Abstract
Fifty different kinds of wheat from 10 provinces in China were chosen as samples to be milled into flour. Dumpling wrappers were made from the flour and their texture qualities and rheological properties, disulfide content, sulfhydryl content, and gluten secondary structure were determined. The web structure formed by the gluten was observed under scanning electron microscopy. These results showed that the flour’s rheological properties, apart from extensibility, were significantly correlated with several aspects of the texture of the dumpling wrapper, and the degree of softening showed negative correlations, most closely with the texture quality of the dumpling wrapper. Disulfide played an important role in forming the gluten web, its content showing significant correlations with the rheological properties of the flour and the texture quality of the dumpling wrapper. The disulfide content in gluten was positively correlated with the hardness and quality of the cooked product; the sulfhydryl content showed no significant correlations with the rheological properties of the flour and the texture of the dumpling wrapper. The gluten secondary structure showed some correlation with the rheological properties of the dough, but little with the texture quality of the dumpling wrapper. Under observation by scanning electron microscopy, the weak structure was loose with large interspaces, while the strong structure was compact with few interspaces.
INTRODUCTION
Wheat is one of the most important arable crops with great financial and economic significance. Wheat grain is a staple food over most of the world with the special rheological properties of gluten allowing wheat flour to be processed into a multitude of food and non-food products.[Citation1] Wheat flour is composed of carbohydrates (70–80%, of which starch is the major component), proteins (8–15%), fat (2–3%), and water (10–12%). When used for food preparation, wheat flour is first mixed with water in the presence of air to form dough. Generally, flour doughs are considered as viscoelastic liquids with elastic properties attributed to the non-covalently bound gluten network.[Citation2] Because of this unique characteristic, wheat flour can be processed into many food products such as bread, biscuits, noodles, and dumplings. Characterizing the rheological properties of dough is effective in predicting its processing behavior and for controlling the quality of food products. The farinograph, mixograph, and extensograph are the most common empirical instruments used for characterizing dough rheology.[Citation3] A gluten network is formed when its protein fractions are hydrated with the input of mechanical energy, processes that are responsible for the rheological properties of dough.[Citation4] Wheat gluten consists mainly of two protein types: Gliadin, which is of low molar mass and soluble in ethanol and gluten in which is of high molar mass and insoluble in ethanol.[Citation5] It is considered that glutenin is the main contributor to foam elasticity, whereas the gliadins contribute to viscous behavior.[Citation6]
The structure of the gluten network is the basis of its characteristics. The precise changes occurring in dough during mixing are still not completely understood, but an increase in dough stiffness occurs that is generally considered to result from “optimization” of the protein–protein interactions within the gluten network. In molecular terms, this “optimization” may include some exchange of disulfide bonds resulting in different effects on the sulfydryl and disulfide content of dough.[Citation7] Both hydrogen bonding and the presence of disulfide linkages have been shown to be important factors in gluten rheology.[Citation8] A high disulfide content in flour increases the toughness and texture of flour products as the free thiol content decreases.[Citation9] The elasticity of gluten maybe due to the presence of an unusual secondary structure that is similar to that previously described for elastin.[Citation10]
Recently, Fourier transform infrared (FTIR) spectroscopy has been used to examine the secondary structure of gluten in dough under various conditions.[Citation11–Citation13] FTIR spectroscopy is an ideal technique for investigating the state of water in dough by monitoring changes in the OH stretch band at 3000–3800 cm–Citation1 and simultaneously, alterations in the gluten structure by monitoring changes in the amide I band at 1600–1700 cm−Citation1.[Citation14–Citation16] A detailed study has also measured the changes in secondary structure in gluten during deformation by biaxial extension,[Citation13] which were consistent with the predictions of “the loop and train model,” which proposes that the extension of gluten should result in changes in protein conformation and that these should take the form of an increase in the β-sheet content.[Citation17]
The dumpling is a traditional food in China and the quality of its wrapper is very important. Food texture is considered to be of increasing importance: it has been defined as “the sensory and functional manifestation of the structural, mechanical, and surface properties of foods detected through the senses of vision, hearing, touch, and kinesthetic.”[Citation18] Compression, bending, and tensile tests have traditionally been used to investigate the mechanical and fracture properties of food.[Citation19] The study is more about the influence of gluten structure and rheological properties of wheat flour dough to the bread[Citation20] and noodle quality,[Citation21] but it is seldom to the dumpling wrapper quality. In this study, 50 different kinds of wheat flour from 10 provinces in China were analyzed for the texture qualities of the dumpling wrappers made from the flour, their rheological properties, disulfide content, sulfhydryl content, and the secondary structure of the gluten. The aim of the present study was to investigate the link between the rheological properties of the wheat flour and gluten structure and the quality of the dumpling wrapper.
MATERIALS AND METHODS
Flour Samples
Fifty wheat samples from 10 provinces in China were used in this study. The wheat samples were cleaned, tempered overnight to a moisture content of 15.5%, and milled on a Buller laboratory mill.
Flour Rheological Properties
For the determination of flour water absorption, dough development time, dough stability time, degree of softening, and quality number, the standard International Association for Cereal Science and Technology (ICC) methods were followed. The extension energy, extension resistance, dough extensibility, and curve configuration ratio were also determined according to the standard ICC methods.
Dumpling Wrapper Preparation
Wheat flour (200.0 ± 5.0 g) and distilled water (80 mL) were placed in the kneading bowl of a pin-type dough making machine. The dough was then kneaded for 2 min and composite rolled three times in a pressing machine (2.4-mm roller spacing). The rolled dough was sealed in a plastic bag and laid aside for 20 min at room temperature, then rolled again three times with a decreasing roller space of 1.4, 1.2, and1.0 mm. The final thickness of the wrapper was 1.0 ± 0.05 mm.
Determination of the Texture of the Boiled Dumpling Wrapper
The texture of the boiled dumpling wrapper was analyzed using a TA.XT2i Texture Analyzer (Stable MicroSystems Ltd., Godalming, Surrey, UK) equipped with a 25 kg load cell, and analyzed using version 2.64 Texture Expert Exceed software. Three dumpling wrappers (5 cm diameter) were put into 500 mL distilled water, boiled for 3.5 min, removed, and then cooled in distilled water for 1 min. Then the wrapper samples were placed in the Texture Analyzer to analyze the hardness, springiness, cohesiveness, chewiness, and resilience. The test parameters were as follows: pre-test speed: 1.00 mm/s, test speed: 0.80 mm/s, post-test speed: 0.80 mm/s, target mode: strain, distance: 10.000 mm, strain: 70.00%, time: 3.00 s, trigger type: auto(force), trigger force: 5.0 g, trigger distance: 2.000 mm.
Determination of the Toughness of the Raw Dumpling Wrapper
The toughness of the raw dumpling wrapper was measured using a TA.XT2i texture analyzer (Stable Micro Systems Ltd.) equipped with a spherical probe (SMSP/1SP). The test parameters were as follows: test mode: compression, pre-test speed: 1.00 mm/s, test speed: 2 mm/s, pre-test speed: 0.00 mm/s, trigger mode: distance, distance: 140 mm.
Determination of the Firmness of the Boiled Dumpling Wrapper
The dumpling wrapper (7 × 3 cm) was boiled for 3.5 min then cooled in distilled water for 1 min. The samples were then placed in the texture analyzer for analysis. The test parameters were as follows: test mode: compression, pre-test speed: 1.00 mm/s, test speed: 0.80 mm/s, pre-test speed: 10.00 mm/s, trigger mode: force, force: 1000.0 g, distance: 5.000 mm, strain: 100.00%, trigger force: 5.0 g, trigger distance: 2.000 mm.
Determination of the Gluten Sulfhydryl and Disulfide Content
The gluten was extracted from the flour by a MS500 gluten washer (Jinan Xucheng Import & Export Co. Ltd., Shandong, China), then frozen and dried before milling into a powder. A 75 mg sample was suspended in 1 mL of Tris-Gly buffer, 4.7 g of Gu HCl was added, and the volume made up to 10 mL with the Tris-Gly buffer. For the sulfhydryl determination, 4 mL of Urea-Gu HCl was added to 1 mL of this slightly turbid solution and then 0.05 mL of Ellman’s reagent was added. For disulfide determination, 0.05 mLof 2-mercaptoethanol and 4 mL of Urea-Gu HCl was added to 1 mL of the flour or gluten solution and the mixture was incubated for 1 h at 25°C. After further 1 h incubation with 10 mL of 12% trichloroacetic acid (TCA), the samples were placed in tubes and centrifuged at 5000 × g for 10 min. The precipitate was twice re-suspended in 5 mL of 12% TCA and centrifuged again to remove 2-mercaptoethanol. The precipitate was dissolved in 10 mL of 8M urea in Tris-Glyand the color was developed using 0.04 mL of Ellman’s reagent.
Determination of the Secondary Structure of Gluten
FTIR spectroscopic measurements were carried out following the method of Wellner et al.[Citation17] FTIR spectra (32 scans) were recorded at a resolution of 4 cm–Citation1 on a Perkin Elmer Spectrum™ 100 FTIR spectrometer equipped with a universal ATR attachment. All raw spectra (range of 400–4000 cm–Citation1) were normalized using sigmalot (version 11.0). Curve fitting was performed using the grams/32 (version 5) software with Gaussian–Lorenzian mix function to examine the amide bands in the region 1600–1700 cm−Citation1of the FTIR spectra. All spectra obtained were subjected to a multipoint linear base-line correction using the OMNIC software (version 7.3, Thermo Electron Co-operation). The relative peak areas of the absorbance bands were expressed as “Percentage of the area of fitted region.” The characteristic mean absorption frequencies of the secondary structural elements in proteins are listed in .[Citation22,Citation23]
TABLE 1 Mean absorption frequencies of various secondary structure elements in proteins[Citation21]
Preparation of the Gluten Samples for Scanning Electron Micrographs (SEMs)
The gluten samples for SEM examination were prepared as described by Kim et al.[Citation24] with slight modification. Fifty percent of 4.0% NaCl solution was added to the gluten and knead with a metal spatula for 5 min and manually for further 5 min. They were then frozen (–70°C) and freeze-dried. The freeze-dried pieces of the samples were fractured into sizes of about 1 × 1 × 0.5 cm using a knife and a hammer. The interior surface of the samples was exposed to gold sputtering. The preparations were then viewed and photographed with Quanta-200 SEM apparatus (JSM 6480LV, JEOL, Japan).
Statistical Analysis
The data were analyzed with analysis of variance (ANOVA; one way) in order to determine statistically significant differences (p < 0.05) among the samples. This was accomplished, employing the software SPSS (version 17.0 for Windows). All the determinations were done in triplicate.
RESULTS AND DISCUSSION
Correlation between dough rheological properties and dumpling wrapper texture quality. The dough rheological properties and the dumpling wrapper texture qualities of the 50 samples from 10 provinces are showed in Appendices 1 and 2. The correlation between dough rheological properties and dumpling wrapper texture qualities are summarized in . The analytical results show that the dough development time was significantly correlated with firmness (r = 0.286*), texture profile analysis (TPA) hardness (r = 0.393**), chewiness (r = 0.390**), and toughness (r = 0.287*); the stability time was significant correlated with firmness (r = 0.383**), TPA hardness (r = 0.423**), and chewiness (r = 0.420**); the degree of softening showed an inverse relationship with firmness (r = −0.545**), TPA hardness (r = −0.463**), chewiness (r = −0.465**), and toughness (r = −0.517**); the farinograph parameters were significantly correlated with firmness, TPA hardness, chewiness, and toughness; among the extensograph parameters, the extension resistance and the extension ratio were significantly correlated with firmness, TPA hardness, chewiness, and toughness. To get an overview of the variability of the data, the degree of softening was most highly correlated with the dumpling wrapper firmness, the TPA parameters and the toughness. The sulfhydryl and disulfide content of the 50 samples from 10 provinces are showed in the Appendix. The correlations between disulfide and sulfhydryl content and dumpling wrapper texture quality are summarized in . The analytical results show that the disulfide content was significantly correlated with the farinograph parameters and with the extensograph parameters except for dough extensibility.
TABLE 2 Correlation between dough rheological properties and dumpling wrapper texture qualitiesa
TABLE 3 Correlation between disulfide and sulfhydryl content and rheological propertiesa
The correlation between disulfide and sulfhydryl content and rheological properties are summarized in . The analytical results show that the disulfide content was significantly correlated with firmness (r = 0.382**), hardness (r = 0.326**), chewiness (r = 0.321*), resilience (r = 0.332*), and toughness (r = 0.407*).
TABLE 4 Correlation between disulfide and sulfhydryl content and dumpling wrapper texture qualitya
These results agree with those of Manu and Prasada Rao,[Citation9] that the disulfide content in gluten is correlated with the firmness quality of wheaten food but sulfhydryl content shows no significant correlations with the rheological properties of wheat and the texture of the dumpling wrapper. This could be because gliadins consist of a heterogeneous mixture of monomeric proteins while glutenins are composed of several subunits crosslinked via inter-chain disulfide bridges.[Citation25] It is generally considered that the hardness and springiness of dough are determined by the amount of glutenin in gluten.[Citation26] Ewart[Citation27] pointed out that elasticity is related to the formation of long disulfide-bonded chains of glutenins. It is now widely accepted that disulfide-linked glutenin chains provide an “elastic backbone” to gluten using evidence from studies using nuclear magnetic resonance and FTIR spectroscopy.[Citation28] It can, therefore, be shown that disulfide plays an important role in the formation of glutenin structure and that the disulfide content of dough influences both the rheological properties of the dough and the dumpling wrapper texture quality.
Secondary Structure of Gluten
The gluten secondary structure of the 50 samples from 10 provinces are showed in the Appendix. The results of the correlation between gluten secondary structure and rheological properties are shown in . This shows that the α-helix and random coil showed no significant correlations with rheological parameters; the β-sheet was significantly correlated with dough stability time (r = 0.377**), degree of softening (r = −0.481**), and quality number (r = 0.390**); β-turn was negatively correlated with dough stability time (r = −0.398**) and quality number (r = −0.413**), but significantly positively correlated with the degree of softening (r = 0.471**). Belton[Citation17] proposed the well-known “loop and train model” as the basis for gluten structure. In this model, gluten chains remain linked in parallel through covalent bonds at low hydration levels. At higher levels of hydration, β-turn changes into the β-sheet in the gluten system as these inter-chain hydrogen bonds break forming open areas, especially between glutamine and water. These changing bonds in gluten bring about a balance between the hydrated zone and zones connected by hydrogen bonds, which is helpful to the production of gluten.
TABLE 5 Correlation between gluten secondary structure and rheological propertiesa
A more detailed study by Wellner et al.[Citation13] measured the changes in secondary structure that occurred in gluten during deformation by biaxial extension. It was observed that the biaxial extension caused an increase in β-sheet conformers in the gluten. The results are consistent with the predictions of the loop and train model,[Citation17] in that the extension of gluten should result in changes in protein conformation and that these should take the form of an increase in the β-sheet content. Pézolet et al.[Citation29] determined the proportions of various secondary structures in gluten and compared this with the content in liquid determined by the circular dichroism (CD) spectrum. It appears that the solubilization of gluten proteins resulted in a large decrease in the amount of β-sheet structures accompanied by an increase in the content of the β-turn and α-helical conformations. These results highlighted for the first time the presence of the β-sheet conformation in domains where intermolecular interactions between the protein polypeptide chains in functional gluten exist. These studies have shown that the dry proteins are disordered with little regular structure, but that their mobility increases and β-sheet structures form on hydration. Further changes occur if hydration continues, with a further increase in protein mobility and the formation of turn-like structures at the expense of the β-sheet.[Citation30]
The correlations between gluten secondary structure and dumpling wrapper texture quality are shown in . The secondary structure in gluten showed little correlation with the texture quality of the dumpling wrapper. The α-helix was significantly correlated with resilience (r = −0.348*), and the β-sheet was significantly correlated with firmness (r = 0.318*). All these correlations may come from the complex transformation of secondary structure changes in gluten during the dough rolling and boiling steps.
TABLE 6 Correlation between gluten secondary structure and dumpling wrapper texture qualitya
The correlations between the disulfide and sulfhydryl content and gluten secondary structure are shown in . The disulfide content was significantly positively correlated with the β-sheet (r = 0.354*) and negatively with the β-turn (r = 0.325*). The inherent ability of glutenin to form disulfide bonds is thought to be determined by the primary and secondary structure of the proteins, which determines whether cysteine residues are present and available to form disulfide bonds, if the capacity of a subunit to fold in the manner that would be required to form the bond is sufficient and if the elasticity of the subunit once in the polymer can provide the viscoelastic properties for the dough.[Citation31]
TABLE 7 Correlation between disulfide and sulfhydryl content and gluten secondary structurea
Gluten Scanning Electron Microscopic Observations
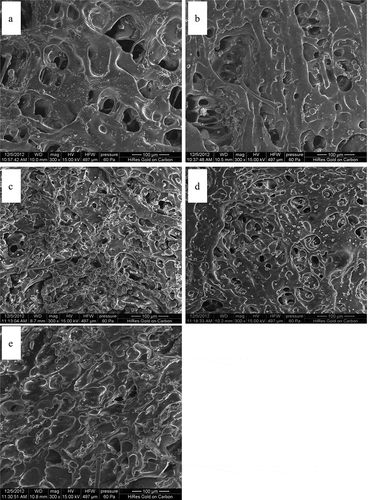
To observe the gluten microstructure, five cultivars were chosen from the 50 samples: their rheological properties are shown in . and their gluten secondary structure, sulfhydryl, and disulfide contents in . shows the gluten microstructure of the five cultivars. From the images, the structures in and appear relatively loose, with interspaces larger and deeper than . However, compared with , the interspaces in are relatively well proportioned and smaller. In and , the gluten webs are relatively compact and proportioned with small interspaces. The structure in is more compact, with a flat surface and no clear interspace, appearing more like a sheet structure. These obvious network structures provide an interpretation of how the discrepancies in gluten quality are influenced by different gluten forces. When the gluten structure is weak, it is easy to rupture and malleable because of its loose structure and relatively large interspaces. When it is strong, it is hard to rupture and not easily shaped because of its compact structure and relatively small interspaces.
TABLE 8 The rheological properties of the five cultivarsa
TABLE 9 The gluten secondary structure and sulfhydryl and disulfide content of the five cultivars
CONCLUSION
The rheological properties of flour, apart from extensibility, were significantly correlated with several aspects of dumping wrapper texture and the degree of softening was negatively correlated, most closely with the texture quality of the dumpling wrapper. Disulfide plays an important role in formation of the gluten web, its level showing relatively significant correlations with the rheological properties of flour and the texture quality of the dumpling wrapper. The disulfide content in gluten was positively correlated with the cooked dough qualities of hardness and quality; the sulfhydryl content was not correlated with the flour rheological properties and texture of the dumpling wrapper. The secondary structure showed some correlation with rheological properties, but little with the texture quality of the dumpling wrapper. The α-helix showed highly significant correlations with resilience, the β-sheet was significantly correlated with firmness. The reasons for this were the transformations in the secondary structure of gluten caused by rolling and boiling the dough. Under observation by scanning electron microscopy, the weak gluten was loose with large interspaces, it was easy to roll and rupture; while the strong gluten was compact and with few interspaces, so it was hard to roll and rupture.
FUNDING
This study was financially supported by the National Natural Science Foundation of China (U1304331), the National Science and Technology Support Program Project Funds (no. 2012BAD37B04), and the Henan Key Science and Technology Project (112102110016).
Additional information
Funding
REFERENCES
- Lagrain, B.; Goderis, B.; Brijs, K.; Delcour, J.A. Molecular Basis of Processing Wheat Gluten Toward Biobased Materials. Biomacromolecules 2010, 11, 533–541.
- MacRitchie, F. The Liquid Phase of Dough and Its Role in Baking. Cereal Chemistry 1976, 53, 318–326.
- Song, Y.; Zheng, Q. Dynamic Rheological Properties of Wheat Flour Dough and Proteins. Trends Food Science & Technology 2007, 18, 132–138.
- Jiang, B.; Kontogiorgos, V.; Kasapis, S.; Goff, H.D. Rheological Investigation and Molecular Architecture of Highly Hydrated Gluten Networks at Subzero Temperatures. Journal of Food Engineering 2008, 89, 42–48.
- Thomson, N.H.; Miles, M.J.; Popineau, Y.; Harries, J.; Shewry, P.R.; Tatham, A.S. Small Angle X-Ray Scattering of Wheat Seed-Storage Proteins: α-, γ-, and ω-Gliadins and the High Molecular Weight (HMW) Subunits of Glutenin. Biochimica Biophysica Acta—Protein Structure and Molecular Enzymology 1999, 1430, 359–366.
- Xu, J.; Bietz, J.A.; Carriere, C.J. Viscoelastic Properties of Wheat Gliadin and Glutenin Suspensions. Food Chemistry 2007, 101, 1025–1030.
- Tsen, C.C.; Bushuk, W. Changes in Sulphydryl and Disulphide Contents of Doughs During Mixing Under Various Conditions. Cereal Chemistry 1963, 40, 399–408.
- Belton, P.S. The Molecular Basis of Dough Rheology. In Bread Making, Improving Quality; Cauvain, S.P.; Ed.; Woodhead: Cambridge, MA, 2003; 273–287.
- Manu, B.T.; Prasada Rao, U.J.S. Influence of Size Distribution of Proteins, Thiol, and Disulfide Content in Whole Wheat Flour on Rheological and Chapati Texture of Indian Wheat Varieties. Food Chemistry 2008, 110, 88–95.
- Venkatachalam, C.M.; Urry, D.W. Development of a Linear Helical Conformation from Its Cyclic Correlate β-Spiral Model of the Elastine Poly (Pentapeptide) (VPGVG) n. Macromolecules 1981, 14, 1225–1229.
- Li, W.; Dobraszczyk, B.J.; Dias, A.; Gil, A.M. Polymer Conformation Structure of Wheat Proteins and Gluten Subfractions Revealed by ATR-FTIR. Cereal Chemistry 2006, 83, 405–410.
- Mejia, C.D.; Mauer, L.J.; Hamaker, B.R. Similarities and Differences in Secondary Structure of Viscoelastic Polymers of Maize A-Zein and Wheat Gluten Proteins. Journal of Cereal Science 2007, 45, 353–359.
- Wellner, N.; Mills, E.N.C.; Brownsey, G.; Wilson, R.H.; Brown, N.; Freeman, J. Changes in Protein Secondary Structure During Gluten Deformation Studied by Dynamic Fourier Transform Infrared Spectroscopy. Biomacromolecules 2005, 6, 255–261.
- Dashnau, J.L.; Nucci, N.V.; Sharp, K.A.; Vanderkooi, J.M. Hydrogenbonding and the Cryoprotective Properties of Glycerol/Water Mixtures. Journal of Physical Chemistry B 2006, 110, 13670–13677.
- Scott, J.N.; Nucci, N.V.; Vanderkooi, J.M. Changes in Water Structure Induced by the Guanidinium Cation and Implications for Protein Denaturation. Journal of Physical Chemistry A 2008, 112, 10939–10948.
- Zelent, B.; Vanderkooi, J.M. Infrared Spectroscopy Used to Study Ice Formation: The Effect of Trehalose, Maltose, and Glucose on Melting. Analytical Biochemistry 2009, 390, 215–217.
- Belton, P.S. Mini Review: On the Elasticity of Wheat Gluten. Journal of Cereal Science 1999, 29, 103–107.
- Szczesniak, A.S. Texture Is a Sensory Property. Food Quality and Preference 2002, 13, 215–225.
- Luyten, H.; van Vliet, T.; Walstra, P. Comparison of Various Methods to Evaluate Fracture Phenomena in Food Materials. Journal of Texture Studies 1992, 23, 245–266.
- Gómez, A.; Ferrero, C.; Calvelo, A.; Añón, M.C.; Puppo, M.C. Effect of Mixing Time on Structural and Rheological Properties of Wheat Flour Dough for Breadmaking. International Journal of Food Properties 2011, 14, 583–598.
- Barak, S.; Mudgil, D.; Khatkar, B.S. Effect of Compositional Variation of Gluten Proteins and Rheological Characteristics of Wheat Flour on the Textural Quality of White Salted Noodles. International Journal of Food Properties 2014, 17, 731–740.
- Dong, A.; Huang, P.; Caughey, W.S. Protein Secondary Structures in Water from Second-Derivative Amide I Infrared Spectra. Biochemistry 1990, 29, 3303–3308.
- Kong, J.; Yu, S. Mini-Review: Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochmica et Biophysica Sinica 2007, 39, 549–559.
- Kim, H.J.; Morita, N.; Lee, S.H.; Moon, K.D. Scanning Election Microscopic Observations of Dough and Bread Supplemented with Gastrodia Elata Blume Powder. Food Research International 2003, 36, 387–397.
- Wieser, H. Chemistry of Gluten Proteins. Food Microbiology 2007, 24, 115–119.
- Wang, Y.G.; Khan, K.; Hareland, G.; Nygard, G. Quantitative Glutenin Composition from Gel Electrophoresis of Flour Mill Streams and Relationship to Breadmaking Quality. Cereal Chemistry 2006, 83, 293–299.
- Ewart, J.A.D. Re-Examination of the Linear Glutenin Hypothesis. Journal of the Science of Food and Agriculture 1977, 28, 191–199.
- Belton, P.S.; Colquhoun, I.J.; Field, J.M.; Grant, A.; Shewry, P.R.; Tatham, A.S.; Wellner, N. FTIR and NMR Studies on the Hydration of a High-Mr Subunit of Glutenin. International Journal of Biological Macromolecules 1995, 17, 74–80.
- Pézolet, M.; Bonen fant, S.; Dousseau, F. Conformation of Wheat Gluten Proteins: Comparison Between Functional and Solution States Determined by Infrared Spectroscopy. FEBS Letters 1992, 299, 247–250.
- Shewry, P.R.; Halford, N.G.; Belton, P.S.; Tatham, A.S. The Structure and Properties of Gluten: An Elastic Protein from Wheat Grain. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 2002, 357, 133–142.
- Shewry, P.R.; Sayanova, O.; Tatham, A.S.; Tamas, L. Structure, Assembly, and Targeting of Wheat Storage Proteins. Journal of Plant Physiology 1995, 145, 620–625.
APPENDIX
Table A1 The dough rheological properties of the 50 samples from 10 provinces in China
Table A2 The dumpling wrapper texture qualities of the 50 samples from 10 provinces in China
Table A3 The sulfhydryl and disulfide content and gluten secondary structure of the 50 samples from 10 provinces in China