Abstract
The multivariate calibration methods—principal component regression and partial least squares—were employed for the prediction of antioxidant capacities of fruit juices. High-performance liquid chromatography and spectrophotometric approaches were used to determine the antioxidant capacities of fruit juices. The importance of calibration design was investigated by calculating the prediction and validation errors. The influences of using independent validation sets were emphasized. Calibration design is shown to have major effect on principal component regression and partial least squares errors. The models developed on the basis of the mean-centered data were able to predict the total antioxidant activity with a precision comparable to that of the reference [2,2-azino-di-(3-ethylbenzothialozine-sulfonic acid)] method. The partial least squares model seems preferable because of its predictive and describing abilities and good interpretability of the contribution of compounds to the antioxidant capacity. The contribution of individual phenolic compounds to the antioxidant capacity was identified by high-performance liquid chromatography.
INTRODUCTION
Fruit and fruit juice are important sources of phenolic compounds such as phenolic acids, flavonoids, and anthocyanins,[Citation1–Citation3] which have demonstrated considerable antioxidant properties in vivo and in vitro,[Citation4,Citation5] which are known to reduce lipid peroxidation mediated deterioration of foods during processing and storage[Citation6] and which can also reduce the rate of mutagenicity resulting from oxidative stress in humans.[Citation7] The measurement of the antioxidant capacity of food products, such as fruit and fruit juice, has become very important for researchers because it may provide information about resistance to oxidation, quantitative contribution of natural antioxidant substances.[Citation8,Citation9] Natural antioxidants are substances that may protect human cells against the effects of free radicals produced. Free radicals can damage cells, and may play a role in heart disease, cancer, and other diseases.[Citation10,Citation11] Therefore, the antioxidant compounds may play an important role in the prevention of certain diseases.[Citation12]
Antioxidant activity has been determined by several assays such as chromium reducing antioxidant capacity (CHROMAC),[Citation13] 2,2-azino-di-(3-ethylbenzothialozine-sulphonic acid; ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging potential, cupric ion reducing antioxidant capacity (CUPRAC), ferric reducing antioxidant power (FRAP), oxygen radical absorbance capacity (ORAC), and total radical absorption potentials (TRAP) in food products.[Citation14] These methods differ in terms of their assay principle and experimental conditions. Each method provides an estimate of antioxidant activity that is dependent on the type of assay selected and experimental conditions. Therefore, there is a need of a simple, convenient method for the fast quantization of antioxidant capacity, suitable for screening in the food and nutraceutical industry.
Multivariate chemometric methods such as partial least squares (PLS) and principal component regression (PCR) allow to extract analytical information from the full spectra/chromatograms, providing so to use simultaneously an elevated number of signals. Moreover, these techniques allow a rapid analytical response with minimum sample preparation, reasonable accuracy and precision, and without a preliminary separation step in complex matrices.[Citation15] The multivariate chemometric methods have been involved in a wide range of studies for measurement of antioxidant capacity of plants and fruit,[Citation16,Citation17] identification, and quality control of herbal medicines.[Citation18]
The aim of this work was to verify the potential use of chromatograms in the construction of multivariate calibration models relating the chromatographic profile with the antioxidant capacity of fruit juice. PCR and PLS multivariate calibration methods were successfully applied to the determination of antioxidant capacities of fruit juices. Also the potential compounds associated with the antioxidant capacity of fruit juice were identified by PLS calibration model.
MATERIALS AND METHODS
Chemicals
Trolox [(±)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid] and ABTS [2,2’-azinobis(3 ethylbenzothiazoline-6-sulphonic acid) diammonium salt] were purchased from Fluka (Buchs, Switzerland). HPLC grade of acetonitrile and formic acid were purchased from Merck (Merck, Darmstadt, Germany).
Fruit Juice Samples
Fruit juices were obtained from local market in Bursa, Turkey. Blueberry, cranberry, vitis vinifera, pomegranate, and cherry juices were used to achieve calibration and validation designs. The samples were stored at 4°C for analysis. The samples were analyzed by high performance liquid chromatography-diode array detection (HPLC-DAD) at 280 nm.
High-Performance Liquid Chromatography (HPLC)
An Agilent 1200 HPLC system (Waldbronn, Germany), consisting of a vacuum degasser, binary pump, autosampler, and a diode array detector, was used. Chromatographic separations were carried out using an XBridge C18 (4.6 × 250 mm, 3.5 µm) column from Waters (USA). Mobile phase consists of 1% formic acid in water (solvent A) and acetonitrile (solvent B). Gradient conditions are as follows: 0–10 min 13% B, 10–20 min 41.5% B, 20–25 min 70% B, 25–35 min 10% B, total run time is 35 min. The column was equilibrated for 10 min prior to each analysis. Flow rate was 0.5 mL/min, and injection volume was 10 mL. Data acquisition and preprocessing were carried out with Chemstation for LC (Agilent). The monitoring wavelength was 280 nm for gallic acid, chlorogenic acid, caffeic acid, rutin, ellagic acid, kaempherol glycoside, and ferulic acid. Peaks were identified on the basis of comparison of retention times and UV-Vis spectra with standards of phenolic compounds.
ABTS Method
The antioxidant capacity of fruit juice was determined with ABTS [2,2-azino-di-(3-ethylbenzothialozine-sulfonic acid)] method as described in our previous work.[Citation3] Spectrophotometric measurements were performed on a UV/vis spectrometer (Varian Cary 50 Conc, Australia) equipped with 10 mm quartz cuvettes. The absorbance was recorded at 734 nm against blank after 6 min. The results were expressed as mg of trolox equivalent (TE) per liter of fruit juice.
Multivariate Calibration
Two multivariate calibration methods were employed for the prediction of antioxidant capacity of fruit juices. Principal component analysis based methods do not require details about the spectra or concentrations of all the compounds in a mixture, although it is important to make a sensible estimate of how many significant components characterize a mixture. The aim is to convert these components to concentrations in multivariate calibration. PCR uses regression to convert principal component scores to concentration.[Citation19] PLS is often presented as the major regression technique for multivariate data to express the relation between x and y. PLS uses the non-linear iterative PLS algorithm (NIPALS).[Citation20–Citation23]
Calibration Design
A calibration design was used for 25 samples to model the multivariate calibrations. The calibration design is based on five levels, which is coded between –2 and +2 for each compound in the mixture. Calibration and validation sets were prepared by adding x mL of blueberry, cranberry, vitis vinifera, pomegranate, cherry juices into 5-mL calibrated flasks and (5-x) mL water was added to the initial mixture so as to make the final volume 5 mL. The levels –2, –1, 0, 1 and 2 relate to 0.2, 0.4, 0.6, 0.8, and 1 mL of fruit juices. The antioxidant capacities of fruit juices at the five coded levels are shown in . The design has a value of r12 = 0, so the two vectors are orthogonal to one another.[Citation24] The difference vector [0231] and cyclical generator –2, –1, 2, 1 were used in the calibration design. The construction of multilevel calibration designs is described in literature.[Citation25] To see how well the calibration set predicts the antioxidant capacities of the five fruit juices, two independent validation sets were generated. The validation set 1 () has a value of r12 = 0 and the validation set 2 () has a value of r12 = 1. The difference vector [1320] and cyclical generator –2, –1, 2, 1 were used in the validation set 1. The two validation sets consist of 25 chromatograms. The prediction of antioxidant capacities of calibration/validation sets by PCR and PLS models was executed using MATLAB version 8.1.0.604 (R2013a; MathWorks, Inc., Natick, MA, USA).
TABLE 1 Antioxidant capacity of calibration set (difference vector: [0 2 3 1], repeater: –2, –1, 2, 1)
TABLE 2 Antioxidant capacity of validation set 1 (difference vector: [1 3 2 0], repeater: –2, –1, 2)
TABLE 3 Antioxidant capacity of validation set 2
RESULTS AND DISCUSSION
Antioxidant Capacities of Fruit Juices
After generation of calibration and validation sets, antioxidant capacities of calibration and validation sets were determined by ABTS method (–). The antioxidant capacity of blueberry was ranged from 0.386 to 1.930, cranberry from 0.488 to 2.442, vitis vinifera from 0.287 to 1.435, pomegranate from 0.449 to 2.243, and cherry juices from 0.301 to 1.504 mg of TE per liter of fruit juice.
Selection of the Optimum Number of Components
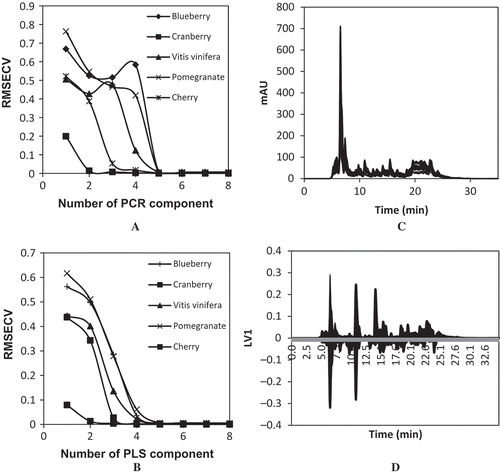
To select the number of factors to be used in the PLS and PCR calibration models a cross-validation procedure was used. In this procedure, calibration is carried out with all calibration samples less one. The process was repeated 25 times for each number of factors until each sample has been left out once. The predicted and known antioxidant capacities of the fruit juices were compared for each giving number of factors. Then the root mean square error of cross-validation (RMSECV) was computed for PCR and PLS models with different number of components.[Citation22]
where, is the measured antioxidant capacity of the ith sample and
is the predicted antioxidant capacity from a calibration equation obtained for the data without the ith sample, M is the number of the calibration samples. The optimum number of factors of the PCR and PLS model corresponds to the number of factors resulting in the lowest RMSECV.
The performance of the calibration model and its prediction ability is measured by the root mean square error (RMSE) obtained on the calibration set and root mean square error of prediction (RMSEP) obtained on the validation set, respectively.
where, M and Mt are the number of samples in the calibration and validation sets, and
denote the experimental value and the predicted value from the model for the ith sample from validation set.
In our case, it was found that the optimum number of factors for each fruit juice was five by the PCR and PLS methods ( and ). It is possible to verify that the lowest value for the RMSECV was obtained using five factors. The number of factors found and the compounds present in the mixture are the same for PCR and PLS calibration methods. In generally, it is expected to get as many factors as compounds are present in the mixture in the case of non-highly overlapped system when standards are used during the calibration step. The results confirm that the system is well modeled by the number of factors selected. The calibration design is seen to be of major importance when assessing the quality of the model.
PCR and PLS Analysis
Multivariate calibration models were built with data matrix x consisting of the 25 chromatograms and a response vector y representing the antioxidant capacity results. The HPLC chromatogram of calibration set was shown in . PCR and PLS calibration models were obtained from calibration set described in experimental design. The aim of PCR calibration model is to convert components to concentrations in multivariate calibration. PLS calibration model was applied for simultaneous determination and removal of the interference effects of one component in the presence of the others. The predictions of antioxidant capacity by ABTS method were calculated based on the RMSE (). It can be seen that the RMSEs of the PLS based calibration model show significant improvement over PCR. The results indicate that the predicted antioxidant capacities by PLS model is close to the real antioxidant capacities for each fruit juice that reveal the validity of the proposed method. The two independent validation sets were generated with values of r12 at 0.0 and 1.0 to see how well the calibration set predicts the antioxidant capacities of five fruit juices. The RMSEs of the predictions by the PCR and PLS calibration models for calibration set and two validation sets are shown in . Both validation sets of results show different trend, in that RMSE values increase as the value of the correlation coefficient of the validation set increases. This indicates that a well-constructed design with r12 at 0.0 would give the lowest error when predicting the validation set with r12 at 0.0 and badly designed validation set with r12 at 1.0 has considerably higher error. This is because a well-constructed validation set is easier to predict by a well-constructed calibration set, whereas less well-constructed validation set will be hard to predict.
TABLE 4 Root mean square errors for the prediction of antioxidant capacity (mg TE/L fruit juice)
One of the major aspects of this study is to identify the compounds in the fruit juices potentially responsible for the antioxidant capacity of the samples. A great deal of information can come from loadings plots. For this purpose, the loading plot for PLS model was evaluated to investigate the contribution of phenolic compounds to the antioxidant capacity (). It can be stated that the larger the loading of a phenolic compound on LV1, the larger the influence on the predicted antioxidant capacity. The peaks with negative loadings correspond to the compounds with high antioxidant capacity. Peaks at 7.7, 8.9, 10.4, 12.1, 13.1, 15.4, 17.6, 20.1, 21.1, 22.4, 22.7, 23.4, 24.5, and 26.2 min with negative PLS loadings contribute to the antioxidant capacity in samples. It was observed that seven compounds are in small and high amount present in the fruit juices. Gallic acid (peak at 7.7 min), chlorogenic acid (peak at 15.4 min), caffeic acid (peak at 20.1 min), rutin (peak at 22.4 min), ellagic acid (peak at 22.7 min), kaempherol glycoside (peak at 23.4 min), and ferulic acid (peak at 24.5 min) have negative PLS loadings. Antioxidant capacity has positive correlation with the concentration of proanthocyanidins, flavonoids, and phenolic compounds.[Citation26] Thus, phenolic compounds might be the major components responsible for antioxidant capacity of fruit juices. The phenolic compounds (gallic acid, chlorogenic acid, caffeic acid, rutin, ellagic acid, kaempherol glycoside, and ferulic acid) determined in fruit juices seem to be responsible for antioxidant capacity. Negative PLS loadings give information about the phenolic compounds that are responsible for antioxidant capacity in fruit juices. However, in the case of mixture of phenolic compounds in fruits/plants, antioxidant capacity may not have correlation with total phenolics because of the different type of phenolic compounds, number of OH groups and their positions in phenolic compound structure. Therefore, in the case of unknown individual phenolic compounds present in the sample, it is better to explain the antioxidant capacity with the presence/absence of certain phenolic compounds. This implies that all phenolic compounds represented by a peak in the chromatogram should give antioxidant capacity proportional to their concentration. Peaks at 8.7 and 13.1 min with negative PLS loadings have high influence on the antioxidant capacity, they show high contribution to the PLS model. However these peaks have not identified.
Determination of Antioxidant Capacity of Real Samples
In order to test the applicability of the proposed method to the analysis of real samples, the method was applied to a mixture containing 25 different fruit juices. The antioxidant capacities of fruit juices were investigated and the results were summarized in . The prediction of RMSEs of PCR and PLS were 1.1245 and 0.4101, respectively. It can be seen that the results obtained by PLS method is very similar with the measured results. The PLS method was rapid, easy, and of low cost for the determination of antioxidant capacity of fruit juices in mixture.
TABLE 5 Measured/predicted antioxidant capacities (mg TE/L fruit juice) of real samples
CONCLUSION
Calibration models of PCR and PLS were constructed to model the antioxidant capacity of fruit juices from the HPLC profile and relate the peaks responsible for the antioxidant capacity. The effectiveness of PLS model for predictions of antioxidant capacities was described in the chromatograms of the fruit juices. PLS calibration model has some advantages over PCR and performs slightly better prediction. Calibration design and construction of validation set are seem to be of major importance when assessing the quality of the model. It is shown that the low correlation coefficient (r12 = 0) between the concentrations in the validation set results much lower prediction error.
By comparing the peaks in the chromatograms with the loadings of the calibration models, peaks responsible for antioxidant capacity can be identified. The application of PLS resulted in better model to predict the antioxidant capacity of fruit juices from chromatograms, and the contribution of each compound to the antioxidant capacity is easy to interpret. Peaks with major negative PLS loadings are responsible for the antioxidant capacity determined by HPLC as gallic acid, chlorogenic acid, caffeic acid, rutin, ellagic acid, kaempherol glycoside, and ferulic acid in fruit juices. The retention time of seven peaks indicated potentially interesting compounds with negative PLS loadings.
REFERENCES
- Šamec, D.; Piljac-Žegarac, J.; Fluctuations in the levels of antioxidant compounds and antioxidant capacity of ten small fruits during one year of frozen storage. International Journal of Food Properties 2015, 18, 21–32.
- Katalinic, V.; Mozina, S.S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic profile, antioxidant capacity, and antimicrobial activity of leaf extracts from six Vitis vinifera L. varieties. International Journal of Food Properties 2013, 16, 45–60.
- Sarıburun, E.; Şahin, S.; Demir, C.; Türkben, C.; Uylaşer, V. Phenolic content and antioxidant activity of raspberry and blackberry cultivars. Journal of Food Science 2010, 75(4), 328–335.
- Heinonen, M.; Lehtonen, P.J.; Hopia, A.I. Antioxidant activity of berry and fruit wines and liquors. Journal of Agricultural and Food Chemistry 1998, 46, 25–31.
- Işık, E.; Şahin, S.; Demir, C.; Türkben, C. Determination of total phenolic content of raspberry and blackberry cultivars by immobilized horseradish peroxidase bioreactor. Journal of Food Composition and Analysis 2011, 24, 944–949.
- Minnunni, M.; Wolleb, U.; Mueller, O.; Pferfer, A.; Aeschbacher, H.U. Natural antioxidants as inhibitors of oxygen species induced mutagenicity. Mutation Research 1992, 269, 193–200.
- Şahin, S.; Demir, C.; Malyer, H. Determination of total phenolic content of Prunella L. by immobilized enzyme bioreactor. Analytical Methods 2011, 3, 944–950.
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chemistry 2009, 114, 310–316.
- Lam, H.S.; Proctor, A.; Howard, L.; Cho, M.J. Rapid fruit extracts antioxidant capacity determination by Fourier transform infrared spectroscopy. Journal of Food Science 2005, 70, C545–C549.
- Shahid, I.; Saba, H.; Mubeena, A.; Muhammad, Z.; Jamshed, A. Efficiency of pomegranate peel extracts in stabilization of sunflower oil under accelerated conditions. Food Research International 2008, 41, 194–200.
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of Drumstick tree (Moringa oleiferea Lam.) leaves. Journal of Agricultural and Food Chemistry 2003, 51, 2144–2155.
- Buettner, G.R.; Jurkiewicz, B.A. Handbook of Antioxidants; Cadenas, E.; Packer, L.; Ed.; Dekker: New York, 1996; 91–115.
- Işık, E.; Şahin, S.; Demir, C. Development of a new chromium reducing antioxidant capacity (CHROMAC) assay for plants and fruits. Talanta 2013, 111, 119–124.
- Özgen, M.; Reese, R.N.; Tulio, A.Z. Jr.; Scheerens, J.C.; Miller, A.R. Modified 2,2- azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (abts) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2’-diphenyl-1-picrylhydrazyl (DPPH) methods. Journal of Agricultural and Food Chemistry 2006, 54, 1151–1157.
- Şahin, S.; Demir, C.; Güçer, Ş. Simultaneous UV-vis spectrophotometric determination of disperse dyes in textile wastewater by partial least squares and principal component regression. Dyes Pigments 2007, 73, 368–376.
- Şahin, S.; Işık, E.; Aybastıer, Ö.; Demir, C. Orthogonal signal correction-based prediction of total antioxidant activity using partial least squares regression from chromatograms. Journal of Chemometrics 2012, 26, 390–399.
- Leopold, L.F.; Leopold, N.; Diehl, H.A.; Socaciu, C. Prediction of total antioxidant capacity of fruit juices using FTIR spectroscopy and PLS regression. Food Analytical Methods 2012, 5, 405–407.
- Liang, Y.Z.; Xie, P.; Chan, K. Quality control of herbal medicines. Journal of Chromatography B 2004, 812, 53–70.
- Brereton, R.G. Chemometrics Data Analysis for the Laboratory and Chemical Plant; John Wiley & Sons: Chichester, UK, 2003; 489.
- Daszykowski, M.; Vander Heyden, Y.; Walczak, B. Robust partial least squares model for prediction of green tea antioxidant capacity from chromatograms. Journal of Chromatography A 2007, 1176, 12–18.
- Nguyen, H.N.; Dejaegher, B.; Tistaert, C.; Nguyen, T.H.V.; Rivière, C; Chataigné, G.; Phan, V.K.; Chau, V.M.; Quetin-Leclercq, J.; Vander Heyden, Y. Development of HPLC fingerprints for Mallotus species extracts and evaluation of the peaks responsible for their antioxidant activity. Journal of Pharmaceutical and Biomedical Analysis 2009, 50, 753–763.
- Tistaert, C.; Dejaegher, B.; Nguyen, H.N.; Chataigné, G.; Rivière, C.; Nguyen, T.H.; Chau, V.M.; Quetin-Leclercq, J.; Vander Heyden, Y. Potential antioxidant compounds in Mallotus species fingerprints. Part I: Indication, using linear multivariate calibration techniques. Analytica Chimica Acta 2009, 649, 24–32.
- Van Nederkassel, A.M.; Daszykowski, M.; Massart, D.L.; Vander Heyden, Y. Prediction of total green tea antioxidant capacity from chromatograms by multivariate modeling. Journal of Chromatography A 2005, 1096, 177–186.
- Zissis, K.D.; Brereton, R.G.; Escott, R. Partial least-squares calibration of two-way diode-array high-performance liquid chromatograms: Influence of calibration design, noise, and peak separation. Analyst 1998, 123, 1165–1173.
- Brereton, R.G. Multilevel multifactor designs for multivariate calibration. Analyst 1997, 122, 1521–1529.
- Wang. H.-C.; Hu, Z.-Q.; Wang, Y.; Chen, H.-B.; Huang, X.-M. Phenolic compounds and the antioxidant activities in litchi pericarp: Difference among cultivars. Scientia Horticulturae 2011, 129, 784–789.