Abstract
Tomato and lettuce are main components of the Mediterranean Diet and it is important to know their content in phenolic compounds, considering they have beneficial effects on human health. In this work, more than 25 phenolic compounds were simultaneously analyzed by ultra-high performance liquid chromatography coupled to triple quadrupole tandem mass spectrometry, including phenolic acids, flavonols, and flavones. Several varieties of lettuce and tomato were evaluated and total phenolic content ranged from 68 to 212 mg/kg dry weight for lettuce, and from 341 to 1296 mg/kg dry weight for tomato, being the chlorogenic acid the compound detected at the highest concentration in both types of vegetables (lettuce from 9 to 126 mg/kg dry weight, tomato from 96 to 538 mg/kg dry weight), although in tomato the content of quercetin-3-O-rutinoside (from 29 to 460 mg/kg dry weight) was also important. On the other hand, the minor compounds detected were quercetin-3-O-derivate (up to 1 mg/kg dry weight) in tomato, and apigenin O derivate, luteolin C glucoside, and isorhamnetin 3-O-glucoside in lettuce. It was observed that the total phenolic content in the plum cherry tomato was significantly higher than in the other varieties, whereas in lettuce, the differences were not statistically different.
INTRODUCTION
Diets rich in fruits and vegetables are consistently associated with a decreased risk of cardiac disfunctions,[Citation1,Citation2] atherosclerosis,[Citation3] cancer,[Citation4,Citation5] diabetes,[Citation6] and neurodegenerative disorders.[Citation7,Citation8] This prevention function of fruit and vegetables is due to their content in phenolic compounds,[Citation9] which have antioxidant effect,[Citation10,Citation11] and they can influence oxidative stress mechanisms, which is a common cause in the pathogenesis of most chronic diseases.[Citation12]
Recent studies have demonstrated that tomato and lettuce are an interesting and cheap source of antioxidant phenolic compounds.[Citation13–Citation15] These vegetables are the most popular vegetables in salads, and the consumption of these products is being increased due to they are considered as “healthy foods.” In this sense, about 162 and 25 million tons of tomato and lettuce, respectively, were produced over the world in 2012.[Citation16] In order to perform a correct valorization of these vegetables, the composition of phenolic compounds in these products must be needed. European labeling regulation[Citation17] requires that nutrition and health claims, which are made on the labels of the products or any form of consumer advertising, should be based on scientific studies, where the composition of phenolic compounds, including qualitative and quantitative characteristics, must be clearly specified. In this sense, it has been reported that caffeic acid derivates have been the main phenolic compounds in green varieties of lettuce, ranging from 14.1 to 102.1 mg/100 g fresh weight (FW), while flavonoids were detected at higher concentration in red varieties of lettuce, ranging between 150 and 322 mg/100 g FW.[Citation18] Although higher concentrations of 61 caffeic acid derivates were also detected (from 146 to 203 mg/100 g FW) in red varieties, other authors also identified caffeoyl derivates as the largest phenolic group (90%) in Romaine lettuce.[Citation19] However, Khanam et al.[Citation20] found neither caffeic acid nor chlorogenic acid in lettuce samples. On the other hand, isoflavones were found in Iceberg lettuce at concentrations ranging from 6.4 to 16.4 μg/100 g dry weight (DW).[Citation21] In relation to tomato, the presence of several phytochemicals in different varieties of tomato, such as Daniela,[Citation14] Raf,[Citation14] Rambo,[Citation14] Purple,[Citation22] Cherry,[Citation23] and in 11 varieties of Korean tomato,[Citation24] has been evaluated. In all varieties phenolic acids have been identified as the most frequently phenolic compounds detected, being chlorogenic acid, the phenolic acid detected at the highest concentrations, from 85 to 805 mg/kg.[Citation14,Citation22–Citation24] Caffeic acid and ferulic acid have also been detected at concentrations ranging from 43 to 160 mg/kg DW, and from 20 to 270 mg/kg DW, respectively.[Citation14,Citation22] Flavonols have also been found in tomato, being quercetin-3-O-rutinoside the flavonol detected at high concentrations (from 36 to 888 mg/kg DW).[Citation14,Citation22–Citation24] This high variability can be explained considering that the content of phenolic compounds in vegetable tissues can be influenced by several factors as variety, crop type, environmental conditions, location, germination, maturity, processing, and storage.[Citation25–Citation27]
Regarding the analysis of phenolic compounds in vegetables, it must be highlighted that the extraction is usually performed using solid liquid extraction (SLE) utilizing as extraction solvent a mixture of methanol and water,[Citation28] adding modifiers such as chlorhidric acid,[Citation29] formic acid,[Citation18] or acetic acid.[Citation20] It has also been used as extractant a mixture of ethanol:water acidified with formic acid.[Citation30] Moreover, microwave assisted extraction using a mixture of methanol:water has been checked[Citation31] as well. It should be highlighted that the quick, easy, cheap, effective, rugged, and safe (QuEChERS) methodology with clean up was also used for the extraction of phenolic compounds in different matrices, using acetonitrile:ethyl acetate (50:50, v/v) containing 1% formic acid as extraction solvent.[Citation32] Ultra-high performance liquid chromatography coupled to triple quadrupole tandem mass spectrometry (UHPLC-QqQ-MS/MS) has been applied for the detection of a wide range of phenolic compounds in different vegetable matrices[Citation33–Citation36] due to its advantages such as selectivity, sensitivity, and speed, allowing the separation and quantification of a high number of compounds in less than 20 min.[Citation33,Citation36] The aim of the present work has been the determination of the polyphenol composition in different varieties of tomato (cherry, cherry plum, vine, plum, raf, kumato, bola, and salad) and lettuce (iceberg, romaine, mini-romaine, trocadero, and heart-mini-romaine) applying SLE and UHPLC-MS/MS determination. For that purpose, a method previously performed has been validated in lettuce.
MATERIAL AND METHODS
Chemicals and Reagents
Commercial phenolic compound standards were purchased from Extrasynthese (Genay, France) and Sigma-Aldrich (Steinheim, Germany). Stock standard solutions of individual compounds (with concentrations between 200 and 300 mg/L) were prepared by precise weighing of the powdered compounds, which were dissolved in 10 mL of HPLC grade methanol or in a mixture of methanol:water (50:50, v/v), and stored at –20ºC in dark bottles. A multicompound working standard solution at a concentration of 5 mg/L of each compound was prepared by appropriate dilution of the stock solutions with methanol and stored in screw-capped glass tubes at –20ºC. The solutions were each prepared for six months. Formic acid (purity > 98%), ethyl acetate (purity ≥ 99.7%), and disodium hydrogencitrate sesquihydrate acetate were provided by Sigma (Madrid, Spain). Ultrapure water was obtained from a Milli-Q Gradient water system (Millipore, Bedford, MA, USA). Ammonium acetate and anhydrous magnesium sulphate were purchased from Panreac (Barcelona, Spain). Sodium chloride and sodium citrate dehydrate were obtained from J.T. Baker (Deventer, The Netherlands). Primary secondary amine (PSA) and acetonitrile were obtained from Scharlab (Barcelona, Spain). Bondesil-C18 was purchased from Agilent Technologies (Santa Clara, CA, USA). Millex-GN nylon filters of 0.20-µm were provided by Millipore (Millipore, Carrightwohill, Ireland).
Equipment and Software
Chromatographic analysis was carried out using an Agilent series 1290 RRLC instrument equipped with a binary pump (G4220A), a high-performance autosampler (G4226A), an autosampler thermostat (G1330B), and a column compartment thermostat (G1316C). The system was coupled to an Agilent triple quadrupole mass spectrometer (6460A) with a Jet Stream ESI ion source (G1958-65138). For the chromatographic separation of the extracts, a Zorbax Eclipse Plus C18 column (100 mm × 2.1 mm, 1.8 µm particle size) from Agilent was used. Chromatographic separation was carried out using a gradient elution with methanol as eluent A, and an aqueous solution of ammonium acetate (30 mM), adjusted to pH 5 with formic acid, was used as eluent B. The elution started at 5% of eluent A for 1.5 min, and then it was increased to 30% of eluent A in 2.5 min. After that, it was increased to 100% in 4 min. This composition was kept constant during 2 min, before being returned to the initial conditions in 0.5 min, keeping this composition 1.5 min prior the next analysis, obtaining a total run time of 12 min. The flow rate was set at 0.2 mL/min. The detection was performed using electrospray (ESI) ionization in positive and negative ion modes. The chromatographic conditions used to evaluate the profile of phenolic compounds in different matrices were optimized previously.[Citation37] shows the MS/MS transitions, as well as the fragmentor voltage and collision energies optimized for each compound.
TABLE 1 UHPLC-MS/MS parameters
An Agilent Mass Hunter Quantitative analysis (Agilent Technologies, Inc.) was used for data acquisition and quantification of samples. Statistical analysis was carried out with IBM SPSS Statistics v21 (Armonk, New York, USA). A Reax-2 rotary agitator from Heidolph (Schwabach, Germany), an ultrasonic equipment from Elma (Singen, Germany), lyophilizer Alpha from Martin Christ (Osterode, Germany), and vacuum pump from Vacuubrand (Wertheim, Germany) were also utilized.
Extraction Procedure
Two methodologies were evaluated to extract phenolic compounds in lettuce, bearing in mind that the methodology was previously validated in tomato.[Citation37] First, the QuEChERS approach was used bearing in mind it has been previously used for the determination of low molecular weight phenols in vegetables.[Citation32] For that purpose, 10 g lettuce were extracted with 10 mL of mixture of acetonitrile:ethyl acetate (50:50, v/v) containing 1% formic acid. The tube was shaken vigorously for 2 min. Trisodium citrate dihydrate (1 g), disodium hydrogencitrate sesquihydrate (0.5 g), sodium chloride (1 g), and anhydrous MgSO4 (4 g) were added and the mixture was centrifuged at 5000 rpm for 5 min. Then, 1 mL of the supernatant was transfer to an Eppendorf tube and 25 mg of PSA, 25 mg of C18 and 150 mg of magnesium sulphate were added for clean-up step. The mixture was shaken in a vortex and centrifuged again at 5000 rpm for 5 min. An aliquot of the extract was evaporated under nitrogen stream to near dryness and the residue was taken up with 500 µL of mobile phase (50:50 v/v of methanol and aqueous solution of ammonium acetate (30 mM), adjusted to pH 5 with formic acid). Finally the sample was filtered prior UHPLC-QqQ-MS/MS analysis.
Second, SLE was evaluated. In this procedure, the homogenized samples were transferred to a Petri dish, weighed, and cooled to –18ºC. Then, all samples were processed according to the following procedure: 150 mg of lyophilized sample were weighed in a 15 mL polypropylene centrifuge tube and 3 mL of methanol:water (80:20, v/v) were added. The mixture was agitated for 30 min using a rotary shaker. After that, the extract was filtered and 300 µL were transferred into a vial containing 200 µL of mobile phase (50:50 v/v of eluent A and B), and 5 μL were injected into the UHPLC system.[Citation37]
Samples
Twenty-four samples of eight varieties of tomato were collected, whereas 27 samples of lettuce from six varieties, some of them bought as fresh-cut samples, were analyzed. In fact, three samples for Trocadero and Mini-Romaine Heart, four samples for Iceberg, five samples for Iceberg fresh-cut, and six samples for Romaine and Mini-Romaine were analyzed in lettuce. For tomato, three samples of each variety (cherry, plum cherry, plum, Raf, vine, Kumato, Bola, and salad) were analyzed. All samples were collected from different supermarkets located in the province of Almería in order to get as many varieties as possible that can be commonly available for consumers. In all cases, the amount of sample used was 1 kg, except for fresh-cut lettuce, using 1 bag (250 g). Samples were chopped and homogenized at room temperature as fast as possible in order to minimize phenolic oxidation. The whole process lasted less than 1 min and after that, homogenized samples were kept freeze at –18°C until lyophilization.
RESULTS AND DISCUSSION
Optimization of the Extraction Procedure and Validation in Lettuce
The aim of this study was the achievement of a simple and fast extraction method to be applied in routine analysis for the simultaneous extraction of phenolic compounds in lettuce. For that purpose QuEChERS[Citation32] and SLE[Citation37] have been evaluated. For the optimization process, Iceberg lettuce was fortified with 5 mg/kg of phenolic compounds. When both procedures were tested, it was observed that the best results were obtained when SLE was carried out, obtaining recovery values higher than 60%, while for the QuECHERS procedure recoveries were lower than 50% for the majority of the compounds included in this study. According to the results, the validation procedure was performed using SLE procedure. Then, a validation protocol of the selected procedure was carried out in order to establish the performance characteristics of the method, ensuring the adequate identification, confirmation and quantification of the target compounds in lettuce, evaluating matrix effect, linearity, trueness, precision (intra and inter-day), and lower limits.
The identification of the phytochemicals was carried out by searching the appropriate retention time windows (RTWs) defined as the retention time ± three times the standard deviation of the retention time of ten standards at 0.5 mg/L. Compounds were confirmed by the acquisition of two MS/MS transitions and comparing the ion ratio of both transitions (quantification and confirmation).
Matrix effect was studied to ensure bias-free analytical results. Lettuce samples were spiked after extraction with the phytochemicals at different concentrations (0.05–2 mg/kg), and the slopes of the calibration plots were compared with results obtained when standard solutions in solvent of the phytochemicals were analyzed. shows slope ratios matrix/solvent for each compound in lettuce, considering a tolerable signal suppression or enhancement effect if the slope ratio ranged between 0.8 and 1.2, whereas values lower than 0.8 or higher than 1.2, implies a strong signal suppression or enhancement, respectively. It can be observed that matrix significantly suppresses the signal for apigenin, apigenin O derivate, chlorogenic acid, gallic acid, and genistein. On the other hand, signal enhancement was observed for apigenin C glucoside, isorhamnetin 3-O-rutinoside, quercetin, quercetin-3-O-glucoside, and quercetin 3-O-rutinoside. The rest of phenolic compounds did not present matrix effect. In order to compensate this effect, standard addition calibration was used for quantification purposes. Then, the linearity of the method was studied, using a sample extract to prepare the calibration curve. In all the cases, determination coefficient was higher than 0.98, and deviation of the individual points from the calibration curve was always lower than 20%.
FIGURE 1 Slope ratios between matrix-matched and solvent calibration. Compliance interval covering the range between 0.8 and 1.2 for tolerable matrix effect has been plotted. Phenolic compound codes are indicated in .
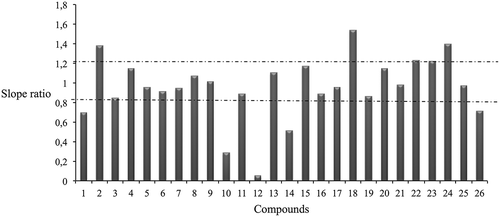
Limits of detection (LODs) and quantification (LOQs) were determined as the lowest concentration level that yielded a signal-to-noise (S/N) ratio of 3 and 10 (when the quantification ion was monitored), respectively, showing the obtained results in lettuce in . LODs ranged from 5 to 25 µg/kg, whereas LOQs ranged from 10 to 50 µg/kg ().
TABLE 2 Validation parameters of the optimized UHPLC-MS/MS method in lettuce
Trueness was estimated through recovery studies. Before extraction, different aliquots of lettuce (n = 5) were spiked at two levels, 2.5 and 5 mg/kg, with the target compounds and they were extracted with the developed method (S1). On the other hand, other aliquots of the same lettuce sample (n = 5) were extracted without spiking (S0) and recovery was calculated as follows: R = 100 × (S1 – S0)/Cspiked. shows the obtained results, and it can be observed that recoveries ranged from 60 (quercetin) to 98% (quercetin 3-O-derivate) for the selected compounds at 2.5 mg/kg, and from 60 (quercetin) to 102 % (quercetin 3-O-derivate) at 5 mg/kg. Precision of the overall method was estimated by performing both repeatability and reproducibility (inter-day precision) studies. Repeatability was evaluated at 2.5 and 5.0 mg/kg of the recovery studies, performing three replicates at each level (). It can be noted that repeatability values (expressed as relative standard deviation, RSD) were always lower than 23%. Inter-day precision was evaluated at 5 mg/kg in five different days (see ), obtaining values lower than 25%.
Analysis of Phenolic Compounds
Lettuce
The most common varieties of lettuce as Iceberg, Romaine, Mini-Romaine, Trocadero, and Mini-Romaine Heart were analyzed. The results are shown in , and it can be observed that the highest concentration of phytochemicals was found in the Mini-Romaine (212 mg/kg DW) and Mini-Romaine Heart (205 mg/kg DW) varieties, followed by Romaine (136 mg/kg DW). On the other hand, fresh Iceberg lettuce presented the lowest level of the assayed compounds (68 mg/kg DW). Phenolic acid was the family of phenolic compounds detected at the highest concentrations in lettuce, except for Iceberg lettuce. For this variety, flavonols were detected at higher values than the other families. The variety with higher concentration of phenolic acids was Mini-Romaine Heart (131 mg/kg DW), followed by Mini-Romaine (120 mg/kg DW), and Romaine (68 mg/kg DW). In general, it should be noted that there is high variability in the content of phenolic acids in the assayed varieties as it can be observed in . Chlorogenic acid was uppermost phenolic acid, and it was detected at concentrations up to 126 mg/kg DW in Mini-Romaine Heart, followed by Mini-Romaine (113 mg/kg DW), and Romaine (60 mg/kg DW) as it can be observed in . Caffeic acid was also detected, although the concentration was lower than chlorogenic acid, and it was found in Romaine and Trocadero varieties at 6 mg/kg DW and 5 mg/kg DW, respectively. Ferulic acid was also common in all varieties of lettuce and the concentration ranged from 1 to 2 mg/kg DW. Iceberg lettuce presented the lowest concentrations of phenolic acids (16 mg/kg DW).
TABLE 3. Content of phenolic compounds (mg/kg DW) in different varieties of lettuce
FIGURE 2 Concentration of the phenolic compounds (mg/kg DW) in: (a) lettuce (a) and (b) tomato. Three samples for Trocadero and Mini-Romaine Heart, four samples for Iceberg, five samples for Iceberg fresh-cut, and six samples for Romaine and Mini-Romaine were analyzed in lettuce. For tomato, three samples of each variety were analyzed.
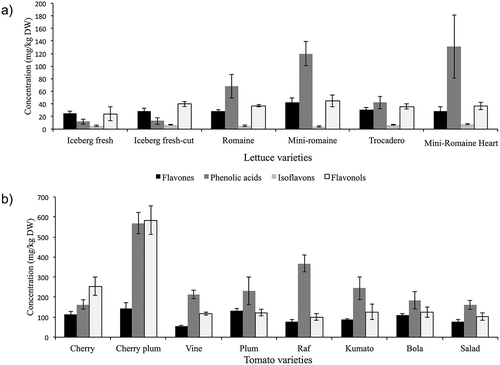
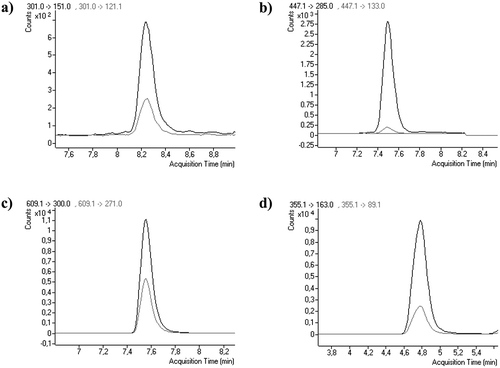
The flavonoids were grouped in flavonols, flavones, and isoflavones. The most common flavonols in lettuce were quercetin (10 mg/kg DW), followed by isorhamnetin (5 mg/kg DW), and tamarexetin (5 mg/kg DW). Quercetin, kaempferol, and isorhamnetin glycosides were also detected in the varieties of lettuce included in this study. Among flavones, luteolin was found as the most abundant, and the detected concentration ranged from 13 mg/kg DW (Iceberg) to 22 mg/kg DW (Mini-Romaine) as it can be observed in . The isoflavones was the family with the lowest concentrations in all varieties of lettuce (). In , some chromatograms of the compounds detected in lettuce are shown.
FIGURE 3 UHPLC-MS/MS MRM chromatograms for tomato and lettuce containing: (a) Quercetin at 20 mg/kg DW in trocadero lettuce; (b) Luteolin-O-glucoside at 17 mg/kg in mini-romaine lettuce; (c) Quercetin-3-O-rutinoside at 25 mg/kg DW in plum tomato; and (d) Chlorogenic acid at 317 mg/kg DW in Raf tomato.
An analysis of variance (ANOVA) was performed in order to check if the observed difference between the content of phenolic compounds depends on the variety evaluated. It was observed that when the total content of phenolic compounds was used as response, there was a significant difference between the varieties evaluated (p = 0.023). In relation to the family of polyphenols, only the content of flavones (p = 0.038) were significantly affected by the variety of lettuce. Although the content of phenolic acids and isoflavones were not affected by variety, these families showed a p-value of 0.072 and 0.084, respectively, which is close to the significant value (p-value < 0.05).
When fresh and fresh-cut Iceberg lettuces were compared, fresh-cut lettuce presented higher concentration of total phenolic compounds than fresh lettuce, with a mean content of 90 and 68 mg/kg DW, respectively. However, when ANOVA was performed it was observed that neither the total content nor the different families of phenolic compounds changed significantly due to minimally processed, being p-value much higher than 0.05 in all the cases, although for isoflavones, which was 0.054.
These results are in agreement with those obtained in other studies. Thus, Llorach et al.[Citation18] observed that Romaine had higher concentration of phenolic compounds than Iceberg, being phenolic acids followed by flavonols and flavones, the families detected at higher concentrations in this variety of lettuce. However, it was noted in this study that this trend is not unique, and there are some varieties, as Iceberg lettuce, where flavonoids content was higher than phenolic acids. Regarding isoflavones, Kuhnle et al.[Citation21] and Konar et al.[Citation38] also detected isoflavones in Iceberg lettuce (up to 0.164 mg/kg DW). Finally, it is important to highlight that no previous studies evaluating the presence of phenolic compounds in other varieties (Trocadero, Mini-Romaine, and Mini-Romaine Heart) were found.
Tomato
The results obtained for different varieties of tomato are shown in , and shows some chromatograms of the compounds detected in tomato. The highest concentration of phenolic compounds was found in plum cherry tomato (1292 mg/kg DW) followed by Raf (553 mg/kg DW), cherry (545 mg/kg DW), and Plum tomato (486 mg/kg DW). On the other hand, Vine (384 mg/kg DW) and Salad tomato varieties (341 mg/kg DW) presented the lowest level of the assayed compounds.
TABLE 4 Content of phenolic compounds (mg/kg DW) in different varieties of tomato
The family of phenolic compounds present at higher concentrations in tomato was phenolic acids, except for cherry and plum cherry tomato. For these varieties, flavonols were the most abundant compounds as it can be observed in . Among phenolic acids, chlorogenic acid was detected at high concentrations ranging from 96 mg/kg DW (salad tomato) to 538 mg/kg DW (plum cherry tomato) as it can be observed in . The second most abundant phenolic acid was caffeic acid, which was detected at concentrations ranging from 21 mg/kg DW in cherry or vine tomato to 62 mg/kg DW in Raf variety.
Regarding flavonols, the flavonol detected at higher concentrations in different varieties of tomato was quercetin 3-O-rutinoside, whose concentration ranged from 29 mg/kg DW (plum) to 460 mg/kg DW (plum cherry), cherry and plum cherry tomato being the varieties with the highest concentration of this compound (149 and 460 mg/kg DW, respectively). In relation to flavones, the concentration ranged from 55 (vine) to 144 mg/kg DW (plum cherry) as it can be observed in .
ANOVA was performed in order to check if the observed difference between the content of phenolic compounds depends on the variety evaluated. It can be observed that all families of phenolic compounds presented significant differences, mainly due to the high content of phenolic compounds in plum cherry tomato. This variety shows a significant difference in the total content of phenolic compounds as well as phenolic acids and flavonols content when it was compared with the other varieties (p < 0.01). However, in relation to flavones content, plum cherry tomato only shown significant differences with vine (p = 0.014) and salad tomato (p = 0.031) varieties.
When these results are compared to those published previously, it can be observed that most of the studies were performed on the cherry tomato.[Citation23,Citation39,Citation40] For instance, Sánchez-Rodríguez et al.[Citation23] analyzed different types of cherry tomato, detecting chlorogenic acid (150–639 mg/kg DW), quercetin-3-O-rutinoside (208–526 mg/kg DW), kaempferol-3-O-rutinoside (12–122 mg/kg DW) in the analyzed samples, which are similar to those obtained in this study. However, Di Lecce et al.[Citation39] obtained the highest concentration of quercetin-3-O-rutinoside (3452.8 mg/kg DW), whereas chlorogenic acid was detected at lower concentrations (39 mg/kg DW) in the cherry tomato than in the present study. Phenolic compounds in the Raf tomato were also analyzed by Gómez-Romero et al.[Citation14] obtaining higher concentrations of chlorogenic acid (531 mg/kg DW) and quercetin-3-O-rutinoside (140 mg/kg DW) than the present results. Martinez-Valverde et al.[Citation41] analyzed the plum and vine tomato among other varieties (Rambo, Senior, Liso, Canario, Durina, Daniella, and Remate). In that study, flavonoids were determined as their aglycones, obtaining concentrations of quercetin and kaempferol, ranging from 166 and 481 mg/kg DW and 19 and 33 mg/kg DW, respectively. Chlorogenic acid (520 mg/kg DW in vine tomato and 286 mg/kg DW in plum tomato) and caffeic acid (260 mg/kg DW in vine tomato and 246 mg/kg in plum tomato) were obtained at higher concentration than in the present work. Finally, it is important to indicate that, to the best of the authors’ knowledge, this is the first time that phenolic compounds were determined in the following varieties of tomato: plum cherry, Kumato, Bola, and salad.
CONCLUSIONS
In this work, the content of phenolic compounds in different varieties of lettuce and tomato was analyzed. The extraction method previously performed for the tomato, was checked for the suitable extraction of these compounds from lettuce, providing better results than the QuEChERS procedure. The results showed that total content of phenolic compounds was not affected by lettuce variety and/or minimally processing. Mini-Romaine and Heart of Mini-Romaine were the varieties with the highest concentration of phenolic compounds. Regarding the tomato, plum cherry tomato was the variety that presented the highest concentration of phenolic compounds, which was significantly higher than the rest of varieties. Therefore, the plum cherry tomato and Mini-Romaine lettuce are the best varieties to prepare a salad, bearing in mind the phenolic compounds content. Due to its highest content in phenolic compounds, the salads should have more tomato than lettuce.
FUNDING
The authors are grateful to Andalusian Regional Government (Regional Ministry of Innovation, Science, and Enterprise) and FEDER for financial support Proyect Ref. P11-AGR-7034. MIAF acknowledges her grant (FPU, Ref: AP 2009-2074) from the Spanish Ministry of Education. RRG is also grateful for personal funding through Ramon y Cajal Program (Spanish Ministry of Economy and Competitiveness-European Social Fund).
Additional information
Funding
REFERENCES
- Fraga, C.G.; Litterio, M.C.; Prince, P.D.; Calabró, V.; Piotrowski, B.; Galleano, M. Cocoa flavanols: Effects on vascular nitric oxide and blood pressure. Journal of Clinical Biochemistry and Nutrition 2011, 48, 63–67.
- Park, S.K.; Tucker, K.L, O’Neill, M.S.; Sparrow, D.; Vokonas, P.S.; Hu, H.; Schwartz, J. Fruit, vegetable, and fish consumption and heart rate variability: The Veterans administration normative aging study. The American Journal of Clinical Nutrition 2009, 89, 778–786.
- Bernardes, S.; Caramori, P.R.A. Stages of change for fruit and vegetable intake among patients with atherosclerotic disease. Appetite 2011, 57, 656–660.
- Gonzalez, C.A.; Lujan-Barroso, L.; Bueno-de-Mesquita, H. B(as), Jenab, M.; Duell, E.J.; Agudo, A.; … Riboli, E. Fruit and vegetable intake and the risk of gastric adenocarcinoma: A reanalysis of the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study after a longer follow-up. International Journal of Cancer 2012, 131, 2910–2919.
- Reiss, R.; Johnston, J.; Tucker, K.; DeSesso, J.M.; Keen, C.L. Estimation of cancer risks and benefits associated with a potential increased consumption of fruits and vegetables. Food and Chemistry Toxicology 2012, 50, 4421–4427.
- Carter, P.; Gray, L.J.; Talbot, D.; Morris, D.H.; Khunti, K.; Davies, M.J. Fruit and vegetable intake and the association with glucose parameters: A cross-sectional analysis of the Let’s Prevent Diabetes Study. European Journal of Clinical Nutrition 2013, 67, 12–17.
- Essa, M.M.; Vijayan, R.K.; Castellano-Gonzalez, G.; Memon, M.A.; Braidy, N.; Guillemin, G.J. Neuroprotective effect of natural products against Alzheimer’s disease. Neurochemical Research 2012, 37, 1829–1842.
- Lau, F.C.; Shukitt-Hale, B.; Joseph, J.A. Mini review beneficial effects of berry fruit polyphenols on neuronal and behavioral aging. Journal of the Science of Food and Agriculture 2006, 86, 2251–2255.
- Pennington, J.A.T.; Fisher, R.A. Food component profiles for fruit and vegetables subgroups. Journal of Food Composition and Analysis 2010, 23, 411–418.
- Galleano, M.; Verstraeten, S.V.; Oteiza, P.I.; Fraga, C.G. Antioxidant actions of flavonoids: Thermodynamic and kinetic analysis. Archives of Biochemistry and Biophysic 2010, 501, 23–30.
- Sellappan, S.; Akoh, C.C. Flavonoids and antioxidant capacity of Georgia-grown Vidalia onions. Journal of Agricultural and Food Chemistry 2002, 50, 5338–5342.
- Nair, U.; Bartsch, H.; Nair, J. Meeting report: Prevention of degenerative diseases: Clues from studies investigating oxidative stress, Brussels, 13 November 2002. Mutagenesis 2003, 18, 477–483.
- Sommano, S.; Caffin, N.; Kerven, G. Screening for antioxidant activity, phenolic content, and flavonoids from Australian native food plants. International Journal of Food Properties 2013, 16 (6) 1394–1406.
- Gómez-Romero, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Metabolite profiling and quantification of phenolic compounds in methanol extracts of tomato fruit. Phytochemistry 2010, 71, 1848–1864.
- Llorach, R.; Tomás-Barberán, F.A.; Ferreres, F. Lettuce and chicory byproducts as a source of antioxidant phenolic extracts. Journal of Agricultural and Food Chemistry 2004, 52, 5109–5116.
- FAO statistics. http://faostat3.fao.org/home/index.html (accessed May, 2014).
- Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December of 2006 with regard to the list of nutrition claims. Official Journal of the European Union L 404/9.
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberan, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chemistry 2008, 108, 1028–1038.
- Ribas-Agustí, A.; Gratacós-Cubarsí, M.; Sárraga, C.; García-Regueiro, J.A.; Castellari, M. Analysis of eleven phenolic compounds including novel p-coumaroyl derivatives in lettuce (Lactuca sativa L.) by ultra high performance liquid chromatography with photodiode array and mass spectrometry detection. Phytochemical Analysis 2011, 22, 555–563.
- Khanam, U.K.S.; Oba, S.; Yanase, E.; Murakami, Y. Phenolic acids, flavonoids, and total antioxidant capacity of selected leafy vegetables. Journal of Functional Foods 2012, 4, 979–987.
- Kuhnle, G.G.C.; Dell’Aquila, C.; Runswick, S.A.; Bingham, S.A. Variability of phytoestrogen content in foods from different sources. Food Chemistry 2009, 113, 1184–1187.
- Li, H.; Deng, Z.; Liu, R.; Young, J.C.; Zhu, H.; Loewen, S.; Tsao, R. Characterization of phytochemicals and antioxidant activities of a purple tomato (Solanum lycopersicum L.). Journal of Agriculture and Food Chemistry 2011, 59, 11803–11811.
- Sánchez-Rodríguez, E.; Moreno, D.A.; Ferreres, F.; Rubio-Wilhelmi, M.M.; Ruiz, J.M. Differential responses of five cherry tomato varieties to water stress: Changes on phenolic metabolites and related enzymes. Phytochemistry 2011, 72, 723–729.
- Choi, S.-H.; Kim, H.-R.; Kim, H.-J.; Lee, I.-S.; Kozukue, N.; Levin, C.E.; Friedman, M. Free amino acid and phenolic contents and antioxidative and cancer cell inhibiting activities of extracts of 11 greenhouse-grown tomato varieties and 13 tomato based foods. Journal of Agriculture and Food Chemistry 2011, 59, 12801–12814.
- Björkman, M.; Klingen, I.; Birch, A.N.E.; Bones, A.M.; Bruce, T.J.A.; Johansen, T.J.; Meadow, R.; Mølmann, J.; Seljåsen, R.; Smart, L.E.; Stewart, D. Phytochemicals of Brassicaceae in plant protection and human health–Influences of climate, environment, and agronomic practice. Phytochemistry 2011, 72, 538–556.
- Ruiz-Rodriguez, A.; Marín, F.R.; Ocaña, A.; Soler-Rivas, C. Effect of domestic processing on bioactive compounds. Phytochemistry Reviews 2008, 7, 345–384.
- Selma, M.V.; Luna, M.C.; Martínez-Sánchez, A.; Tudela, J.A.; Beltrán, D.; Baixauli, C.; Gil, M.I. Sensory quality, bioactive constituents, and microbiological quality of green and red fresh-cut lettuces (Lactuca sativa L.) are influenced by soil and soilless agricultural production systems. Postharvest Biology and Technology 2012, 63, 16–24.
- Romani, A.; Pinellia, P.; Galardia, C.; Sani, G.; Cimato, A.; Heimler, D. Polyphenols in greenhouse and open-air-grown lettuce. Food Chemistry 2002, 79, 337–342.
- Ordidge, M.; García-Macías, P.; Battey, N.H.; Gordon, M.H.; Hadley, P.; John, P.; Lovegrove, J.A.; Vysini, E.; Wagstaffe, A. Phenolic contents of lettuce, strawberry, raspberry, and blueberry crops cultivated under plastic films varying in ultraviolet transparency. Food Chemistry 2010, 119, 1224–1227.
- Degl`Innoocenti, E.; Pardossi, A.; Tattini, M.; Guindi, L. Phenolic compounds and antioxidant power in minimally processed salad. Journal of Food Biochemistry 2008, 32, 642–653.
- Jokić, S.; Cvjetko, M.; Božić, D.; Fabek, S.; Toth, S.; Vorkapić-Furač, J.; Radojčić Redovniković, I. Optimisation of microwave-assisted extraction of phenolic compounds from broccoli and its antioxidant activity. International Journal of Food Properties 2012, 47 (12), 2613–1619.
- Silva, C.L.; Haesen, N.; Câmara, J.S. A new and improved strategy combining a dispersive-solid phase extraction-based multiclass method with ultra high pressure liquid chromatography for analysis of low molecular weight polyphenols in vegetables. Journal of Chromatography A 2012, 1260, 154–163.
- Ceymann, M.; Arrigoni, E.; Schärer, H.; Bozzi Nising, A.; Hurrell, R.F. Identification of apples rich in health-promoting flavan-3-ols and phenolic acids by measuring the polyphenol profile. Journal of Food Composition and Analysis 2012, 26, 128–135.
- Medina-Remón, A.; Tulipani, S.; Rotchés-Ribalta, M.; Mata-Bilbao, M.D.L.; Andres-Lacueva, C.; Lamuela-Raventos, R.M. A fast method coupling ultrahigh performance liquid chromatography with diode array detection for flavonoid quantification in citrus fruit extracts. Journal of Agricultural and Food Chemistry 2011, 59, 6353–6359.
- Prokudina, E.A.; Havlíček, L.; Al-Maharik, N.; Lapčíkm O.; Strnad, M.; Gruz, J. Rapid UPLC-ESI-MS/MS method for the analysis of isoflavonoids and other phenylpropanoids. Journal of Food Composition and Analysis 2012, 26, 36–42.
- Ortega, N.; Romero, M.P.; Maclà, A.; Reguant, J.; Anglès, N.; Morelló, J.R.; Motilva, M.J. Obtention and characterization of phenolic extracts from different cocoa sources. Journal of Agriculture and Food Chemistry 2008, 56, 9621–9627.
- Alarcón-Flores, M.I.; Romero-González, R.; Martinez Vidal, J.L; Garrido Frenich, A. Multiclass determination of phytochemicals in vegetables and fruits by ultra high performance liquid chromatography coupled to tandem mass spectrometry. Food Chemistry 2013, 141, 1120–1129.
- Konar, N.; Poyrazoğlu, E.S.; Demir, K.; Artik, N. Effect of different sample preparation methods on isoflavone, lignan, coumestan, and flavonoid contents of various vegetables determined by triple quadrupole LC-MS/MS. Journal of Agricultural and Food Chemistry 2012, 26, 26–35.
- Di Lecce, G.; Martínez-Huélamo, M.; Tulipani, S.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Setup of a UHPLC-QqQ-MS method for the analysis of phenolic compounds in cherry tomatoes, tomato sauce, and tomato juice. Journal of Agricultural and Food Chemistry 2013, 61, 8373–8380.
- Stewart, A.J.; Bozonnet, S.; Mullen, W.; Jenkins, G.I.; Lean, M.E.J.; Crozier, A. Occurrence of flavonols in tomatoes and tomato-based products. Journal of Agricultural and Food Chemistry 2000, 48, 2663–2669.
- Martínez-Valverde, I.; Periago, M.J.; Provan, G.; Chesson, A. Phenolic compounds, lycopene, and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum). Journal of the Science of Food and Agriculture 2002, 82, 323–330.
