabolism were very complex during germination.
The specific surface area is a comprehensive reflection of the parameters of the particle size distribution, surface structure, and internal voids of aggregates. It could be used effectively to measure the activity and adsorption capacity of the starch granules during chemical reactions. The greater the specific surface area, the stronger is adsorption capacity, and the higher is the reactivity. As can be seen in , the specific surface area of starch first decreased and then increased with increasing ethanol concentration. In 70% ethanol, the starch surface area was smallest, and the maximum surface area appeared in 50% ethanol, which corresponded with the changes in particle size. In 50% ethanol, the surface area of starch from oat germinated for 144 h increased to approximate 100 m2/kg, whereas starch from raw oat reached 236.28 m2/kg.
The changes in volume-average diameter and area-average diameter of starch particles are shown in . These changes were similar to those of D50, and volume-average diameter of all samples > D50 > area average diameter. This indicated the asymmetry distribution of starch granule size.
Original starch could be transformed into soluble starch by acid and alcohol treatment, while generating the least amount of low-molecular-weight dextrin. Ma and Robyt successfully prepared soluble starch from potato and waxy-maize starches by acid hydrolysis in different alcohols.[Citation19] All of the modified starches were readily soluble in hot water, and their molecular weights decreased progressively from methanol-modified starches to 1-butanol modified starch. The modified starches showed uniform granular appearance. Most studies on alcohol and acid-modified starches used anhydrous ethanol acidified with HCl; some used alcohol in various proportions.[Citation20−Citation22] Chang et al. also attempted using different alcohol concentrations.[Citation23] Chun et al. treated rice starch with ethanol containing various concentrations of 2–4% HCl.[Citation24] The results showed that in the same acid concentration, the degree of starch hydrolysis and the average degree of polymerization of modified starch were reduced as the alcohol concentration increased. It is postulated that acid hydrolysis is not of the same degree in different alcohol solution concentrations. Navdeep et al. observed that degradation of amylopectin to low molecular weight molecules was due to hydrolysis caused by acid-alcohol treatment.[Citation25] This may be attributed to the fact that alcohols are breaking the hydrophobic and hydrogen bonds that occur between the double helical starch chains in the granule, thereby disrupting the crystalline micelles and unwinding the double helices so that acid can penetrate into the interior of the helix and hydrolyze the glycosidic linkages. Along with the formation of limit dextrins, there are physically different “kinds” of acid susceptible a-(1→4) glycosidic linkages in the starch granules and that the alcohols are inducing the different kinds of linkages to become more chemically susceptible to acid hydrolysis. This suggested that the alcohols are affecting something more than the concentration of acid inside the granules.[Citation21] Although this study did not involve the acidification in the case of starch hydrolysis, the ethanol concentration caused changes in the size of starch granules; such changes can facilitate the interpretation of the results of the above study.
CONCLUSIONS
The morphology and granular diffraction properties of oat starch during germination were investigated. Additionally, the particle size distributions were studied. The shape of starch granules was almost intact, as shown by low-magnification SEM. However, surface fissures were found on the end germination sample under high magnification. Germination could affect the changes in two peak shapes of the starch X-ray diffraction pattern (at 2θ 17° and 18°), but the crystal form did not change. After soaking, the relative crystallinity of the samples was significantly reduced, and the starch crystallinity increased then decreased, reaching a maximum crystallinity of 16% at the end of germination. In various concentrations of ethanol, the sizes of starch granules produced in different germination phases differed slightly, but this difference was not easy to detect with the naked eye in the scanning electron microscope images. Overall, the particle size of oat starch generally decreased and the specific surface area increased significantly during oat germination.
FUNDING
This work was sponsored by the national support program of the 11th Five-Year Plan of the ministry of science and technology, P.R. China (Grant Number 2006BAD27B09). We thank the senior researchers Chengxiong Li and Gang Li for supplying high-quality oat grains for this research project.
Additional information
Funding
REFERENCES
- Liu, S.W.; Alavi, S.; Abughoush, M. Extruded moringa leaf-oat flour snacks: Physical, nutritional, and sensory properties. International Journal of Food Properties 2011, 14, 854–869.
- Giannini, A.N.; Krokida, M.K.; Bisharat, G.I. Structural properties of corn-based extrudates enriched with plant fibers. International Journal of Food Properties 2013, 16, 667–683.
- Drzikova, B.; Dongowski, G.; Gebhardt, E.; Habel, A. The composition of dietary fibre-rich extrudates from oat affects bile acid binding and fermentation in vitro. Food Chemistry 2005, 90 (1–2), 181–192.
- Nie, L.; Wise, M.L.; Peterson, D.M.; Meydani, M. Avenanthramide, a polyphenol from oats, inhibits vascular smooth muscle cell proliferation and enhances nitric oxide production. Atherosclerosis 2006, 186 (2), 260–266.
- Skoglund, M.; Peterson, D.M.; Andersson, R.; Nilsson J.; Dimberg, L.H. Avenanthramide content and related enzyme activities in oats as affected by steeping and germination. Journal Cereal Science 2008, 48 (2), 294–303.
- Lim, W.J.; Liang, Y.T.; Seib, P.A.; Rao, C.S. Isolation of oat starch from oat flour. Cereal Chemistry 1992, 69, 233–236.
- Galdeano, M.C.; Mall, S.; Grossmann, M.V. Effects of plasticizers on the properties of oat starch films. Materials Science and Engineering: C 2009, 29, 532–538.
- Singh, V.; Gopalkrishne, U.R.; Somashekarppah, A.S.Z.; Somashekar, R. X-ray analysis of different starch granules. Bulletin of Materials Science 1995, 18 (5), 549–555.
- Wu, J.; Xie, B.J.; Xiong, H.G. Effect of particle size of starch on the properties of thermoplastic starch. Transactions of the Chinese Society of Agricultural Engineering 2003, 19, 37–40. (in Chinese)
- Beta, T.; Rooney, L.W.; Waniska, R.D. Malting characteristics of sorghum cultivars. Cereal Chemistry 1995, 72 (6), 533–538.
- Pablo, D.R.; Silvia, C.; Alberto, E.L.; María, C.A. The staling of bread: An X-ray diffraction study. European Food Research and Technology 2004, 218 (3), 219–223.
- Wang, L.; Yin, Z.H.; Zhang, Y.; Xie, B.J.; Sun, Z.D. Morphological, physicochemical and textural properties of starch separated from Chinese water chestnut. Starch 2008, 60 (3–4), 181–191.
- Lineback, D.R. The starch granule—organization and properties. Bakers Digest 1984, 58 (2), 16–21.
- Hoover, R.; Vasanthan, T. Studies on isolation and characterisation of starch from oat (Avena nuda) grains. Carbohydrate Polymers 1992, 19 (4), 285–297.
- Hartunian-Sowa, M.; White, P.J. Characterization of starch isolated from oat groats with different amount of lipid. Cereal Chemistry 1992, 69, 521–527.
- Salmenkallio-Marttila, M.; Heiniö, R.L.; Myllymäkl, O. Relating microstructure, sensory, and instrumental texture of processed oat. Agricultural and Food Science 2004, 13, 124–137.
- Gorinstein, S.; Lii, C.Y. The effects of enzyme hydrolysis on the properties of potato, cassava, and amaranth starches. Starch 1992, 44 (12), 461–466.
- Hoover, R.; Senanayake, S.P.J.N. Composition and physicochemical properties of oat starches. Food Research International 1996, 29 (1), 15–26.
- Ma, W.P.; Robyt, J.F. Preparation and characterization of soluble starches having different molecular sizes and composition, by acid hydrolysis in different alcohols. Carbohydrate Research 1987, 166 (2), 283–297.
- Yiu, P.H.; Loh, S.L.; Rajan, A.S.; Wong, C.; Bong, C.F.J. Physiochemical properties of sago starch modified by acid treatment in alcohol. American Journal Applied Science 2008, 5 (4), 307–311.
- Robyt, J.F.; Choe, J.Y.; Fox, J.D.; Hahn, R.S.; Fuchs, E.B. Acid modification of starch granules in alcohols: Reactions in mixtures of two alcohols different ratios combined in different ratios. Carbohydrate Research 1996, 283, 141–150.
- Lin, J.H.; Lee, S.Y.; Chang, Y.H. Effect of acid-alcohol treatment on the molecular structure and physicochemical properties of maize and potato starches. Carbohydrate Polymers 2003, 53 (4), 475–482.
- Chang, Y.H.; Lin, J.H.; Lii, C.Y. Effect of ethanol concentration on the physicochemical properties of waxy corn starch treated by hydrochloric acid. Carbohydrate Polymers 2004, 57 (1), 89–96.
- Chun, J.; Lim, S.; Takeda, Y.; Shoki, M. Properties of high-crystalline rice amylodextrins prepared in acid-alcohol media as fat replacers. Cereal Foods World 1997, 42 (10), 813–819.
- Navdeep, S.S.; Chang, Y.H.; Nimratbir, K.; Kaoru, K. Effect of acid–methanol treatment on the molecular structure and physicochemical properties of lentil (Lens culinaris Medik) starch. Food Hydrocolloids 2009, 23, 2219–2225.