Abstract
The antioxidant compounds in the stems and leaves of Gynura bicolor were studied. 1,1-diphenyl-2-picrylhydrazyl and 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging assays were employed to evaluate antioxidant capacity. By solvent extraction and Sephadex LH-20 column chromatography in sequence, ethanol extracts of Gynura bicolor stems and leaves were fractionated to obtain their active fractions, which were further separated to obtain 12 compounds: 1–8 from stems, and four, 8–12, from leaves. Their structures were elucidated on the basis of spectroscopic data (nuclear magnetic resonance and mass spectrometry). Among these substances, compounds 1, 2, 3, 4, and 8 with significant antioxidant activity were determined to be responsible active components for stems, and compounds 4, 8, and 12 for leaves.
INTRODUCTION
Oxidative stress, mediated by excessive free radicals in organisms, resulted from the imbalance of oxidants (superoxide anion and peroxynitrite as examples) and antioxidants (superoxide dismutase and catalase for instances) in favor of oxidants, could induce the occurrence of cancer, neurodegenerative disorders, cardiovascular diseases, and especially the diabetes.[Citation1] The overproduction of superoxide in high hyperglycemic condition by the mitochondrial electron-transport chain seems to be the first and key event in the activation of all other pathways involved in the pathogenesis of diabetes complications, which took “antioxidant therapy” for granted as a new strategy to treat such diseases.[Citation2] Though classic antioxidants, such as vitamin E, failed to show any effect in clinical trials, some plant extracts were reported to control diabetics for their antioxidant efficiency, which greatly stimulated the interest in antioxidants from natural resources.[Citation3Citation4]
Gynura bicolor, containing a characteristic reddish purple color with potential as a natural food color, is a perennial herb native to the tropics of East Asia.[Citation5,Citation6] It is also cultivated as a vegetable on account of its nutritive value. Chemical investigations revealed the presence of terpenoids,[Citation7–Citation12] flavonoids,[Citation12–Citation14] anthocyanins,[Citation5] and phenolics.[Citation12] The anti-hyperglycemic[Citation15] and antioxidant activities[Citation5,Citation16] were observed in a series of pharmacological experiments along with the effects including immunity enhancement,[Citation17] anti-inflammation,[Citation18] and apoptosis induction.[Citation19] Apart from anthocyanins,[Citation5] polyphenols were responsible for the antioxidant activity of G. bicolor.[Citation20] However, a detailed antioxidant profile was insufficient. On the other hand, anti-diabetic effect of G. bicolor may be attributed to its antioxidant activity for their close correlations, which stirred up our interest in antioxidant profile of G. bicolor. Thus, a chemical investigation guided by antioxidant activity (1,1-diphenyl-2-picrylhydrazyl [DPPH] and 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) [ABTS]) radical scavenging capacity) was performed to establish the antioxidant profiles of G. bicolor stems and leaves. The isolation and purification of active fractions from stems and leaves led to 12 compounds (1–12; ). Most of them showed significant radical scavenging capacity on DPPH and ABTS.
MATERIAL AND METHODS
Chemicals and Equipment
Vitamin C was obtained from Tianjin Bodi Chem. Inc. Ltd., and potassium persulphate from Aladdin Reagent. DPPH and ABTS were purchased from Sigma-Aldrich. Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance 600 or Ascend 400 spectrometer with chemical shifts δ in ppm. Electrospray ionization mass spectrometry (ESI-MS) spectra were obtained on a Waters Xevo TQ (tandem quadrupole) mass spectrometer, and high resonance electrospray ionization mass spectrometry (HR-ESI-MS) on a Bruker Bio TOF IIIQ (quadrupole time of flight) mass spectrometer. Column chromatography was performed on Silica gel (200–300 mesh, Qingdao Haiyang Chemical Co. Ltd., P. R. China (QHCC)), and Sephadex LH-20 (GE Healthcare, U.S.A.). Thin layer chromatography (TLC) was conducted on plates precoated with 10 ~ 40 μm of silica gel GF254 from QHCC. Preparative high-performance liquid chromatography (HPLC) was carried out on a LC3000 HPLC system (Beijing Chuangxin Tongheng Science and Technology Co. Ltd.) with a YMC C18 column (20 × 250 mm; 10 μm), and a semi-preparative HPLC on a Perkin-Elmer 200 HPLC system with a Welch C18 column (10 × 250 mm; 5 μm), and detected at 208 nm. All other solvents were commercially purchased and distilled under normal atmospheric pressure prior to use.
Plant Materials
The fresh aerial parts of G. bicolor (100 Kg), cultivated in the vegetable base of Pengzhou County, were purchased in July 2013 from a vegetable market at Chengdu City, China. A voucher specimen (2013-07) was identified by professor Fading Fu at Chengdu Institute of Biology, Chinese Academy of Sciences (CIBCAS), and was deposited in the herbarium of CIBCAS. The material was then manually divided into stems and leaves. The stems and leaves were separately cut up prior to extraction.
Extraction and Activity Guided Fractionation
The results of our preliminary study (data not shown) indicated that the antioxidant activity of both stems and leaves showed positive correlations with ethanol concentration of solvent. Consequently, 95% EtOH was chosen as the appropriate solvent for extraction. The treated stems was percolated with 95% EtOH (80 L × 3, and once for 7 d) at room temperature. After concentration under reduced pressure, the crude extract was suspended in water (1.5 L), and was fractionated successively with petroleum ether (1.5 L × 3), EtOAc (1.5 L × 3), and n-BuOH (1.5 L × 3) to give four fractions. The fraction with better radical scavenging effect was then subjected to Sephadex LH-20 column eluted with CHCl3-MeOH (1:1/, v/v). The eluates (50 mL/bottle) were pooled into a series of fractions based on TLC analyses. The fractions with significant radical scavenging capacity were selected as active parts for further separation. The same procedures were applied on the leaves to obtain its active fractions.
Isolation and Purification of Compounds from Active Fractions
EtOAc fraction of the 95% ethanol extract of either stems (XJB, 22 g) or leaves (XYB, 25 g) was screened out for further fractionation. According to the TLC analyses, XJB was divided into eight fractions (XJB1-XJB8) over Sephadex LH-20 column eluted with CHCl3-MeOH (1:1, v/v). The last two fractions (XJB7 and XJB8) were active fractions. XJB7 (2.45 g) was separated with preparative HPLC using MeOH-H2O (46:54, v/v; 15 mL/min) as solvents to afford compound 1 (tR = 32 min; 177 mg) and several fractions including XJB7B (tR = 10 min; 330 mg), XJB7E (tR = 17 min; 200 mg), XJB7F (tR = 22 min; 125 mg), and XJB7G (tR = 27.5 min; 79 mg). XJB7B was separated over silica gel column eluted with petroleum ether-acetone (2:1, v/v) to give a subfraction XJB7B2, which was recrystallized from methanol to give compound 2 (2 mg). Further purification of the mother liquor led to compound 3 (tR = 16 min; 2 mg) by semi-preparative HPLC with MeOH-H2O (27:73, v/v; 4 mL/min) as solvents. XJB7E was subjected to silica gel column eluted with CHCl3-MeOH (20:1, v/v) to afford fraction XJB7E3, which was recrystallized from methanol to give compound 4 (3 mg). Compound 5 (tR = 16 min; 4 mg) was obtained by separation of XJB7F with preparative HPLC taking MeOH-H2O (45:55, v/v; 4 mL/min) as solvents. XJB7G was separated by semi-preparative HPLC eluted with MeOH-H2O (25:75, v/v; 4 mL/min) to give fraction XJB7G2, which was further separated over silica gel column eluted with petroleum ether-acetone (2:1, v/v) to afford compound 6 (7 mg). XJB8 (1.64 g) was divided into fractions XJB8A and XJB8C over silica gel column eluted with CHCl3-MeOH (10:1, v/v). XJB8A was further separated by semi-preparative HPLC eluted with MeOH-H2O (39:61, v/v; 3 mL/min) to give fraction XJB8A1 (tR = 20 min), which was then subjected to silica gel column eluted with petroleum ether-acetone (2:1, v/v) to afford compound 7 (1 mg). Compound 8 (4 mg) was obtained by recrystallization of XJB8C from methanol.
XYB was divided into six fractions (XYB1-XYB6). The last two fractions (XYB5 and XYB6) were determined as active fractions. The recrystallization of XYB5 (2.5 g) from methanol afforded compound 9 (67 mg). The mother liquor of compound 9 was separated by preparative HPLC eluted with MeOH-H2O (38:62, v/v; 15 mL/min) to afford several fractions including XYB5B3 (tR = 5 min), XYB5B4 (tR = 8 min), and XYB5B5 (tR = 10 min). Compounds 10 (1 mg) and 11 (3 mg) were obtained from the separation of XYB5B3 over silica gel column eluted with CHCl3-MeOH (1:1, v/v). XYB5B4 was separated by semi-preparative HPLC with MeOH-H2O (23:77, v/v; 4 mL/min) as solvents to give compound 12 (tR = 17 min; 3 mg). Compound 8 (tR = 29 min) was obtained from XYB5B5 separated by semi-preparative HPLC with MeOH-H2O (23:77, v/v; 4 mL/min) as solvents. Compound 4 was obtained by recrystallization of XYB6 from methanol.
Antioxidant Activity Assays
DPPH radical scavenging assay
The DPPH radical scavenging effect was determined according to the method reported previously with little modification.[Citation21] In brief, DPPH stock solution (1.49 mM) was prepared using 95% EtOH and kept at −20ºC in the dark. DPPH working solution was prepared freshly for use by diluting the stock solution 20 times with 95% EtOH. The mixture containing 10 μL of sample solution and 290 μL of DPPH working solution in a 96-well plate was allowed to stand for 30 min at room temperature in the dark. Absorbance was measured at 517 nm using a Varioskan Flash Reader (Thermo Fisher Scientific, USA). The DPPH radical scavenging effect (%) was calculated as:
where, Ae is the A517 in the presence of sample, As is the A517 of sample, and Ac is the A517 of negative control solution. Vitamin C was used as positive control. Moreover, all analyses were done in triplicate.
ABTS radical scavenging assay
The ABTS radical scavenging effect was determined in line with the previously reported method with minor modification.[Citation22] In brief, the aqueous mixture of ABTS (7 mM) and potassium persulphate (2.45 mM) was incubated for 16 h under room temperature in the dark, which was then stored as ABTS stock solution at 4ºC in the dark. ABTS working solution was prepared freshly for use by diluting 350 μL of stock solution to 25 mL with 80% EtOH. The mixture containing 10 μL of sample solution and 250 μL of ABTS working solution in a 96-well plate was allowed to stand for 5 min at room temperature in the dark. Absorbance was measured at 734 nm using a Varioskan Flash Reader. The ABTS radical scavenging effect (%) was calculated as:
where, Ae is the A734 in the presence of sample, As is the A734 of sample, and Ac is the A734 of negative control solution. Vitamin C was used as a positive control. Moreover, all analyses were done in triplicate.
RESULTS AND DISCUSSIOIN
Activity Guided Fractionation
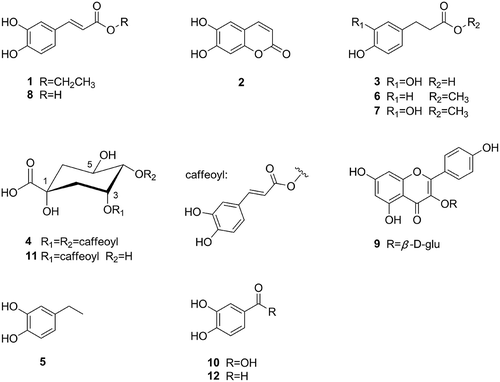
DPPH and ABTS radical scavenging assays were employed to perform activity guided fractionation. The ethanol extract of stems was divided into four fractions on the basis of polarity, whose antioxidant capacity were evaluated at 5 mg/mL (). Fraction XJB (EtOAc fraction) showed better radical scavenging capacity with inhibition rate of 93.6% on DPPH and 94.8% on ABTS, which was in accordance with those reported by Teoh et al.[Citation20] XJB was then subjected to Sephadex LH-20 column to give eight subfractions XJB1-XJB8 after TLC analyses. XJB7 and XJB8 were determined as active fractions based on its radical scavenging capacity at 1 mg/mL (): inhibition rate of 91.7 and 93.3% on DPPH, respectively; 88.9 and 90.9% on ABTS, respectively.
FIGURE 2 DPPH and ABTS radical scavenging capacity of different polar fractions at 5 mg/mL: XJ (XY) for stems (leaves) extract; XJA (XYA), XJB (XYB), XJC (XYC), XJD (XYD) for stems (leaves), separately representing petroleum ether, EtOAc, n-BuOH, and water fraction.
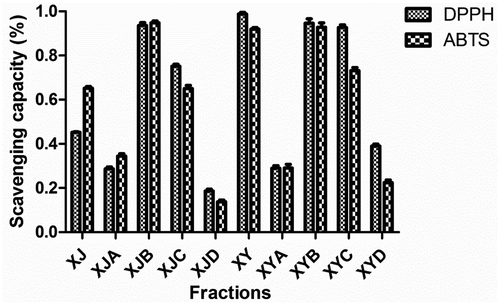
FIGURE 3 DPPH and ABTS radical scavenging capacity of fractions (XJB, XYB, and their corresponding fractions separated by Sephadex LH-20) at 1 mg/mL.
The same procedure was applied on ethanol extract of leaves. Fraction XYB, with scavenging rate of 94.6% on DPPH and 92.8% on ABTS at 5 mg/mL (), was screened out for further fractionation over Sephadex LH-20 column. Six fractions XYB1-XYB6 were then obtained. The results of DPPH and ABTS radical scavenging assays at 1 mg/mL () indicated that XYB5 and XYB6 were the active fractions of G. bicolor leaves: scavenging rate of 91.6 and 89.1% on DPPH, respectively; 74.2 and 67.5% on ABTS, respectively.
Separation and Purification of Active Fractions
Twelve compounds were isolated from the active fractions of G. bicolor stems (1–8) and leaves (4, 8, 9–12; ). Based on the NMR and MS spectroscopic data, they were identified as ethyl caffeate (1),[Citation23] esculetin (2),[Citation24] dihydrocaffeic acid (3),[Citation25] 3,4-O-dicaffeoyl-quinic acid (4),[Citation26] 3,4-dihydroxyphenylethane (5),[Citation27] p-hydroxyphenylpropionic methyl ester (6),[Citation28] methyl dihydrocaffeate (7),[Citation29] caffeic acid (8),[Citation30] kaempferol-3-O-β-D-glucoside (9),[Citation31] 3,4-dihydroxylbenzoic acid (10),[Citation32] 3-O-caffeoyl-quinic acid (11),[Citation26] and 3,4-dihydroxybenzaldehyde (12).[Citation33] Their NMR and MS data, including typical original spectrum, were shown in supporting information. All these compounds were first isolated from this plant, except for 9 and 11.
Antioxidant Activity of the Isolated Compounds
In the DPPH radical scavenging assay, compounds 3 and 4 showed significant scavenging capacity with IC50 values of 0.44 and 0.33 mM, respectively (), which were better than positive control vitamin C with IC50 value of 0.57 mM. Compounds 2 (IC50 value of 0.62 mM) and 12 (IC50 value of 0.58 mM) displayed comparative effects as vitamin C. The order of DPPH scavenging capacity was 4 > 3 > 12 > 2 > 1 ≈ 8 > 7 > 11 > 5 > 6 > 10 > 9. Compounds 2, 3, and 12 (successive IC50 value of 0.51, 0.49, and 0.51 mM) behaved similar capacity as vitamin C (IC50 value of 0.51 mM) in ABTS radical scavenging assay. The order of ABTS scavenging activity was 2 ≈ 3 ≈ 12 > 8 > 4 > 5 > 6 > 7 ≈ 1 > 10 > 11 > 9.
TABLE 1 Scavenging effects (IC50) on DPPH and ABTS of compounds 1–12.
The free radical scavenging activity of antioxidants was attributed to its hydrogen-donating ability, which mainly depended on the amounts and position of free phenolic hydroxyl groups, and the potential for the stabilization of the newly formed phenol-oxyradicals.[Citation34–Citation36] Most of the isolated compounds (1, 2, 5, 7, 12) with a substructure of o-diphenol showed much higher antioxidant activity than that of compound 9 even with three separate phenolic hydroxyl groups, which further confirmed the effect of location of phenolic hydroxyl group on antioxidant activity. Detailed analysis of antioxidant capacity of cinnamic acid derivatives led to a conclusion that: (1) free carboxyl group displayed moderate antioxidant activity (comparison of 3 and 5, 3 and 7, 1 and 8, respectively); (2) the double bond between C-7 and C-8 showed negative effect on antioxidant activity when carboxyl group was free (comparison of 3 and 8), and positive effect when esterified (comparison of 1 and 7). The effect of aldehyde group was better than that of carboxyl group when connected directly to aromatic ring (comparison of 10 and 12), which may be related to their capacity to stabilize the newly formed phenol-oxyradicals.
According to the data depicted in , compounds 1, 2, 3, 4, and 8 were the antioxidant components for G. bicolor stems, and compounds 4, 8, and 12 for G. bicolor leaves. A literature survey indicated that all the responsible active composition (1,[Citation37] 2,[Citation38] 3,[Citation39] 4,[Citation40] 8,[Citation41] 12[Citation42]) displayed their potential to counteract oxidative stress, a key mechanism in the development of diabetic complications,[Citation2] which indicated that antioxidant effect of G. bicolor may be involved in its mechanisms to treat diabetics.
CONCLUSION
Gynura bicolor is a popular vegetable in South China. Activity guided isolation of the ethanol extracts from G. bicolor stems and leaves led to 12 compounds (1–12). All compounds showed scavenging effects on DPPH and ABTS radicals. The compounds with significant radical scavenging capacity were determined to be responsible active components: 1, 2, 3, 4, 8 for stems, and 4, 8, 12 for leaves. The antioxidant effect of G. bicolor may be involved in its mechanisms to treat diabetics.
Supplemental Data
Supplemental data for this article can be accessed on the publisher’s website.
Supplementary_file.pdf
Download PDF (1,003.2 KB)REFERENCES
- Ratnam, D.V.; Ankola, D.D.; Bhardwaj, V.; Sahana, D.K.; Kumar, M.N.V.R. Role of Antioxidants in Prophylaxis and Therapy: A Pharmaceutical Perspective. Journal of Controlled Release 2006, 113, 189–207.
- Ceriello, A. Controlling Oxidative Stress As a Novel Molecular Approach to Protecting the Vascular Wall in Diabetes. Current Opinion in Lipidology 2006, 17, 510–518.
- Hafizur, R.M.; Babiker, R.; Yagi, S.; Chishti, S.; Kabir, N.; Choudhary, M.I. The Antidiabetic Effect of Geigeria Alata Is Mediated by Enhanced Insulin Secretion, Modulation of β-Cell Function, and Improvement of Antioxidant Activity in Streptozotocin-Induced Diabetic Rats. Journal of Endocrinology 2012, 214, 329–335.
- Dewanjee, S.; Maiti, A.; Sahu, R.; Dua, T.K.; Mandal, V. Effective Control of Type 2 Diabetes Through Antioxidative Defense by Edible Fruits of Diospyros Peregrina. Evidence-Based Complementary and Alternative Medicine 2011, 2011, 675397–675403.
- Shimizu, Y.; Imada, T.; Zhang, H.L.; Tanaka, R.; Ohno, T.; Shimomura, K. Identification of Novel Poly-Acylated Anthocyanins from Gynura Bicolor Leaves and Their Antioxidative Activity. Food Science and Technology Research 2010, 16, 479–486.
- Shimizu, Y.; Imada, T.; Ohno, T.; Zhang, H.L.; Shimomura, K. Characteristics of Gynura Bicolor DC. Color and Its Applications As a Novel Natural Food Color. Nippon Shokuhin Kagaku Kogaku Kaishi 2010, 57, 539–545.
- Shimizu, Y.; Imayoshi, Y.; Kato, M.; Maeda, K.; Iwabuchi, H.; Shimomura, K. Volatiles from Leaves of Field-Grown Plants and Shoot Cultures of Gynura Bicolor DC. Flavour and Fragrance Journal 2009, 24, 251–258.
- Shimizu, Y.; Imayoshi, Y.; Kato, M.; Maeda, K.; Iwabuchi, H.; Shimomura, K. New Eudesmane-Type Sesquiterpenoids and Other Volatile Constituents from the Roots of Gynura Bicolor DC. Flavour and Fragrance Journal 2011, 26, 55–64.
- Chen, J.; Adams, A.; Mangelinckx, S.; Ren, B.R.; Li, W.L.; Wang, Z.T.; De Kimpe, N. Investigation of the Volatile Constituents of Different Gynura Species from Two Chinese Origins by SPME/GC-MS. Natural Product Communications 2012, 7, 655–657.
- Chen, J.; Mangelinckx, S.; Adams, A.; Li, W.L.; Wang, Z.T.; De Kimpe, N. Chemical Constituents from the Aerial Parts of Gynura Bicolor. Natural Product Communications 2012, 7, 1563–1564.
- Yin, M.C.; Lin, M.C.; Mong, M.C.; Lin, C.Y. Bioavailability, Distribution, and Antioxidative Effects of Selected Triterpenes in Mice. Journal of Agricultural and Food Chemistry 2012, 60, 7697–7701.
- Zhuo, M.; Lv, H.; Ren, B.R.; Li, W.L.; Zhao, Z.Q.; Zhang, H.Q. Study on Chemical Constituents of Gynura Bicolor DC. Chinese Traditional and Herbal Drugs 2008, 39, 30–32.
- Lv, H.; Pei, Y.P.; Li, W.L.; Xu, D.R. Detection of Flavonoids in Gynura Bicolor DC. by HPLC-MSn. Lishizhen Medicine and Materia Medica Research 2011, 22, 2582–2583.
- Lv, H.; Pei, Y.P.; Li, W.L. Studies on Flavonoids from Gynura Bicolor DC. Chinese Journal of Modern Applied Pharmacy 2010, 27, 613–614.
- Li, W.L.; Ren, B.R.; Zhuo, M.; Hu, Y.; Lu, C.G.; Wu, J.L.; Chen, J.; Sun, S. The Anti-Hyperglycemic Effect of Plants in Genus Gynura Cass. The American Journal of Chinese Medicine 2009, 37, 961–966.
- Suda, I.; Oki, T.; Nishiba, Y.; Masuda, M.; Kobayashi, M.; Nagai, S.; Hiyane, R.; Miyashige, T. Polyphenol Contents and Radical-Scavenging Activity of Extracts from Fruits and Vegetables in Cultivated in Okinawa, Japan. Nippon Shokuhin Kagaku Kogaku Kaishi 2005, 52, 462–471.
- Hsieh, S.L.; Wu, C.C.; Liu, C.H.; Lian, J.L. Effects of the Water Extract of Gynura Bicolor (Roxb. & Willd.) DC on Physiological and Immune Responses to Vibrio Alginolyticus Infection in White Shrimp (Litopenaeus Vannamei). Fish & Shellfish Immunology 2013, 35, 18–25.
- Wu, C.C.; Lii, C.K.; Liu, K.L.; Chen, P.Y.; Hsieh, S.L. Anti-Inflammatory Activity of Gynura Bicolor (Hong Feng Cai) Ether Extract Through Inhibits Nuclear Factor Kappa B Activation. Journal of Traditional and Complementary Medicine 2013, 3, 48–52.
- Hayashi, M.; Iwashita, K.; Katsube, N.; Yamaki, K.; Kobori, M. Kinjiso (Gynura bicolor DC.) Colored Extract Induce Apoptosis in HL60 Leukemia Cells. Nippon Shokuhin Kagaku Kogaku Kaishi 2002, 49, 519–526.
- Teoh, W.Y.; Sim, K.S.; Moses Richardson, J.S.; Abdul Wahab, N.; Hoe, S.Z. Antioxidant Capacity, Cytotoxicity, and Acute Oral Toxicity of Gynura Bicolor. Evidence-Based Complementary and Alternative Medicine 2013, 2013, 958407–958416.
- Thoo, Y.Y.; Ho, S.K.; Liang, J.Y.; Ho, C.W.; Tan, C.P. Effects of Binary Solvent Extraction System, Extraction Time, and Extraction Temperature on Phenolic Antioxidants and Antioxidant Capacity from Mengkudu (Morinda Citrifolia). Food Chemistry 2010, 120, 290–295.
- González, E.A.; García, E.M.; Nazareno, M.A. Free Radical Scavenging Capacity and Antioxidant Activity of Cochineal (Dactylopius Coccus C.) Extracts. Food Chemistry 2010, 119, 358–362.
- Cao, Y.; Wang, L.; Chen, Y.Z.; Ren, G.J.; Xu, N. Isolation and Identification of Phenols Constituents from Whole Plant of Leontopodium Leontopodioides. Journal of Shenyang Pharmaceutical University 2012, 29, 606–609.
- Wang, Z.W.; Tan, X.J.; Ma, T.T.; Chen, X.H.; Bi, K.X. Isolation and Identification of Chemical Constituents from Artemisia Capillaries Thumb. Journal of Shenyang Pharmaceutical University 2008, 25, 781–785.
- Wang, N.; Wang, J.H.; Cheng, J.; Li, X. Chemical Constituents of Pyrrosia Petiolosa (Christ) Ching. Journal of Shenyang Pharmaceutical University 2003, 20, 425–428.
- Teng, R.W.; Zhou, Z.H.; Wang, D.Z.; Yang, C.R. Four Caffeoylquinic Acids from Morina Nepalensis Var. alba Hand.-Mazz. Chinese Journal of Microwave & Radio-Frequency Spectroscopy 2002, 19, 167–174.
- Wu, J.L.; Wang, P.; Gao, P.; Zeng, N.; Liu, X.F.; Wang, S.Y.; Sheng, Y.; Xu, C.D. Studies on the Chemical Constituents in Leaves of Rosa Laevigata Michx. Journal of Pharmaceutical Practice 2012, 30, 275–279.
- Liu, D.L.; Pang, F.G.; Zhang, J.X.; Wang, N.L.; Yao, X.S. Studies on the Chemical Constituents Of Bulbophyllum Odoratissimum Lindl. Chinese Journal of Medicinal Chemistry 2005, 15, 103–108.
- Etzenhouser, B.; Hansch, C; Kapur, S.; Selassie C.D. Mechanism of Toxicity of Esters of Caffeic and Dihydrocaffeic Acids. Bioorganic & Medicinal Chemistry 2001, 9, 199–209.
- Feng, M.L.; Wang, S.F.; Zhang, X.X. Chemical Constituents in Fruits of Lycium Barbarum. Chinese Traditional and Herbal Drugs 2013, 44, 265–269.
- Zhou, R.G.; Yang, Z.X.; Wang, J.; Tian, L.W.; Zhang, Y.J. Chemical Constituents from Leaves of Mangifera Persiciformis. Natural Product R&D 2012, 24, 1217–1219.
- Wang, S.M.; Kadota, S.; Liu, Z.Q.; Liu, S.Y. Studies on the Anti-Free Radical Compounds in Tuocha (Camellia Sinensis Var. Assamica). Natural Product R&D 2005, 17, 131–137.
- Zhao, Q.C.; Hua, W.; Fu, Y.H.; Du, Z.Q.; Guo, T.; Wu, L.J. Isolation and Identification of the Non-Alkaloid Constituents from Whole Plant of Gelsemium Elegans Benth. (III). Journal of Shenyang Pharmaceutical University 2010, 27, 516–519.
- Pei, Y.X.; Wang, S.S.; Wang, W.W.; Zhou, Y.H.; Zhao, H.H.; Jia, L. Isolation and Structure-Activity Relationship of the Antioxidant Chemical Constituents from the Flowers of Rosa Chinensis Jacq. International Journal of Food Properties 2014, 17, 38–44.
- Jin, S.W.; Chen, G.L.; Du, J.J.M.; Wang, L.H.; Ren, X.; An, T.Y. Antioxidant Properties and Principal Phenolic Compositions of Cynomorium Songaricum Rupr. International Journal of Food Properties 2014, 17, 13–25.
- Lagouri, V.; Prasianaki, D.; Krysta Lli, F. Antioxidant Properties and Phenolic Composition of Greek Propolis Extracts. International Journal of Food Properties 2014, 17, 511–522.
- Garrido, J.; Gaspar, A.; Garrido, E.M.; Miri, R.; Tavakkoli, M.; Pourali, S.; Saso, L.; Borges, F.; Firuzi, O. Alkyl Esters of Hydroxycinnamic Acids with Improved Antioxidant Activity and Lipophilicity Protect PC 12 Cells Against Oxidative Stress. Biochimie 2012, 94, 961–967.
- Prabakaran, D.; Ashokkumar, N. Protective Effect of Esculetin on Hyperglycemia-Mediated Oxidative Damage in the Hepatic and Renal Tissues of Experimental Diabetic Rats. Biochimie 2013, 95, 366–373.
- Verzelloni, E.; Pellacani, C.; Tagliazucchi, D.; Tagliaferri, S.; Calani, L.; Costa, L.G.; Brighenti, F.; Borges, G.; Crozier, A.; Conte, A.; Del Rio, D. Antiglycative and Neuroprotective Activity of Colon-Derived Polyphenol Catabolites. Molecular Nutrition & Food Research 2011, 55, S35–S43.
- Abdallah, H.M.; Ezzat, S.M.; EI Dine, R.S.; Abdel-Sattar, E.; Abdel-Naim, A.B. Protective Effects of Echinops Galalensis Against CCl4-Induced Injury on the Human Hepatoma Cell Line (Huh7). Phytochemistry Letters 2013, 6, 73–78.
- Nakajima, Y.; Nishida, H.; Nakamura, Y.; Konishi, T. Prevention of Hydrogen Peroxide-Induced Oxidative Stress in PC12 Cells by 3,4-dihydroxybenzalacetone Isolated from Chaga (Inonotus Obliquus (Persoon) Pilat). Free Radical Biology and Medicine 2009, 47, 1154–1161.
- Jeong, J.B.; Hong, S.C.; Jeong, H.J. 3,4-Dihydroxybenzaldehyde Purified from the Barley Seeds (Hordeum Vulgare) Inhibits Oxidative DNA Damage and Apoptosis Via Its Antioxidant Activity. Phytomedicine 2009, 16, 85–94.