Abstract
Polyphenolic composition of lemon balm (Mellisa officinalis L.) aqueous extracts prepared as infusion and decoction were evaluated by high performance liquid chromatography/tandem mass spectrometry. All the samples showed a similar phenolic profile but presenting differences in the quantities found of each compound. Higher content was found in plant infusion extract compared to decoction process. During the prolonged time of extractions the contents of some compounds were changed. Rutin and caffeic acid contents were decreased in the infusion mode, while increased concentration was observed during decoction process. The antioxidant activities measured by Folin-Ciocalteu and CUOPRAC assays were higher for the extract prepared in decoction mode (1245 mg/g GAE and 4.91 mmol/g TRE, respectively) in comparison to infusion process (267 mg/g GAE and 2.62 mmol/g TRE).
INTRODUCTION
Lemon balm (Melissa officinalis L.), a perennial herb belonging to Lamiaceae family, is cultivated in many countries for its carminative and antispasmodic properties. It is used for treatment of headache, rheumatism, indigestion, and hypersensitivities.[Citation1] Natural essential oils (mixtures of fragrant chemical) obtained from various parts of plant are efficient active antimicrobial agents[Citation2] and due to its citrus fragrance is used in the cosmetic industry and perfumery. All of these properties of lemon balm have been related to the high levels of polyphenolic compounds.[Citation3−Citation5]
Antioxidative properties of polyphenolic compounds are manifested particularly by their abilities to inhibit free radical generation, scavenge free radicals, and chelate transition metal ions, which are catalysts of free radical reactions. Polyphenols also prevent free radical generation by inhibiting activity of existing enzymes participating in their generation or by increasing the activity of enzymes with antioxidative properties.[Citation6] The antioxidant capacities of many polyphenolic compounds are much stronger than those of vitamin C and E.[Citation7]
There is an increasing interest in the antioxidant effects of natural compounds from medicinal plants and pharmaceutical products. Herbal therapies are perceived by many as natural, safer, gentler, and lower in cost than synthetic pharmaceuticals. Several different species and aromatic herbs have been investigated for their chemical composition and antioxidant activity.[Citation8−Citation10]
Infusion and decoction are the most consumed preparation of Melissa officinalis. During these processes, hydrophilic compounds will be released, including flavonoids and phenolic acids. The objective of this study was to compare the content of some phenolic compounds in aqueous extracts of lemon balm prepared as infusion and decoction. These processes were examined using different brewing time. Additionally, antioxidant properties of the prepared extracts were studied by Folin-Ciocalteu (FC) and cupric reducing ability (CUPRAC) assays.
MATERIALS AND METHODS
Reagents and Apparatus
The commercial standards of flavonoids and polyphenolic acids, FC’s reagent as well as the rest of the chemicals were purchased from Sigma (Steinheim, Germany). Acetonitrile were of HPLC gradient grade from Merck (Darmstadt, Germany). Ultrapure water from Milli-Q system (Millipore, Bedford, MA, USA) with a conductivity of 18 MQ was used in all experiments.
Chromatographic analysis was performed with the Shimadzu LC system with 3200 QTRAP Mass spectrometer (Applied Biosystem/MDS SCIEX). A mass spectrometry (MS) system was equipped with electrospray ionization source (ESI). ESI in negative mode conditions were used. For each compound the optimum conditions of selected reaction mode (SRM) were determined in infusion mode. Continuous mass spectra were obtained by scanning m/z from 50 to 650.[Citation11]
Compounds were separated on SeQuantTM ZIC-HILIC column (100 × 2.1 mm, 3.5 μm) from Merck (Darmstadt, Germany) at 30°C. Ten mM ammonium acetate (pH 7) as eluent A and acetonitrile as eluent B were used. The mobile phase was delivered at 0.2 mL/min in gradient mode: 0–4 min 98%B, 6–7 min 90% B, 8–8.4 min 80% B, 8.4–12 min 50% B, and 13–20 min 98% B. The analytes were identified by comparing retention time and m/z values obtained by MS and MS2 with the mass spectra.[Citation11] Quantification of compounds was done from the calibration curves obtained in SRM mode.
Brewing Processes
Lemon balm tea bags were purchased from a local market. In order to simulate household brewing conditions, the samples were prepared using an aqueous extraction procedure. Infusion was carried out by pouring 2 g of dried plant in 50 mL of freshly boiled distilled water and allowed steep for appropriate interval time at room temperature. For decoction, 50 mL of distilled water was added to 2 g of a herb and the liquid was heated on the heating plate under the cover and boiled for appropriate interval time. The extracts were then filtered and analyzed. For each experiment the brewing process was conducted separately from three bags.
FC Assay
The FC assay was conducted according to Singleton et al.[Citation12] with a slight modification. Briefly, 1 mL of extract was introduced into test tubes followed by 0.1 mL of FC’s reagent and 0.9 mL of water. The tubes were allowed to stand for 5 min. Then, 1 mL of Na2CO3 (7%, w/v) and 0.4 mL of water were added and 10 more min was allowed for stabilization of the formed color. The absorbance against a reagent blank was measured at 765 nm. The data were expressed as gallic acid equivalent (GAE) in mg per gram of dry matter.
CUPRAC
For CUPRAC method, the assay described by Apak et al. was adopted.[Citation13] One mL of CuCl2 solution (1.0 × 10−2 M) was mixed with 1 mL of neocuproine solution (7.5 × 10−3 M) and 1 mL of 1 M NH4AC buffer (pH 7), followed by mixing 0.5 mL of extract and 0.6 mL of water. The reagents were then incubated in a water bath at 50°C for 20 min. Absorbance against the reagent blank was measured at 450 nm. The antioxidant activity of the extracts were expressed as trolox equivalent (mmol TR/g of dry matter).
Statistical Analysis
The results were expressed as mean ± standard deviation at least three independent determinations. Statistical analysis was conducted using the software package STATISTICA 8.0 for Windows from Statsoft. A p-value of less than 0.05 was considered statistically different.
TABLE 1 The content of polyphenols in the extracts of lemon balm
RESULTS AND DISCUSSION
HPLC Analysis
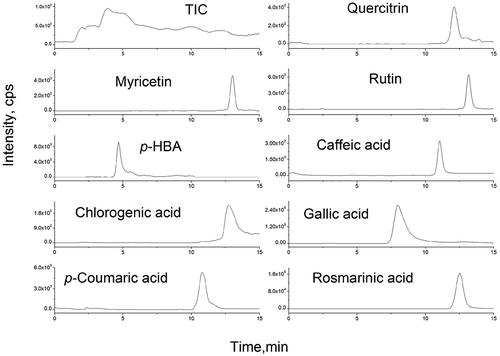
The data from the HPLC analysis of the aqueous lemon balm extracts prepared as infusion and decoction is presented in , while the chromatogram of this herb infusion is shown in . Rosmarinic acid was the major phenolic compound present in the plant extract, which is in accordance with the previous studies.[Citation5,Citation14] From the flavonoid family, high content of rutin was found as well as traces of quercetrin and myricetin. The presence of rutin in the aqueous extract of lemon balm was not reported, although, its aglycones quercetin was found in the hydrolyzed extract.[Citation15] Borros et al.[Citation5] found only one flavonoid, luteolin-3-O-glucoronide, in cultivated, in vitro cultured and commercial samples of Melissa officinalis. Some hydroxycinnamic acids (caffeic acid, chlorogenic acid, and p-coumaric acid) and hydroxybenzoic acid derivatives (gallic acid and p-HBA) were also found. From the quantified phenolic acids, caffeic, and coumaric acids have already been reported in the lemon balm extracts.[Citation13,Citation5,Citation14] The differences in chemical composition may be due to a variety of reason ranging from climate and geography to difference in the specificity of the extraction procedures.[Citation3]
FIGURE 1 Total ion chromatogram (TIC) in SRM mode of lemon balm water extract and extracted ion chromatogram of polyphenolic compounds. Column: SeQuantTM ZIC-HILIC (100 × 2.1 mm, 3.5 μm). Eluent: CH3COONH4/acetonitrile.
The amounts of the phenolic compounds found in water extracts of lemon balm varied among the different preparation processes. Generally, higher content of determined polyphenolic compounds was found in plant infusion compared to decoction process. The same was observed in chamomile (Matricaria recutita L.) extracts.[Citation16] During the prolonged time of extraction the contents of some polyphenolic compounds were changed. Rutin and caffeic acid contents were decreased in the infusion mode, while increase of their concentration was observed during decoction process. Myricetin content was increasing in both brewing processes. Polyphenols are present in the plant matrix at different sites, some of which can be readily extracted, while others are tightly bound. Therefore, the longer time of extraction and increase in temperature may have influenced the release of the tightly bound compounds and improves the efficiency of extraction. However, degradation of some termolable compounds also could occur as a result of polyphenol oxidase activity.[Citation17]
Antioxidant Activity
For the evaluation of antioxidant properties of studied lemon balm extracts FC and CUPRAC assays were used as no single assay accurately reflects the mechanism of action of all radial sources or all antioxidants in a complex sample.[Citation18] These assays used different chromogenic redox reagents as well as different conditions for measurement. FC assay has been used for many years by the food and agricultural industries to determine the phenolic content of plant products. However, FC reagent reacts with other antioxidants besides phenols and possible contributiors include proteins, carbohydrates, amino acids, thiols, vitamins, amines, aldehydes, and ketones.[Citation19] The chemistry behind this assay counts on the transfer of electrons in alkaline medium from phenolic compounds and other reducing species to molybdenum, forming blue complexes that can be monitored spectrophotometrically. CUPRAC method is based on reduction of Cu(II) to Cu(I) at neutral pH by reductants (antioxidants) present in a sample utilizing the copper(II)-neocuproine reagent as the chromogenic oxidant.[Citation13]
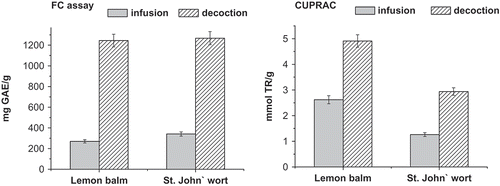
The antioxidant capacities of lemon balm extracts using both reagents are presented in . The values in FC assay increased with prolonged time of brewing, particularly for infusion mode. However, during decoction process more compounds with antioxidant (reductative) properties, are released and the highest value for so-called total phenols was obtained for this brewing mode. Some organic acids (oxalic or ascorbic acid) released from the plant during brewing, may contribute to the antioxidant activity of the samples studied herein. As literature data for lemon balm infusion in FC assay are expressed in different units – as 8240 mg GAE/100 g,[Citation20] 959.5 mg GAE/mL,[Citation14] or 268.0 mg GAE/g (dry weight) extract,[Citation3] thus, it is difficult to compare them. The values for antioxidant activity in CUPRAC method decreased slightly when the extraction time was increased. Apak et al.[Citation13] reported CUPRAC value for Melissa officinalis infusion as 0.99 mmol TR/g. No values were found for antioxidant activity of this herb prepared in decoction mode.
The obtained value for the antioxidant capacities of lemon balm using both FC and CUPRAC assays were compared with the aqueous extracts of St. John’s wort (Hypericum perforatum), popular medicinal plant, using the same procedures (). In FC assay very similar results were obtained for both plants in decoction mode, while the infusion of St. John’s wort exhibited higher value. Using the CUPRAC method the values for lemon balm extracts were higher despite of kind of brewing process. Similar results were reported by Kratachanova et al.,[Citation20] who compared the results for these two plants using FC and ORAC assays.
CONCLUSIONS
The present study showed that the aqueous extracts of lemon balm can be a source of phenolic compounds such as rutin, quercetrin, and myricetin from the flavonoid family as well as some phenolic acids, known by their bioactive effects. A higher content of these compounds was found in plant infusion extract compared to decoction process. However, higher antioxidant activity was obtained for the extract prepared in decoction mode.
REFERENCES
- Petersen, M.; Simmonds, M.S.J. Molecules of interest: Rosmarinic acid. Phytochemistry 2003, 62, 121–125.
- Allahverdiyev, A.; Duran, N.; Ozguven, M.; Koltas, S. Antiviral activity of the volatile oils of Melissa officinalis L. against Herpes simplex virus type-2. Phytomedcine 2004, 11, 657–661.
- Dastmalchi, K.; Dorman, H.J.D.; Oinonen, P.P.; Darwis, Y.; Laakso, I.; Hiltumen, R. Chemical composition and in vitro antioxidative activity of a lemon balm (Melissa officinalis L.) extract. LWT–Food Science and Technology 2008, 41, 391–400.
- Dias, M.I.; Barros, L.; Sousa, M.J.; Ferreira, I.C.F. Systematic comparison of nutraceuticals and antioxidant potential of cultivated, in vitro cultured, and commercial Melissa officinalis samples. Food and Chemical Toxicology 2012, 50, 1866–1873.
- Barros, L.; Dueñas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C.F. Phenolic profiles of cultivated, in vitro cultured, and commercial samples of Melissa officinalis L. infusions. Food Chemistry 2013, 136, 1–8.
- Galleano, M.; Verstraeten, S.V.; Oteiza, P.I.; Fraga, C.G. Antioxidant actions of flavonoids: Thermodynamic and kinetic analysis. Archives of Biochemistry and Biophysics 2010, 501, 23–30.
- Prochazková, D.; Bouŝová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoteriapia 2011, 82, 513–523.
- Jaberian, H.; Piri, K.; Nazari, J. Phytochemical composition and in vitro antimicrobial and antioxidant activities of some medicinal plants. Food Chemistry 2013, 136, 237–244.
- Alinezhad, H.; Azimi, R.; Zare, M.; Ebrahimzadeh, M.A.; Eslami, S.; Nabavi, S.F.; Nabavi, S.M. Antioxidant and antihemolytic activities of ethanolic extract of flowers, leaves, and stems of hyssopus officinalis L. Var. angustifolius. International Journal of Food Properties 2013, 16, 1169–1178.
- Nabavi, S.F.; Nabavi, S.M.; Ebrahimzadeh, M.A.; Eslami, B.; Jafari, N. In vitro antioxidant and antihemolytic activities of hydroalcoholic extracts of Allium scabriscapum Boiss. & Ky. aerial parts and bulbs. International Journal of Food Properties 2013, 16, 713–722.
- Sentkowska, A.; Biesaga, M.; Pyrzynska, K. Effects of the operation parameters on HILIC separation of flavonoids on zwitterionic column. Talanta 2013, 115, 284–290.
- Singleton, V.L.; Orthofer, R.; Lamuela-Ravwntos, R.M. Analysis of total phenolics and other oxidation substrates and antioxidants by means of Folin-Ciocaltea reagent. Methods in Enzymology 1999, 299, 152–178.
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E.; Erçag, E. The cupric reducing antioxidant capacity and polyphenolic content of some herbal teas. International Journal of Food Science and Nutrition 2006, 57, 292–304.
- Fecka, I.; Turek, S. Determination of water-soluble polyphenolic compounds in commercial herbal teas from Lamiaceae: Peppermint, melisa, and sage. Journal of Agricultural and Food Chemistry 2007, 55, 10908–10917.
- Komes, D.; Beščak-Cvitanović, A.; Horžić, D.; Rusak, G.; Likić, S.; Berendika, M. Phenolic composition and antioxidant properties of some traditionally used medicinal plants affected by the extraction time and hydrolysis. Phytochemical Analysis 2010, 22, 172–180.
- Guimarães, R.; Barros, L.; Dueňas, M.; Calhelha, R.; Carvallh, A.M.; Santos-Buelga, C.; Queiroz, M.J.; Ferreira, I.F.F. Infusion and decoction of wild German chamomile: Bioactivity and characterization of organic acids and phenolic compounds. Food Chemistry 2010, 136, 947–954.
- Marete, E.N.; Jacquier, J.C.; O’Riordan, D. Effects of extraction temperature on the phenolic and parthenolide contents, and colour of aqueous feverfew (Tanacetum parthenium) extracts. Food Chemistry 2009, 117, 226–231.
- Magalhâes, L.M.; Segundo, M.A.; Reis, S.; Lim, J.L.F. Methodological aspects about in vitro evaluation of antioxidant properties. Analytica Chimica Acta 2008, 613, 1–19.
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Through study of reactivity of various compound classes toward the Folin-Ciocalteu reagent. Journal of Agricultural and Food Chemistry 2010, 58, 8139–8144.
- Kratchanova, M.; Denev, P.; Ciz, M.; Lojek, A.; Atanas Mihailov, A. Evaluation of antioxidant activity of medicinal plants containing polyphenol compounds. Comparison of two extraction systems. Acta Biochimica Polonica 2010, 57, 229–234.