Abstract
Fumonisins are mycotoxins primarily produced by Fusarium verticillioides that grow on food and feedstuffs. In the present investigation, the antifumonisin activity of 2-hydroxy-4-methoxybenzaldehyde isolated from Decalepis hamiltonii was evaluated. The results demonstrated that the compound 2-hydroxy-4-methoxybenzaldehyde exhibited dose-dependent antifungal activity with the zone of inhibition, minimum inhibitory concentration and minimum fungicidal concentration values 14.8 mm (at 500 μg/disc), 100 and 500 μg/mL, respectively. The fumonisin B1 production was completely inhibited at 400 mg/L under in vitro and 750 mg/kg under in vivo. There were no adverse effects observed in treated seed samples. The present findings indicate the possible use of 2-hydroxy-4-methoxybenzaldehyde as an alternative agent for management of fusarial growth and mycotoxins contamination.
INTRODUCTION
Fumonisins are mycotoxins primarily produced by Fusarium verticillioides that grow on agricultural commodities in the field or during storage.[Citation1,Citation2] Mycotoxins are toxic fungal metabolites that can contaminate agricultural products, and they significantly impact human and animal health[Citation3] More than ten types of fumonisins have been isolated and characterized from agricultural commodities, but fumonisin B1 (FB1), fumonisin B2 (FB2), and fumonisin B3 (FB3) are of great concern due to their widespread contamination and their adverse effects on animal and human health.[Citation4] The most prevalent fumonisins in contaminated corn is FB1, which is known to be the most toxic fumonisin produced by F. verticillioides.[Citation1,Citation2,Citation5−Citation7] Fumonisins are associated with a variety of adverse health effects in livestock and experimental animals, such as esophageal cancer, equine leukoencephalomalacia (ELEM), neurotoxicity, hepatotoxicity, nephrotoxicity, immune suppression, immune stimulation, developmental abnormalities, liver tumors, kidney tumors, and other abnormalities.[Citation8,Citation9] The extent of contamination of maize with fumonisins varies with geographical location, and is found to be highest in the warmer regions of the world.
Many recent reports stated that accumulation of these toxins in maize-based food and feedstuffs are increasing worldwide, possibly due to climate changes, use of different plant varieties of high yield, but which are susceptible to mold and mycotoxin contaminations, and agricultural practices.[Citation2,Citation7,Citation8,Citation10,Citation11] Several strategies are used to control the growth of F. verticillioides and the FB1 biosynthesis in grains and feedstuffs by chemical treatments and food preservatives, but many of the chemical fungicides pose adverse effects viz., environmental pollution, health hazard, and affects the natural ecological balance.[Citation12] Use of plant-based natural compounds with bioactivity would be helpful in avoiding application of synthetic chemicals against F. verticillioides and fumonisin contamination in maize.[Citation13] Keeping these points of view, some plants which are edible have been selected to evaluate the antifungal activity against F. verticillioides and to find out new sources of natural antifungal and the scientific validation of their uses in management of fumonisins and biodeterioration caused by F. verticillioides. D. hamiltonii Wight and Arn (Asclepiadaceae), an edible plant,[Citation14] showed highly significant in vitro antifungal activity against many field and storage fungi. The active compound responsible for antifungal activity was 2-hydroxy-4-methoxybenzaldehyde (HMB).[Citation15] The antifumonisin activity of aqueous and solvent extracts of D. hamiltonii have been previously reported.[Citation11] In the present study, the antifumonisin potency of HMB isolated from D. hamiltonii is being reported.
MATERIALS AND METHODS
Chemicals and Culture Media
Sabouraud dextrose agar/broth (SDA/SDB), dimethyl sulfoxide (DMSO), all analytical grade solvents, reagents, and iodonitro tetrazolium (INT) were purchased from Hi-Media (Mumbai, India). Mancozeb (Dithane M-45) was purchased from Indofil Chemicals, Mumbai. Carbendazim (Bavistin) was procured from Saraswathi Agro Chemicals, Jammu, India. Microtiter-plates (96-well) were purchased from Axiva (New Delhi, India). The standard FB1 was obtained from Sigma, Germany. Silica gel 60 F254 coated preparative thin layer chromatography (TLC) plates were obtained from Merck, Germany.
Plant Sample Collection
Fresh rhizome of D. hamiltonii was collected from BR Hills region, Karnataka (India) and the plant sample was authenticated by Dr. Seetharam, Professor, Department of Biological Sciences, Bangalore University, Bangalore (India). The authenticated voucher specimen of this plant was deposited at the Herbarium, Department of Microbiology and Biotechnology, Bangalore University, Bangalore (Voucher numbers: BUB/MB-BT/DCM/JU10/16).
Isolation and Identification of HMB
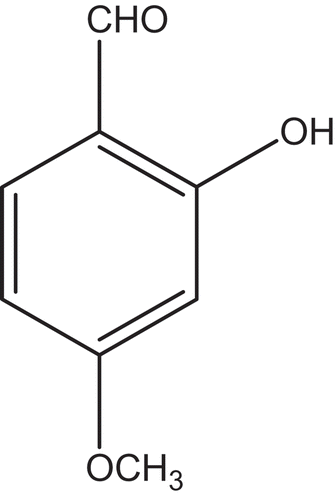
The compound HMB responsible for antifungal activity was isolated from phenolic fraction of petroleum ether extract using TLC and characterized as described in previous reports.[Citation15,Citation16] The 1H NMR analysis of the compound showed at δ 3.85 (s,- OCH3), 6.52 (dd, J=2 HZ; 3-H), 6.55 (d, J=7 HZ, 5-H), 7.40 (d, J=7 HZ; 6-H), 9.70 (s, CHO), 11.6 (s; -OH) functional groups and 13C NMR analysis showed eight carbon signals at δ 135.6 (1-CH), 108.7 (3-CH), 167.2 (C of carbonyl), 101.05 (5-CH), 164.8 (2-C), 115.5 (C), 194.7 (6-CH), 56.09 (CH3), and its identity confirmed by the mass spectral analysis [m/z] with percent abundance: 57(48), 95(46), 108(24), 121(20), 151(100), 152(70). The strong molecular ion peak (m/z 151) in negative mode [M+H]− confirmed that the molecular weight of the compound was 152 corresponding to the molecular formula C8H8O3. Based on these results and reported literatures, the compound was identified as (HMB; ).[Citation15,Citation17]
Determination of Antifungal Activity
Isolation of FB1 producing F. verticillioides
Isolation and identification of FB1 producing F. verticillioides was carried out as reported in an earlier report.[Citation11] The toxigenic F. verticillioides (M8) isolate from maize was identified using fungal key manuals and used as test fungi for evaluation.[Citation18,Citation19] FB1 production was confirmed by comparing with standard FB1 on TLC plate. The isolated culture was maintained on SDA and the seven-day-old culture was used for assay.
Determination of ZOI, MIC, and MFC
The disc diffusion method was employed for the determination of zone of inhibition (ZOI) and the broth microdilution method was used to determine the minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) according to the procedures of Thippeswamy et al. and Makni et al.[Citation11,Citation20] DMSO served as a negative control, and carbendazim and mancozeb served as positive controls. Four replicates were maintained for each treatment. The ZOI diameters were measured in millimeters (mm). The values of the compound were detected by the addition of 50 μl of INT (2 mg/mL) according to the procedure of Hajji et al.[Citation21] and the MFC values were determined following the procedure of Dung et al.[Citation22] MIC was defined as the lowest concentration at which no visible fungal growth was observed, and the complete absence of growth on the compound free agar surface at the lowest concentration was defined as the MFC.
In vitro and in vivo efficacy of HMB on MDW losses and FB1 production by F. verticillioides
The in vitro efficacies of HMB on mycelial dry weight (MDW) losses and FB1 production were determined according to a previous report[Citation11] with some modifications. Briefly, 100 μl of a spore suspension (104 spores/mL) of F. verticillioides was inoculated into SDB and SDA media, containing the requisite amount of HMB and incubated at 28 ± 2°C for ten days. The F. verticillioides culture along with SDA medium was used to estimate FB1. The amount of FB1 was estimated qualitatively and quantitatively using spectrophotodensitometer (Biorad, Universal Hood II 720BR/02170) at 600 nm with different concentrations of standard FB1. The mycelial mat of F. verticillioides obtained after the filtration of the SDB media was used for the estimation of MDW losses following the procedure of Shukla et al.[Citation23]
The in vivo efficacies of HMB on FB1 production, seed-borne mycoflora and seedling vigor in maize seeds were determined following the standard procedures[Citation7,Citation24] with some modifications. Briefly, freshly harvested maize samples were collected, surface sterilized under UV and the water activity (aw) was adjusted to 0.95 by adding sterile distilled water. The maize samples were treated with desired different concentrations of HMB separately, and made into two sets. The first set was inoculated with 100 μl of a spore suspension (104 spores/mL) of toxigenic F. verticillioides, stored in sterile plastic containers (200 g/pack), incubated at 25°C for up to 15 days and subjected to FB1 extraction and quantification. Whereas, second set was subjected to seed-germination and seedling-vigour index analysis.
Statistical Analysis
Values were expressed as means ± standard error. Analysis of variance was conducted, and the differences between values were tested for significance by ANOVA and Tukey’s multiple comparison tests with the SPSS 20 (IBM, USA) program. Differences at p ≤ 0.05 were considered as statistically significant.
RESULTS AND DISCUSSION
The inhibitory activity of HMB against F. verticillioides growth was determined by recording the ZOI, MIC, and MFC. The negative control, DMSO, did not show any inhibitory activity. The compound HMB exhibited dose-dependent strong inhibitory activity with the ZOI, MIC, and MFC values 14.8 mm (at 500 μg/disc), 100 and 500μg/mL, respectively (). The obtained results were compared to the synthetic fungicides carbendazim and mancozeb. The order of inhibitory activity was carbendazim > HMB > mancozeb.
TABLE 1 Determination of ZOI, MIC, and MFC value of HMB against FB1 producing strain of F. verticillioides
The mycelial growth of F. verticillioides was strongly inhibited by HMB in a dose-dependent manner and the complete inhibition of the mycelial growth was observed at 450 μg/mL under in vitro (). Similarly, the FB1 production was in declining trend with increasing concentration. The FB1 production was completely inhibited at 400 mg/L under in vitro and 750 mg/kg under in vivo. The effect of HMB on the inhibition of the seed-borne natural mycoflora of maize seeds was concentration-dependent. In the control group, species of Aspergillus (89%) and Fusarium (67%) were found to be the dominant fungi, followed by species of Penicillium (55%), Alternaria (25%), and Curvularia (12%), whereas the percent incidences of these fungi in HMB treated maize seed were 22, 0, 12, 0, and 5%, respectively, at 750 mg/kg. Compared to the control, seed-germination and seedling-vigor increased with increasing concentrations of HMB. The seedling-vigor indices in control and HMB treated (750 mg/kg) maize seeds were 1890 and 2379, respectively, after seven days incubation. These results confirmed that HMB was effective in inhibiting the growth and FB1 production by F. verticillioides both in vitro and in vivo conditions.
TABLE 2 In vitro and in vivo efficacy of HMB on MDW losses and FB1 production by F. verticillioides
Rhizome of D. hamiltonii is largely used in south Indian dishes for pickling along with curd or lime juice.[Citation14] Earlier reports on the phytochemical analysis of the rhizome revealed that HMB as an important and major component.[Citation17] Antimicrobial, insecticidal, anticancer, and antioxidant properties of D. hamiltonii have been reported.[Citation7,Citation15,Citation16,Citation25−Citation30] However, there are no reports available on the antifumonisin activity of HMB. To the best of the authors’ knowledge, the antifumonisin activity of HMB has reported here for the first time. Mycotoxigenic fungi are significant contaminants and destroyers of agricultural crops in the field, during storage, processing and in the markets, thereby reduce their nutritive value.[Citation23,Citation31] Major food commodities affected are cereals, nuts, dried fruit, coffee, cocoa, spices, oil seeds, dried peas, beans, and fruit.[Citation3] The mycotoxins can also enter the human food chain via meat or other animal products, such as, eggs, milk, and cheese, as the result of livestock eating contaminated feed. Tropical conditions, such as, high temperatures and moisture, monsoons, and unseasonal rains during harvest, and flash floods, lead to fungal proliferation and production of mycotoxins.[Citation32] According to the Food and Agriculture Organization (FAO) of the United Nations, over 25% of the agricultural commodities worldwide are significantly contaminated by mycotoxins.[Citation33] Out of the 300–400 mycotoxins known, the most important are aflatoxins and fumonisins. Fumonisins are a group of mycotoxins produced by several agriculturally important fungi, including F. verticillioides, which is a common fungal contaminant of corn- and maize-derived products worldwide. The most important method of protecting plants against fungal attack is the use of fungicides that are toxic to fungi. The use of chemicals has been found very effective, but widespread and indiscriminate use of chemical preservatives or pesticides has significant drawbacks in not being economical, handling hazards, toxic residues on the grains, and more importantly the emergence of resistant foodborne microorganisms. Therefore, it is important to search a practical, cost effective, and non-toxic method to prevent fungal incidences and mycotoxin contaminations in stored grains. In recent decades, developing formulation to control molds and mycotoxins using phytochemicals as alternative to synthetic chemicals has become a major priority of scientists worldwide.[Citation31] Use of natural products obtained from edible plants provides an opportunity to avoid chemical preservatives for eco-friendly management of plant diseases and safe storage of food and feedstuffs. The results of the present investigation are an important step in developing the plant based eco-friendly product for the management of fusarial diseases and fumonisins.
CONCLUSION
The development of antimycotoxigenic agents from edible plant sources would be helpful for decreasing the negative impact of synthetic chemicals. In the present study, the dose-dependent anti-F. verticillioides and antifumonisin activities of HMB have been reported for the first time. Hence, this bioactive compound could be exploited as an alternative source/agent for the management of fusarial contaminations and their toxins in food and feedstuffs. Further studies are required to investigate the safety and practical applicability in food preservation.
FUNDING
This work was financially supported by the Science and Engineering Research Board, Department of Science and Technology, Government of India and University Grant Commission, New Delhi, India.
Additional information
Funding
REFERENCES
- Bankole, S.A.; Adebanjo, A. Mycotoxins in food in West Africa: Current situation and possibilities of controlling it. African Journal of Biotechnology 2003, 2 (9), 254–263.
- Covarelli, L.; Beccari, G.; Salvi, S. Infection by mycotoxigenic fungal species and mycotoxin contamination of maize grain in Umbria central Italy. Food and Chemical Toxicology 2011, 49, 2365–2369.
- Ocak, I.; Celik, A.; Ozel, M.Z.; Korcan, E.; Konuk, M. Antifungal activity and chemical composition of essential oil of Origanum Hypericifolium. International Journal of Food Properties 2012, 15, 38–48.
- Yazar, S.; Omurtag, G.Z. Fumonisins, trichothecenes, and zearalenone in cereals. International Journal of Molecular Sciences 2008, 9, 2062–2090.
- Shim, W.; Woloshuk, C.P. Regulation of fumonisin B1 biosynthesis and conidiation in fusarium verticillioides by a cyclin-like (C-Type) gene FCC1. Applied and Environmental Microbiology 2001, 1607–1612.
- CAC (Codex Committee on Food Additives and Contaminants). 32nd Session Beijing, People’s Republic of China. Food and Agriculture Organization and World Health Organization, 2001; 1–13.
- Garcia, D.; Ramos, A.J.; Sanchis, V.; Marin, S. Effect of Equisetum arvense and Stevia rebaudiana extracts on growth and mycotoxin production by Aspergillus flavus and Fusarium verticillioides in maize seeds as affected by water activity. International Journal of Food Microbiology 2012, 153, 21–27.
- Domijan, A.M.; Zeljezic, D.; Peraica, M.; Kovacevic, G.; Gregorovic, G.; Krstanac, Z.; Horvatin, K.; Kalafatic, M. Early toxic effects of fumonisin B1 in rat liver. Human and Experimental Toxicology 2008, 27, 895–900.
- Fandohan, P.; Hell, K.; Marasas, W.F.O.; Wingfield, M.J. Infection of maize by Fusarium species and contamination with fumonisin in Africa. African Journal of Biotechnology 2003, 2 (12), 570–579.
- Quiroga, E.N.; Sampietro, D.A.; Sgariglia, M.A.; Soberon, J.R.; Vattuone, M.A. Antimycotic activity of 5′-prenylisoflavanones of the plant Geoffroea decorticans, against Aspergillus species. International Journal of Food Microbiology 2009, 132, 42–46.
- Thippeswamy, S.; Mohana, D.C.; Abhishek, R.U.; Manjunath, K. Effect of plant extracts on inhibition of Fusarium verticillioides growth and its toxin fumonisin B1 production. Journal of Agricultural Technology 2013, 9 (4), 889–900.
- Reddy, K.R.N.; Raghavender, C.R.; Reddy, B.N.; Salleh, B. Biological control of Aspergillus flavus growth and subsequent aflatoxin B1 production in sorghum grains. African Journal of Biotechnology 2010, 9 (27), 4247–4250.
- Yassin, M.A.; Moslem, M.A.; El-Samawaty, A.E.M.A. Mycotoxins and non-fungicidal control of corn grain rotting fungi. Journal of Plant Sciences 2012, 7 (3), 96–104.
- CSIR (Council of Scientific & Industrial Research, India). A dictionary of Indian raw materials and industrial products, Raw materials: III. New Delhi: Council of Scientific and Industrial Research 1952.
- Mohana, D.C.; Raveesha, K.A.; Lokanath Rai, K.M. Herbal remedies for the management of seed-borne fungal pathogens by an edible plant Decalepis hamiltonii (Wight & Arn). Archives of Phytopathology and Plant Protection 2008, 41 (1), 38–49.
- Mohana, D.C.; Raveesha, K.A. Antimycotic antibiodeteriorative and antiaflatoxigenic potency of 2- hydroxy-4-methoxybenzaldehyde isolated from Decalepis hamiltonii on fungi causing biodeterioration of maize and sorghum grains. Journal of Mycology and Plant Pathology 2010, 40 (2), 197–206.
- Nagarajan, S.; Rao, L.J.M.; Gurudatt, K.N. Chemical composition of the volatiles of Decalepis hamiltonii (Wight & Arn). Flavour and Fragrance Journal 2001, 16, 27–29.
- Nagamani, A.; Kunwar, I.K.; Manoharachary, C. Handbook of Soil Fungi, 1st Ed; IK International Pvt Ltd.: New Delhi, 2006; 227–232.
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi: Morphologies of Cultured Fungi and Key To Species, 2nd Ed; CRC Press LLC: Boca Raton, FL, 2002, 270–278.
- Makni, M.; Haddar, A.; Kriaa, W; Zeghal, N. Antioxidant, free radical scavenging, and antimicrobial activities of Ajuga iva leaf extracts. International Journal of Food Properties 2013, 16 (4), 756–765.
- Hajji, M.; Masmoudi, O.; Souissi, N.; Triki, Y.; Kammoun, S.; Nasri, M. Chemical composition angiotensin I-converting enzyme (ACE) inhibitory antioxidant and antimicrobial activities of the essential oil from Periploca laevigata root barks. Food Chemistry 2010, 121, 724–731.
- Dung, N.T.; Kim, J.M.; Kang, S.C. Chemical composition antimicrobial and antioxidant activities of the essential oil and the ethanol extract of Cleistocalyx operculatus (Roxb) Merr. and Perry buds. Food and Chemical Toxicology 2008, 46, 3632–3639.
- Shukla, R.; Kumar, A.; Singh, P.; Dubey, N.S. Efficacy of Lippa alba (Mill.) N. E. Brown essential oil and its monoterpene aldehyde constituents against fungi isolated from some edible legume seeds and aflatoxin B1 production. International Journal of Food Microbiology 2009, 135, 165–170.
- Mohana, D.C.; Satish, S.; Raveesha, K.A. Antifungal activity of 2-hydroxy-4-methaxybenzaldehyde isolated from Decalepis hamiltonii (Wight & Arn) on seed-borne fungi causing biodeterioration of paddy. Journal of Plant Protection Research 2009, 39, 570–571.
- Elizabeth, K.M.; Vimala, Y.; Devarapalli, H.C.P. Antimicrobial activity of Decalepis hamiltonii. Asian Journal of Microbiology, Biotechnology, and Environmental Sciences 2005, 7, 151–153.
- George, J.; Bais, H.P.; Ravishankar, G.A. Biotechnological production of plant-based insecticides. Critical Reviews in Biotechnology 2000, 49, 49–77.
- George, J.; Ravishankar, G.A.; Keshava, N.; Udayasankar, K. Antibacterial activity of supercritical extract from Decalepis hamiltonii root. Fitoterapia 1999a, 70, 172–174.
- George, J.; Ravishankar, G.A.; Pereira, J.; Divakar, S. Bioinsecticide from swallowroot (Decalepis hamiltonii) Wight & Arn protects food grains against insect infestation. Current Science 1999b, 77, 501–502.
- Phadke, N.Y.; Gholap, A.S.; Ramakrishnan, K.; Subbulakshmi, G. Essential oil of Decalepis hamiltonii as an antimicrobial agent. Journal of Food Science and Technology 1994, 31, 472–475.
- Thangadurai, D.; Anita, S.; Pullaiah, T.; Reddy, P.N.; Ramachandraiah, O.S. Essential oil constituents and in vitro antibacterial activity of Decalepis hamiltonii roots against food-borne pathogens. Journal of Agricultural and Food Chemistry 2002, 50, 3147–3149.
- Reddy, B.N.; Raghavender, C.R. Outbreaks of aflatoxicoses in India. African Journal of Food Agriculture, Nutrition, and Development 2007, 7 (5), 1–15.
- Bhat, R.V.; Vasanthi, S. Food safety in food security and food trade. Mycotoxin food safety risk in developing countries. International Food Policy Research Institute, Focus 2003, 10, 3–17.
- Reddy, K.R.N.; Salleh, B.; Saad, B.; Abbas, H.K.; Abel, C.A.; Shier, W.T. An overview of mycotoxin contamination in foods and its implications for human health. Toxin Reviews 2010, 29 (1), 3–26.