Abstract
Desalting is an important process of bone collagen extraction. In this present study, collagens were prepared from tilapia bone using acetic acid and pepsin. The extracted collagen was partially characterized. Prior to extraction, tilapia bone was desalted by soaking in ethylenediaminetetraacetic acid solution and hydrochloric acid. Thermo-gravimetric analysis, X-ray diffraction, Fourier transform infrared spectroscopy, and scanning electron microscopy were performed to analyze the structural characteristics. The extracted protein was classified as collagen by Fourier transform infrared spectroscopy, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and circular dichroism. Results showed that desalination with HCl was fast, but collagen yield was low. No acid-soluble collagen and only 0.5% pepsin-soluble collagen were obtained from HCl-treated fish bone. By comparison, the yields of acid-soluble collagen and pepsin-soluble collagen from ethylenediaminetetraacetic acid-treated fish bone were 3.5 and 6.0%, respectively. These values were observed higher than those from HCl-treated fish bone. In HCl-treated fish bone, the properties of collagen, such as amino acid composition, denaturation temperature, and molecular weight distribution, were different from those of collagen in ethylenediaminetetraacetic acid-treated fish bone. Collagens extracted from ethylenediaminetetraacetic acid-treated fish bone showed more integrated secondary structure. Therefore, ethylenediaminetetraacetic acid can be used more effectively than HCl to extract collagen from tilapia bone.
INTRODUCTION
Collagen is a primary protein from animals and has a wide range of applications in various industries, including leather, film, pharmaceuticals, cosmetics, food, and biomedical materials.[Citation1] Collagen is usually extracted from the skin and bones of vertebrate species, particularly pigs and cattle. In recent years, studies on collagen from aquatic animals have increased[Citation2−Citation5] because of the safety of collagen from aquatic sources compared with livestock sources. However, these studies have concentrated on various aquatic animals, and many aquatic animals have very low populations.[Citation6,Citation7] Therefore, these sources cannot support the requirements of the collagen industry. Nevertheless, tilapia is a satisfactory candidate for farming because this fish can provide high amounts of inexpensive protein. Tilapia has also been considered as the second most important farm-raised fish. China is among the largest tilapia producers worldwide. In 2010, the tilapia produced in China accounted for roughly 55% of approximately three million tons of tilapia produced worldwide. Tilapia meat is also exported from China to America and Europe. Although waste products from tilapia processing have been used initially, large amounts of fish heads and skeletons are disposed of every year.
Tilapia bone can be a useful source of collagen and gelatin. Bone is a complex tissue composed of a fibrous type I collagen scaffold, in which hydroxyapatite (HA) crystals [(Ca)10(PO4)6(OH)2] are deposited.[Citation8] The collagen in the bone is difficult to extract if HA is partially removed. Nagai and Suzuki[Citation9] used an ethylenediaminetetraacetic acid (EDTA) solution alone to remove HA from the bone for 5 d. Weak HCl solution (0.25 to 1.0 M), which is commonly used in collagen extraction in archeology and gelatin production, is possibly more useful and effective than EDTA solution. Urist et al.[Citation10] used 0.6 mol/L HCl to remove HA from the bone. Other researchers extracted collagen directly by using 0.5 mol/L acetic acid.[Citation11] A modified traditional method used to remove minerals from fish bone by using 0.6 mol/L HCl solution[Citation12] has been applied in fish bone gelatin extraction. To determine a more effective desalination method, a 0.5 mol/L EDTA solution and 0.6 mol/L HCl was used. In particular, these solutions were used to remove HA from tilapia bone in the current study. The desalting methods and the properties of tilapia bone collagen extracted using these methods were then compared.
MATERIALS AND METHODS
Materials and Pretreatment
Tilapia skeletons containing bones, residual meat, fins, and small portions of skins were provided by Guangxi Nanning Baiyang Food Co., Ltd. (Guangxi Zhuang Autonomous Region, China) and stored at –20°C. The tilapia skeletons were thawed at room temperature before use. Residual meat, fins, and small portions of skins in the skeletons were removed using a scalpel at 0°C. The bones were then extracted using 0.1 mol/L NaOH to remove non-collagenous proteins and washed with distilled water. Afterward, the bones were filtered, washed with tap water, dried at room temperature, crushed to obtain a small diameter by a crusher (Tianjin Taisite Instrument Co., Ltd., China), and passed through a 40-mesh screen. The insoluble bone was decalcified with 0.5 mol/L EDTA (pH 7.4, 4 C) for 9 d; the EDTA solution was changed once each day.[Citation9] The residue and the bone were washed with distilled water (4°C). Afterward, the bone was lyophilized. Another part of the insoluble bone was treated with 0.6 mol/L HCl for 9 h at 4°C, and the acid solution was changed at an interval of 1.5 h.[Citation12] After the acid solution was removed, the bone was washed again with distilled water (4°C) until a neutral pH was obtained and then lyophilized. Uncrushed opercular bones of tilapia were desalinated using the two methods to compare surface structures and microstructures.
Collagen Extraction from Tilapia Bone
Dry, desalinated bone powder (10 g) was extracted with 200 mL of 0.5 mol/L acetic acid (1 g of bone powder per 20 mL acetic acid) for 24 h. The soaking solution was centrifuged at 5,000 × g for 20 min at 4°C. The residues were mixed and re-extracted with 0.5 mol/L acetic acid for 24 h. The re-extracted residues were then centrifuged under the same conditions. The supernatants were combined and salted out by adding NaCl at a final concentration of 0.9 mol/L. The precipitated acid-soluble collagen (ASC) was separated by centrifugation at 10,000 × g for 20 min at 4°C and subsequently re-dissolved in 0.5 mol/L acetic acid. Salting out and solubilization were repeated three times. The residues were collected and extracted with 200 mL of 0.5 mol/L acetic acid and 1000 U pepsin (1200 U/g, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) at 4°C for 96 h. The viscous solution was centrifuged at 10,000 × g for 20 min at 4°C. Pepsin-soluble collagen (PSC) was obtained using the same method as in ASC preparation. The precipitated collagens (ASC and PSC) were dialyzed against distilled water and then lyophilized. Collagen yield was calculated as follows: yield (%) = weight of dried collagen (g) × 100/weight of tilapia bone powder used (g). The acid solution used in the removing of bone minerals were collected and centrifuged at 10,000 × g for 20 min at 4°C. The supernatant was adjusted to pH 6 using 0.1 mol/l NaOH with stiring, then was dialyzed against distilled water using dialysis tubings which had a molecular weight cut-off of 1000 Da, and then lyophilized.
Thermogravimetric Analysis (TGA) of Bone
TGA was performed to quantitatively estimate the inorganic content by using Mettler-Toledo TGA/SDTA851e. The untreated bone powder was used as the control sample for TGA. The TGA of the demineralized bone powders was also performed after the samples were collected at different time intervals (3, 6, and 9 d for EDTA; 2, 3, and 9 h for HCl) to determine the extent of demineralization. Approximately 10 mg of the sample was treated with heat from room temperature to 700°C at a heating rate of 20°C min−1 under nitrogen flow.[Citation13] All of the graphs were plotted on the basis of temperature (°C, X-axis) against residue (%, Y-axis).
X-ray Diffraction (XRD)
The XRD patterns of xerogels were obtained using a DX-2700 X-ray diffractometer (Dandong Haoyuan Instrument Co., Ltd, China) equipped with Cu Kα radiation (λ = 0.15406 nm) and operated at 40 kV and 40 mA. Data were recorded in an angular range of 2θ (3 to 90°) at a rate of 4° min−1 and a step size of 0.02°. The divergence slit (DS), scatter slit (SS), and receiving slit (RS) was set at 1, 1, and 0.1 mm. Each sample was analyzed once. MDI Jade 5.0 was used to analyze the diffractograms.
Fourier Transform Infrared Spectroscopy (FTIR)
Bone and collagen samples were analyzed by the attenuated total reflectance FTIR. The spectra were obtained using a Nicolet Nexus FTIR spectrometer (Thermo Electron Corporation) with an absorption mode at intervals of 4 cm−1 and 16 times scanning frequency at the wave number region from 600 to 4000 cm−1.[Citation14] HA (Sinopharm Chemical Reagent Co., Ltd, China) was used to determine the peak of the phosphate anion.
Scanning Electron Microscopy (SEM)
The bone samples were gold-coated using a sputter coater (BAL-TEC, SCD 005; Witten, Germany) prior to observation. The SEM micrographs of each specimen were obtained using a scanning electron microscope (QUANTA-200, FEI).
Amino Acid Analysis
The collagen samples were hydrolyzed in 6 mol/L HCl at 110°C for 22 h, and the hydrolysates were analyzed using an amino acid analyzer (HP1100, Agilent, USA).
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
SDS-PAGE was performed using 50 and 40 g/L resolving and stacking gels, respectively. The samples (10 μL) were applied and subjected to electrophoresis. Afterward, the gel was stained with Coomassie Brilliant Blue R-250. Type I collagen and protein standards for SDS-PAGE were obtained from Boster Biology Engineering Industry Co., Ltd. (Wuhan, China).
Circular Dichroism (CD) Analysis
CD spectrometry was performed to assess the secondary structure of collagen by using a Bio-Logic MOS-450 CD spectrometer (Bio-Logic, France). The samples were dissolved in 0.5 mol/L acetic acid to obtain a 0.125 mg/mL concentration. CD measurements were then obtained at 15, 20, 25, 30, 35, 40, 45, 50, and 60°C by using a 1 mm path-length quartz cell at wavelengths ranging from 190 to 260 nm, and a scan speed of 100 nm min−1 at an interval of 1 nm. Data were accumulated three times. Each sample was maintained at the scanning temperature for 10 min before scanning. Collagen denaturation temperatures (Td) were determined on the basis of the rotary angle at a fixed wavelength of 223 nm at 15, 20, 25, 30, 35, 40, 45, 50, and 60°C.[Citation15] Td was determined as the temperature at which the change in ellipticity (h) was half complete.
RESULTS AND DISCUSSION
XRD
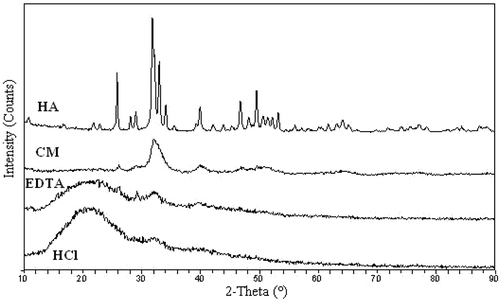
XRD was performed to analyze untreated and desalinated tilapia bone. The untreated bone showed similar XRD patterns of HA (), in which an intense peak of approximately 32.01° corresponds to the reflection of a crystallographic plane. By contrast, HCl- and EDTA-treated samples presented very low peak intensities of approximately 32.03 and 32.76°. However, broad reflections at 22.7° associated with the single left-hand helix chain of protein were observed in the X-ray diffractogram of HCl- and EDTA-treated samples. This result indicated that HA content decreased in HCl- and EDTA-treated bones. Simultaneously, the corresponding d001 value of EDTA-treated bone (2.76 Å), which represents the basal spacing of phosphate, was lower than that of HA (2.81 Å), untreated (2.79 Å), and HCl-treated bone (2.79 Å). This finding indicated that the structure of HA lattice became more compact. A possible explanation was that calcium ions in HA lattice were removed or replaced with other ionic compounds during desalting with EDTA.
FTIR of Tilapia Bone
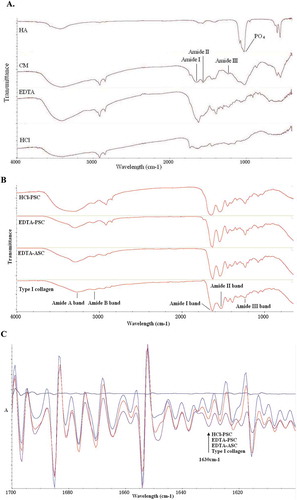
FTIR was performed to analyze the desalting methods of tilapia bone. In , FTIR images of PO43– band was found at 1030 cm−1 which is contained in hydroxylapatite.[Citation16] However, the peaks of the phosphate anion were difficult to determine in the infrared spectra of desalinated bones. These results indicated that the content of HA decreased in HCl- and EDTA-treated bones. In addition, the amide I of HCl-treated bone was evidently weaker and wider than that of EDTA-treated bone. The result also suggested that the secondary structure in collagen was partially broken because the amide I band is the characteristic coil structure of collagen.[Citation17]
FIGURE 2 (a) FTIR spectra of hydroxyapatite (HA), tilapia bone (CM), EDTA treatment tilapia bone (EDTA), and HCl treatment tilapia bone (HCl); (b) Fourier transform infrared spectrum of HCl-PSC, EDTA-PSC, EDTA-ASC, and type I collagen; (c) Second derivative of amide I band from FTIR spectra of HCl-PSC, EDTA-PSC, EDTA-ASC, and type I collagen.
TGA of Bone
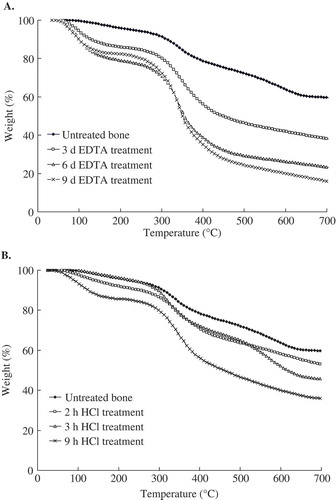
The thermal degradation of intact and desalted tilapia bones is presented in . All of the samples showed a two-step transition. The first notable weight loss occurred at approximately 90°C and was associated with the removal of physically absorbed water. The second transition, which corresponds to collagen decomposition, was observed at approximately 350°C. The total ash content in the intact tilapia bone was 55.2 g/100 g bone, in which the moisture content of the bone was 12.3 g/100 g bone. The desalination method of EDTA was effective (). Approximately 15% residue was obtained from the EDTA-treated sample for 9 d. Considering the time involved, 9 d was selected as the desalination time. Weak HCl solution (0.25 to 1.0 M) was more effective in desalination than other treatments. However, the desalting effect of 0.6 mol/L HCl was not as satisfactory as expected (). This effect may be appropriate for gelatin extraction.
SEM
shows the SEM micrographs of the surface morphology of intact and desalted opercular bones of tilapia. In contrast to intact opercular bone with a smooth surface, HCl-treated opercular bone showed a considerably rough surface with huge cracks and holes. By comparison, EDTA-treated opercular bone showed a slightly rough surface. The inner microstructure of intact bone was compacted and homogeneous. The section of opercular bone treated with EDTA showed a layered structure with cracks, tiny holes, and fibers. By contrast, HCl-treated opercular bone contained a mass of sponge-like pores. Greater damage was observed in HCl-treated opercular bones than in EDTA-treated opercular bones.
Yields of Collagen
The yield of EDTA-ASC (extracted from tilapia bone desalinated with EDTA) was 2.5%, which was lower than that of EDTA-PSC (7.3%; extracted from tilapia bone desalinated with EDTA). However, no HCl-ASC (extracted from tilapia bone desalinated by HCl) was obtained in this study. In addition, the yield of HCl-PSC (extracted from tilapia bone desalinated by HCl) was only 0.5%. These results could be attributed to collagen that was degraded during desalination with HCl.
Amino Acid Composition of Collagen
The amino acid composition of collagen samples are shown in . In this study, glycine was the most abundant amino acid (361 per 1000 residues, EDTA-ASC; 366 per 1000 residues, EDTA-PSC). The amount of glycine in tilapia bone was also higher than that in black drum bone (338 per 1000 residues) and sheephead bone (342 per 1000 residues),[Citation3] as well as the mean content of cold water fish bone collagen (340 per 1000 residues) and warm water fish bone collagen (337 per 1000 residues).[Citation18] Half cysteine was found in low amounts in tilapia bone collagen. Methionine, histidine, and tyrosine were observed in amounts similar to the amount of collagen from other fish species.[Citation3,Citation18] The total imino acid contents (160 per 1000 residues, EDTA-ASC; 156 per 1000 residues, EDTA-PSC) were lower than the mean in warm water fish bone collagen.[Citation18] The degrees of proline hydroxylation were approximately 50% of EDTA-ASC and EDTA-PSC; these results are higher than those in warm water fish bone collagen (41%).[Citation18] Hydroxyproline contents (80 per 1000 residues in EDTA-ASC; 78 per 1000 residues in EDTA-PSC) were similar to those in warm water fish bone collagen (76 per 1000 residues)[Citation18] but lower than those in black drum bone (85 per 1000 residues) and sheephead bone (88 per 1000 residues).[Citation3] HCl-PSC showed a different amino acid composition from EDTA-ASC and EDTA-PSC. HCl-PSC contained less glycine (306 per 1000 residues). In particular, the total imino acid content of HCl-PSC was higher than that of other collagens.
TABLE 1 Mean amino acid compositions for tilapia bone collagen (residues/1000 residues)a
FTIR of Collagen
FTIR is applied to analyze the differences in protein structure. The amide A band position in type I collagen was 3272 cm−1 () because of the hydrogen-bonded hydroxyl groups. The amide B band was found at 3068 cm−1, where the band always appears in collagen.[Citation19] The spectrum of type I collagen dispersions also demonstrated the characteristic pattern that corresponds to the amide I band at 1634 cm−1, the amide II band at 1532 cm−1, and the amide III band at 1241 cm−1.[Citation20] The amide I band is the characteristic coil structure of collagen[Citation17] and the amide III band indicates the presence of a helical structure.[Citation21,Citation22] The FTIR curves of EDTA-ASC, EDTA-PSC, and HCl-PSC are similar to those of type I collagen (). The shift of the amide A band positions of the HCl-PSC (3330 cm−1) to higher frequencies was also observed. This result indicated that the hydrogen bonds in HCl-PSC decreased considerably. Furthermore, the differences between the FTIR spectra of tilapia bone collagens and type I collagen may be due to the difference in the organisms used. The amide I band positions of the EDTA-ASC, EDTA-PSC, and type I collagen were the same (1634 cm−1) but greatly differed from HCl-PSC (1650 cm−1). This result indicated that the content of the secondary structure in HCl-PSC decreased. The second derivative was used to visualize the amide I band region spectrum. Prystupa and Donald[Citation23] reported that the peaks near 1630, 1652, and 1660 cm−l indicate related random coil regions, single α-helix, and triple helix, respectively. EDTA-ASC, EDTA-PSC, and type I collagen showed the corresponding characteristic peaks of a secondary structure (). However, HCl-PSC showed an almost straight spectral line of the second derivative. The result showed that HCl-PSC exhibits only a partially ordered secondary structure.
SDS-PAGE
The SDS-PAGE patterns of EDTA-ASC and EDTA-PSC are shown in . Two distinct species were observed at different positions and mobility of the α regions of the samples. These patterns indicated that tilapia bone collagens are trimers containing two distinct α chains (α1 and α2) that are similar to those of a typical triple-helix structure of type I collagen with two distinct α chains. This finding indicated that EDTA-ASC and EDTA-PSC are type I collagen. The molecular weights of α1 and α2 EDTA-ASC and EDTA-PSC subunits were approximately 110 kDa and <100 kDa, respectively, which are different from those of carp bone collagen (α2 is exactly 116 kDa),[Citation24] alligator (Alligator mississippiensis) bone collagen (α1 and α2 chains are 123 and 110 kDa, respectively),[Citation11] backbone collagen of Baltic cod (α1 and α2 chains are visible near 116 kDa),[Citation25] and black drum and sheephead sea bream bone collagen (α1 and α2 chains are 130 and 110 kDa, respectively).[Citation3] Moreover, high molecular weight components were observed in EDTA-ASC and EDTA-PSC, including β and γ components.
FIGURE 5 The SDS-PAGE pattern of EDTA-ASC, EDTA-PSC, and HCl-PSC. Lane 1: EDTA-ASC; Lane 2: EDTA-PSC; Lane 3: standard protein; Lane 4: type I collagen; Lane 5: residues in acid solution changed at 1.5 h; Lane 6: residues in acid solution changed at 3.0 h; Lane 7: residues in acid solution changed at 4.5 h; Lane 8: HCl-PSC. Molecular weights from top to bottom in the standard protein are 300, 250, 180, 130, 100, and 70 kDa.
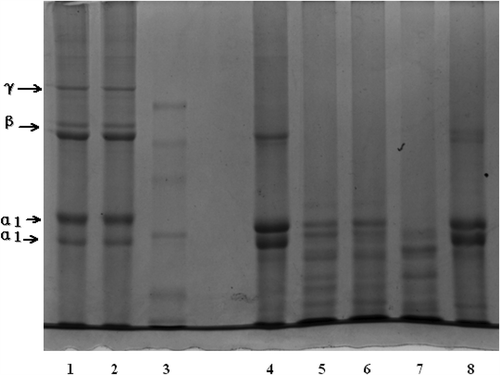
HCl-PSC contained higher proportions of β components with molecular weights of >200 kDa, peptides with molecular weights (<α-chains, whose molecular weights were approximately 100 kDa) of <97 kDa were also found. These results indicated that a greater degradation of α-chains was observed in HCl-PSC than in other collagens. Moreover, HCl-PSC was not a collagen similar to gelatin or collagen peptides. The SDS-PAGE pattern of residues in acid solution used in the removing of bone minerals is also shown in . Higher proportions, such as β and γ components, were not found. On the contrary, a lot of fragments of peptides were determined. This result indicated that a part of collagen in the bone was dissolved and degraded in HCl solution during desalting.
Thermal Properties
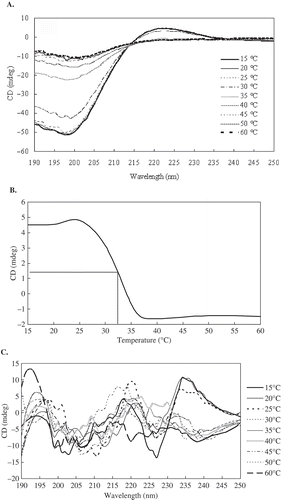
The CD spectra of the protein solutions provide information regarding the secondary structure of proteins.[Citation26] The CD spectrum of EDTA-PSC was in agreement with earlier reported spectra.[Citation27] EDTA-PSC exhibited the characteristic CD spectra with negative peaks at 199 nm and positive peaks at 223 nm (). These EDTA-PSC spectral characteristics are distinctive of the collagen triple helix.[Citation28] A zero rotation point was observed at approximately 213 nm, which is a characteristic of the triple helical conformation of this protein.[Citation29] Td of EDTA-PSC was 32.5°C (). Td of EDTA-PSC is lower than that of collagen from land animals, such as porcine skin collagen (37°C)[Citation6] and calf skin collagen (37°C).[Citation3] Furthermore, Td of EDTA-PSC is lower than that of alligator (A. mississippiensis) bone collagen (38.2°C),[Citation11] black drum bone collagen (35.7°C), and sheephead bone collagen (34.8 °C).[Citation3] By comparison, Td of EDTA-PSC is higher than that of carp bone collagen (28°C).[Citation23] Td decreased as the number of imino residues in the collagen decreased, and a strong linear correlation was observed between the degree of crosslinking and the hydroxyproline content.[Citation30] Hydroxyproline is important to maintain the stability of a collagen trimer. Td likely increases as hydroxyproline content increases. In , the imino acid and hydroxyproline contents of EDTA-PSC are lower than those of black drum and sheephead bone collagen. Thus, the degree of crosslinking and Td of EDTA-PSC are lower than those of the black drum bone collagen and sheephead bone collagen. This result is consistent with the expected Td. In , HCl-PSC does not exhibit an integrated secondary structure.
CONCLUSION
Collagens were extracted from tilapia bone desalting by different methods, and these collagens were partially characterized. The results indicated that EDTA can be used to remove HA from tilapia bone. The characteristics of EDTA-ASC and EDTA-PSC from tilapia bone are similar to those of standard type I collagen. By contrast, tilapia bone collagen can be degraded by desalting with 0.6 mol/L HCl. The results of FTIR and CD indicated that HCl-PSC exhibited only a partially ordered secondary structure. On the contrary, EDTA-ASC and EDTA-PSC showed integrated secondary structure. In conclusion, 0.6 mol/l HCl was not suitable for the desalination of tilapia bone. Collagen is a very useful protein extracted from tilapia-processing wastes. This study provided useful information for further utilization of tilapia bone.
FUNDING
This study was supported by Fundamental Research Funds for the Central Universities with research grant no. JUSRP21010 and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Additional information
Funding
REFERENCES
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chemistry 2005, 89, 363–372.
- Sadowska, M.; Kolodziejska, I.; Niecikowska, C. Isolation of collagen from skins of Baltic cod (Gadus morhua). Food Chemistry 2003, 81, 257–262.
- Ogawa, M.; Portier, R.J.; Moody, M.W.; Bell, J.; Schexnayder, M.A.; Losso, J.N. Biochemical properties of bone and scale collagens isolated from the subtropical fish black drum (Pogonia cromis) and sheepshead seabream (Archosargus probatocephalus). Food Chemistry 2004, 88, 495–501.
- Bae, I.; Osatomi, K.; Yoshida, A.; Osako, K.; Yamaguchi, A.; Hara, K. Biochemical properties of acid-soluble collagens extracted from the skins of underutilised fishes. Food Chemistry 2008, 108, 49–54.
- Pati, F.; Adhikari, B.; Dhara, S. Isolation and characterization of fish scale collagen of higher thermal stability. Bioresource Technology 2010, 101, 3737–3742.
- Nagai, T.; Araki, Y.; Suzuki, N. Collagen of the skin of ocellate puffer fish (Takifugu rubripes). Food Chemistry 2002, 78, 173–177.
- Huang, Y.R.; Shiau, C.Y.; Chen, H.H.; Huang, B.C. Isolation and characterization of acid and pepsin-solubilized collagens from the skin of balloon fish (Diodon holocanthus). Food Hydrocolloids 2011, 25, 1507–1513.
- Baht, G.S.; Hunter, G.K.; Goldberg, H.A. Bone sialoprotein-collagen interaction promotes hydroxyapatite nucleation. Matrix Biology 2008, 27, 600–608.
- Nagai, T.; Suzuki, N. Isolation of collagen from fish waste material—Skin, bone, and fins. Food Chemistry 2000, 68, 277–281.
- Urist, M.R.; Iwata, H.; Ceccotti, P.L.; Dorfman, R.L.; Boyd, S.D.; McDowell, R.M.; et al. Bone morphogenesis in implants of insoluble bone gelatin. Proceedings of the National Academy of Sciences of the United States of America 1973, 70, 3511–3515.
- Wood, A.; Ogawa, M.; Portier, R.J.; Schexnayder, M.; Shirley, M.; Losso, J.N. Biochemical properties of alligator (Alligator mississippiensis) bone collagen. Comparative Biochemistry and Physiology B-Biochemistry & Molecular Biology 2008, 151, 246–249.
- Liu, H.Y.; Han, J.; Guo, S.D. Characteristics of the gelatin extracted from channel catfish (Ictalurus Punctatus) head bones. LWT-Food Science and Technology 2009, 2, 540–544.
- Asran, A.S.; Henning, S.; Michler, G.H. Polyvinyl alcohol-collagen-hydroxyapatite biocomposite nanofibrous scaffold: Mimicking the key features of natural bone at the nanoscale level. Polymer 2010, 51, 868–876.
- Guerrero, P.; Stefani, P.M.; Ruseckaite, R.A.; Caba, K. Functional properties of films based on soy protein isolate and gelatin processed by compression molding. Journal of Food Engineering 2011, 105, 65–72.
- Falguni, P.; Basudam A.; Santanu D. Isolation and characterization of fish scale collagen of higher thermal stability. Bioresource Technology 2010, 101, 3737–3742.
- Suda, H. K.; Kajiwara, M.; Matsumoto, N.; Murayama, H.; Yamato, H. Characterization of apatite and collagen in bone with FTIR imaging. International Journal of Food Properties 2009, 505, 64–69.
- Yakimets, I.; Wellner, N.; Smith, A.C.; Wilson, R.H.; Farhat, I.; Mitchell, J. Mechanical properties with respect to water content of gelatin films in glassy state. Polymer 2005, 46, 12577–12585.
- Szpak, P. Fish bone chemistry and ultrastructure: Implications for taphonomy and stable isotope analysis. Journal of Archaeological Science 2011, 38, 3358–3372.
- Kaminska, A.; Sionkowska, A. Effect of UV radiation on the infrared spectra of collagen. Polymer Degradation and Stability 1996, 51, 19–26.
- Hammed, A.M.; Irwandi, J.; Hamzah, M.S.; Hassan, A.A. Effects of pretreatment on properties of gelatin from perch (lates niloticus) skin. International Journal of Food Properties 2014, 17, 1224–1236.
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chemistry 2004, 86, 325–332.
- Kumar, N.S.S.; Nazeer, R.A. Characterization of acid and pepsin soluble collagen from the skin of horse mackerels (Magalaspis cordyla) and croaker (Otolithes ruber). International Journal of Food Properties 2013, 16, 613–621.
- Prystupa, D.A.; Donald, A.M. Infrared study of gelatin conformations in the gel and sol states. Polymer Gels and Networks 1996, 4, 87–110.
- Duan, R.; Zhang, J.J.; Dua, X.Q.; Yao, X.C.; Konno, K. Properties of collagen from skin, scale, and bone of carp (Cyprinus carpio). Food Chemistry 2009, 112, 702–706.
- Żelechowska, E.; Sadowska, M.; Turk, M. Isolation and some properties of collagen from the backbone of Baltic cod (Gadus morhua). Food Hydrocolloids 2010, 24, 325–329.
- Kelly, S.M.; Price, N.C. The application of circular dichroism to studies of protein folding and unfolding. Biochimica et Biophysica Acta-Biomembranes 1997, 1338, 161–185.
- Piez, K.A.; Sherman, M.R. Equilibrium and kinetic studies of the helix-coil transition in α1-CB2, a small peptide from collagen. Biochemistry-U 1970, 9, 4134–4140.
- Engel, J. Folding and unfolding of collagen triple helices. Advances in Meat Research 1987, 4, 145–161.
- Heidemann, E.; Roth, W. Synthesis and investigation of collagen model peptides. Advances in Polymer Science 1982, 42, 143–203.
- Ikoma, T.; Kobayashi, H.; Tanaka, J.; Walsh, D.; Mann, S. Physical properties of type I collagen extracted from fish scales of Pagrus major and Oreochromis niloticas. International Journal of Biological Macromolecules 2003, 32, 199–204.