Abstract
The starchy kernel of Euryale ferox seed has attracted much attention because of its edible and medicinal values. In this article, physicochemical properties of E. ferox kernel starches were investigated from two different regions. Dry kernel contained about 70% starch. The starch was polyhedral in shape, had very small size of about 1~2 μm in diameter, and exhibited A-type crystallinity. The native starches of kernels from two different regions showed similar amylose content, molecular weight distribution, relative crystallinity, short-range ordered structure, swelling power, and digestion properties. The kernel starch from Weishan Lake had higher gelatinization temperature, enthalpy, and water solubility, contained higher resistant starch in gelatinized and retrograded starch, and showed higher resistant ability to acid and amylase hydrolysis than that from Shaobo Lake. These above results could help in promoting the use of E. ferox kernel as an alternative starchy source.
INTRODUCTION
Euryale ferox Salisb. (Nymphaeaceae), an aquatic plant, is the only species of the genus Euryale native to eastern Asia, and has been found from India to Korea and Japan.[Citation1,Citation2] In China, the seeds of E. ferox, locally known as Qianshi as well as cock’s head, are consumed medicinally or as food.[Citation2] Qianshi is used to be a traditional Chinese medicine to treat some diseases, such as kidney problems, chronic diarrhea, excessive leucorrhea, and hypofunction of the spleen.[Citation3] In southern China, the seeds are served as food by adding them to soup or porridge.[Citation2] Currently, researches on E. ferox seeds are mainly focused on the isolation, purification and identification of chemical constituents.[Citation2–Citation6] The edible part of the seed is starchy kernel.[Citation7] However, to the best of our knowledge, few studies have been reported about the physicochemical properties of kernel starch from E. ferox seeds but there has been one previous study investigating physicochemical properties of kernel starch from one E. ferox variety in India.[Citation8]
Starch has been extensively studied and discussed in literatures. Physicochemical properties of starch determined its application in food and non-food industries. Starches from different botanical sources have diverse physicochemical properties.[Citation9–Citation13] In this study, starches were isolated from mature kernels of E. ferox seeds from two different regions. The morphology, molecular structure, crystal structure, swelling power, water solubility, thermal property, hydrolysis property, and digestion property of starch were investigated. The objective of this study was to determine the physicochemical properties of E. ferox kernel starch in order to provide useful information for its utilizations as an alternative starchy source.
MATERIALS AND METHODS
Plant Materials
Mature seeds of E. ferox were collected from Shaobo Lake of Jiangsu Province and Weishan Lake of Shandong Province, China in October, 2013. The hard seed coat was removed with a stainless steel knife, and the edible kernel was used to isolate starch.
Morphology, Starch Content, and Soluble Sugar Content
Fresh fruits, seeds, and peeled kernels were photographed with a Canon digital IXUS 750 camera. The contents of starch and soluble sugar in kernel flour were determined following the method described by Gao et al.[Citation14]
Starch Isolation
The dry kernels were steeped in distilled water at 4°C overnight. The softened kernels were homogenized with ice-cold water in a home blender. The homogenate was squeezed through four layers of cheesecloth. The residue was homogenized and squeezed twice more to facilitate the release of starch. The extract was filtered with 100, 200, 300, and 400-mesh sieves. The starch slurry was centrifuged at 5000 g for 10 min. After centrifugation, the supernatant was discarded and the upper non-white layer was scraped off. The white starch sediment was resuspended in distilled water. After five cycles of centrifuging and resuspending repeatedly, the starch sediment was further washed twice with anhydrous ethanol, dried at 40°C, ground into powders, and passed through a 100-mesh sieve.
Scanning Electron Microscope Observation
The dried starch was suspended in anhydrous ethanol. Twenty μL of the starch-ethanol suspension was applied to an aluminum stub using double-sided adhesive tape, and the starch was coated with gold before viewing with an environmental scanning electron microscope (Philips XL-30).
Apparent Amylose Content Determination
Apparent amylose content was determined using the iodine colorimetric method of Man et al.[Citation15] Briefly, starch was defatted with 85% (v/v) methanol and dissolved in urea dimethyl sulphoxide (UDMSO) solution. The starch-UDMSO solution was treated with iodine solution. Apparent amylose content was evaluated from absorbance at 620 nm.
Gel-Permeation Chromatography (GPC) Analysis
Starch was deproteinizated with protease and sodium bisulfite, and debranched with isoamylase following the methods of Tran et al.[Citation16] and Li et al.[Citation17] The molecular weight distributions of debranched starches were analyzed using a PL-GPC 220 high temperature chromatograph (Agilent Technologies UK Limited, Shropshire, UK) with three columns (PL110-6100, 6300, 6525) and a differential refractive index detector according to the method of Cai et al.[Citation18]
X-Ray Powder Diffraction (XRD) Analysis
Crystal structure of starch was analyzed on an X-ray diffractometer (D8, Bruker, Germany) spectroscope. The XRD analysis and determination of relative crystallinity were carried out using the method described by Gao et al.[Citation14]
Infrared (IR) Analysis
Starch was analyzed on a Varian 7000 Fourier transform infrared (FTIR) spectrometer with a deuterated triglycine sulphate (DTGS) detector equipped with an attenuated total reflectance (ATR) single reflectance cell containing a germanium crystal (45° incidence-angle; PIKE Technologies, USA) as previously described by Gao et al.[Citation14] Original spectra were corrected by subtraction of the baseline in the region from 1200 to 800 cm–1 before deconvolution was applied using Resolutions Pro. The assumed line shape was Lorentzian with a half-width of 19 cm–1 and a resolution enhancement factor of 1.9. Intensity measurements at 1045, 1022, and 995 cm–1 were performed on the deconvoluted spectra by recording the height of the absorbance bands from the baseline using Adobe Photoshop 7.0 image software.
Swelling Power and Water Solubility Determination
The swelling power and water solubility were determined by heating starch-water slurries in a water bath at temperatures ranging from 50 to 95°C according to the procedures of Wei et al.[Citation19]
Thermal Property Analysis
Five milligrams of starch was precisely weighed and mixed with 15 μL of distilled water. The mixture was sealed in an aluminum pan overnight at 4°C. After equilibrating for 1 h at room temperature, the starch sample was then heated from 30 to 110°C at a rate of 10°C/min using a differential scanning calorimetry (DSC; 200-F3, NETZSCH, Germany).
Hydrolysis Property Analysis
The starch was hydrolyzed by HCl, porcine pancreatic α-amylase (PPA), and Aspergillus niger amyloglucosidase (AAG). The hydrolysis degree of starch by HCl was analyzed using the method of Gao et al.[Citation14] Twenty milligrams of starch was suspended in 2 mL of 2.2 M HCl. The hydrolyses were conducted in a shaking water bath at 35°C with continuous shaking (100 rpm) for 0.5, 1, 2, 4, 6, 8, 10, 12, and 14 d. The hydrolysis degrees of starch by PPA and AAG were determined using the method of Li et al.[Citation20] with some modifications. For PPA hydrolysis, 10 mg of starch was suspended in 2 mL of enzyme solution (0.1 M phosphate sodium buffer, pH 6.9, 25 mM NaCl, 5 mM CaCl2, 0.02% NaN3, 50 U PPA [Sigma A3176]). For AAG hydrolysis, 10 mg of starch was suspended in 2 mL of enzyme solution (0.05 M acetate buffer, pH 4.5, 5 U AAG [Sigma A7095]). The hydrolyses of PPA and AAG were conducted in a shaking water bath at 37 and 55°C, respectively, with continuous shaking (100 rpm) for 1, 2, 4, 8, 12, 24, 48, and 72 h. After hydrolysis, starch slurry was quickly centrifuged (5000 g) at 4°C for 5 min. The supernatant was used for measurement of the soluble carbohydrates to quantify the hydrolysis degree using the anthrone-H2SO4 method.
In Vitro Digestion Analysis
In vitro digestion was analyzed following the method of Cai et al.,[Citation18] using raw starch, gelatinized starch (98ºC, 12 min), and retrograded starch (gelatinized starch re-crystallized for 36 h at 4ºC). Ten milligrams of starch was incubated in 2 mL of enzyme solution (20 mM sodium phosphate buffer, pH 6.0, 6.7 mM NaCl, 0.01% NaN3, 2.5 mM CaCl2, 4 U PPA [Sigma A3176], 4 U AAG [Megazyme E-AMGDF]). The digestion was conducted in a shaking water bath at 37°C with continuous shaking (100 rpm) for 20 min and 2 h. Enzyme treatment was terminated by adding 240 μL 0.1 M HCl and 2 mL of 50% ethanol on ice and centrifuged (14,000 g, 5 min). The glucose content in the supernatant was determined by the D-Glucose (GOPOD Format) assay kit (Megazyme, K-GLUC). Starch nutritional fractions based on the rate of hydrolysis were rapidly digestible starch (RDS, digested within 20 min), slowly digestible starch (SDS; digested between 20 and 120 min) and resistant starch (RS; undigested after 120 min).
Statistical Analysis
The data reported in all tables were mean values and standard deviation. Analysis of variance (ANOVA) by Tukey’s test (p < 0.05) was evaluated using the SPSS 16.0 Statistical Software Program.
RESULTS AND DISCUSSION
Morphology, Starch Content, and Soluble Sugar Content of Kernel
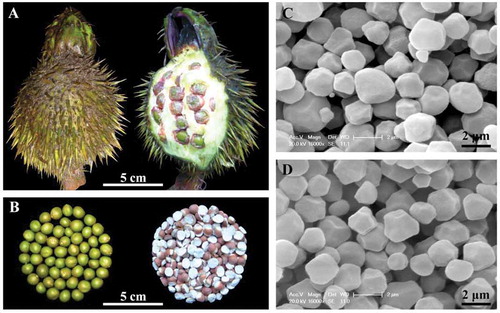
Euryale ferox fruits, seeds and kernels are shown in and . The seed shells were very hard, resulting that it was very difficult to obtain whole peeled kernels. The contents of starch and soluble sugar of dry kernel flour are shown in . The starch contents were about 70% and similar in kernels from two different regions. The soluble sugar content in the kernels from Shaobo Lake was significantly lower than that from Weishan Lake. The high starch content indicated that E. ferox kernel was rich in starch and a good resource for starch.
TABLE 1 Contents of starch and soluble sugar of dry kernels, and amylose content, relative crystallinity, and IR ratio of isolated starch
Morphology of Starch
Scanning electron micrographs of E. ferox starch granules showed regular polyhedron with a size of 1~2 μm (, ), which was in agreement with the report of Jha et al.[Citation1] that the size of E. ferox starch granules was very small (1~3 μm). Euryale ferox starch granules were significantly smaller than the other botanical starches,[Citation14,Citation18,Citation21] and their shape and size were homogeneous. The granule surface was all smooth and did not show any pores and fissures. The morphology of starch granules did not show significant difference in kernels from two different regions, and was in agreement with the previous report that the size and shape of starch granule were attributed to the biological origin.[Citation21]
Amylose Content and Molecular Weight Distribution of Starch
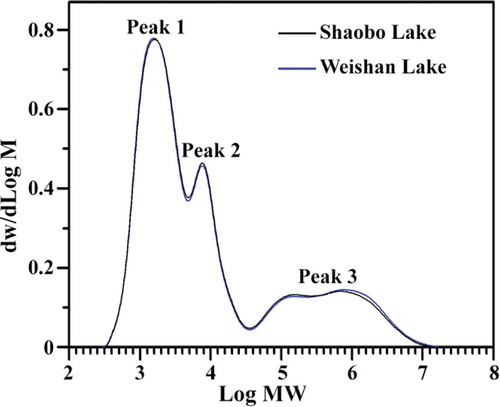
The amylose content determined based on iodine and starch affinity is described as apparent amylose content. The apparent amylose contents were similar between kernel starches from two different regions (). Nath and Chakraborty[Citation8] also found that E. ferox starch contained about 25% amylose as determined by potentiometric titrations, which was in agreement with the present result. The molecular weight distribution of isoamylase-debranched starch as determined by GPC is shown in . A trimodal distribution of low, middle, and high molecular weight peaks, designated peak 1, peak 2, and peak 3, respectively, was observed. Peak 1 and peak 2 consisted of short (A and short B chains) and long (long B chain) branch-chains of amylopectin, respectively. Peak 3 included amylose.[Citation22] The GPC result suggested that the contents of amylopectin short branch-chain, amylopectin long branch-chain and amylose were about 53.7, 22.1, and 24.2%, respectively, for kernel starch from Shaobo Lake, and 53.8, 21.6, and 24.6%, respectively, for kernel starch from Weishan Lake. Starches from two different regions showed similar molecular weight distribution. In the present study, the amylose content determined by GPC was significantly lower than apparent amylose content obtained by iodine colorimetric method, which was in agreement with the report of Shi et al.[Citation23] and indicated that the intermediate chain and amylopectin long branch-chain could bind iodine to increase the value of apparent amylose content.
Crystal Structure
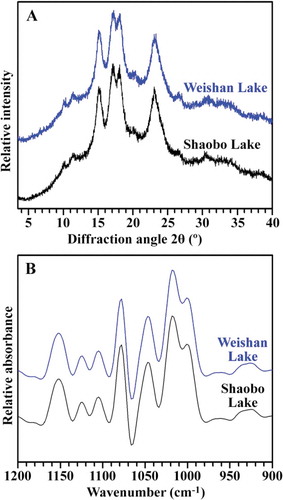
XRD has been used to reveal the crystal structure of starch. According to XRD patterns, there are three types of starch crystallinity reported, known as A-, B- and C-type.[Citation24] The XRD spectra of kernel starches from two different regions were shown in . The patterns were identical to that of A-type starch with strong diffraction peaks at about 15 and 23º, and an unresolved doublet at around 17 and 18º 2θ. The relative crystallinities of E. ferox kernel starches are given in . The relative crystallinities were similar in kernel starches from two different regions.
ATR-FTIR Analysis
The deconvoluted ATR-FTIR spectra in the region 1200–900 cm–1 of starches are shown in . The bands at 1045 cm–1 are linked with order/crystalline regions in starch. The 1022 cm–1 absorbance band arises as a result of absorption by stretching modes in amorphous starch, and is, therefore, sensitive to amorphous structure. The band at 995 cm–1 results from bonding in hydrated carbohydrate helices. The ratio of absorbance 1045/1022 cm–1 is used to quantify the degree of order, and that of 1022/995 cm–1 can be used as a measure of the proportion of amorphous to ordered carbohydrate structure in the starch. The intensity ratios of 1045/1022 and 1022/995 cm–1 are, therefore, useful as a convenient index of FTIR data in comparisons with other measures of starch conformation.[Citation14,Citation25] The ratios for 1045/1022 and 1022/995 cm–1 of starch are shown in . Based on both the spectra and calculated data, kernel starches from two different regions showed the similar short-range ordered structure.
Thermal Property
Thermal property of starch was determined by using DSC, the thermal parameters are summarized in . The gelatinization temperature and enthalpy were higher for kernel starch from Weishan Lake than for that from Shaobo Lake.
TABLE 2 DSC parameters of starch
Swelling Power and Water Solubility
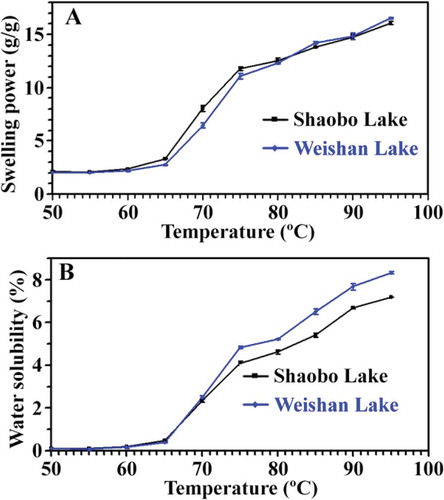
The swelling power of starch indicates the degree of water absorption of starch granules and the water solubility reflects the degree of dissolution during the starch swelling procedure.[Citation26] Swelling power and water solubility are characteristics of the starches. In this study, the swelling power and water solubility of starch were investigated from 50° to 95ºC in 5°C intervals (). Before gelatinization, swelling power and water solubility slightly increased with increasing temperature. After 65°C, they quickly increased. The results were in agreement with gelatinization onset temperature determined by DSC. After 75°C, the swelling powers were similar between kernel starches from two different regions, but the water solubility was higher in kernel starch from Weishan Lake than in that from Shaobo Lake.
Hydrolysis Property
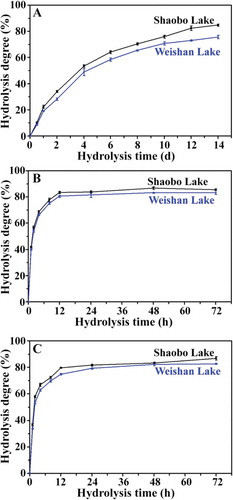
Most uses of starch in food and non-food applications require the disruption of starch granules through acid and enzyme treatments.[Citation27] The hydrolysis degree of starch by HCl is presented in . The hydrolysis degree increased gradually with increasing acid hydrolysis time. The kernel starch from Weishan Lake showed higher resistant ability to the acid than the kernel starch from Shaobo Lake. The time courses of PPA and AAG hydrolysis of starch are presented in and , respectively. The hydrolysis was biphasic, a very rapid rate at the initial stage, followed by a progressively decreased rate thereafter. A biphasic trend of amylase hydrolysis has also been observed in starches.[Citation20] The kernel starch from Shaobo Lake had slightly higher hydrolysis degrees of PPA and AAG than the kernel starch from Weishan Lake.
Digestion Property
In vitro digestion by both PPA and AAG is employed to simulate the effects of small intestine hydrolysis and subsequent glycemic response of starch.[Citation28] The RDS, SDS, and RS contents of starch were calculated in native, gelatinized, and retrograded starches and shown in . For native starch, the digestion properties were similar between kernel starches from two different regions. For gelatinized starch, RDS and SDS were similar between kernel starches from two different, but the starch from Weishan Lake had higher RS than the starch from Shaobo Lake. For retrograded starch, starch from Weishan Lake had higher RDS and RS than that from Shaobo Lake, but starch from Shaobo Lake had higher SDS than that from Weishan Lake. Native starch had significant higher RS than gelatinized and retrograded starches, and gelatinized starches had higher RDS and lower RS than retrograded starches. When starch granules in water are exposed to heat, the inter- and intra-molecular hydrogen bonds between starch chains are disrupted, allowing the granules to swell and then disintegrate. Thus, the availability of starch chains to the digestive enzymes is increased as gelatinization progresses.[Citation29] During gelatinized starch retrogradation, the amylose chains associate to form the amorphous matrix and amylopectins recrystallize to form the crystallites, which increase the resistance to digestive enzymes.[Citation30]
TABLE 3 Digestion properties of starches
CONCLUSION
In conclusion, Euryale ferox kernel contained about 70% starch. The starch was polyhedral, had very small size of about 1–2 μm, and exhibited A-type crystallinity. The kernel starches from two different regions showed similar amylose content, molecular weight distribution, relative crystallinity, short-range ordered structure, and swelling power. The kernel starch from Weishan Lake had higher gelatinization temperature, enthalpy and water solubility, contained higher RS in gelatinized and retrograded starch, and showed higher resistant ability to acid and amylase hydrolysis than that from Shaobo Lake. These results would be useful for E. ferox kernel as the potential of starchy source.
FUNDING
This study was financially supported by grants from the National Natural Science Foundation of China (31170299), the New Century Talent Project of Yangzhou University, and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Additional information
Funding
REFERENCES
- Jha, V.; Kargupta, A.N.; Dutta, R.N.; Jha, U.N.; Mishra, R.K.; Saraswati, K.C. Utilization and Conservation of Euryale ferox Salisbury in Mithila (North Bihar), India. Aquatic Botany 1991, 39, 295–314.
- Song, C.W.; Wang, S.M.; Zhou, L.L.; Hou, F.F.; Wang, K.J.; Han, Q.B.; Li, N.; Cheng, Y.X. Isolation and Identification of Compounds Responsible for Antioxidant Capacity of Euryale ferox Seeds. Journal of Agricultural and Food Chemistry 2011, 59, 1199–1204.
- Han, Z.; Luo, J.; Kong, L.Y. Two New Tocopherol Polymers from the Seeds of Euryale ferox. Journal of Asian Natural Products Research 2012, 14, 743–747.
- Jha, S.N.; Sharma, R. Physical, Gravimetric, and Functional Characterization of Various Milling Fractions of Popped Gorgon Nut (Euryale ferox). Journal of Food Science and Technology 2010, 47, 564–570.
- Liu, Y.; Wei, S.; Liao, M. Optimization of Ultrasonic Extraction of Phenolic Compounds from Euryale ferox Seed Shells Using Response Surface Methodology. Industrial Crops and Products 2013, 49, 837–843.
- Zhang, C.; Li, M.; Jia, X.L. Preliminary Separation and Identification of the Ethyl Acetate Extract from Gorgon Nut (Euryale ferox Salisb.) Seed Coat. Science and Technology of Food Industry 2012, 33, 223–225.
- Jha, S.N.; Prasad, S.N. Determination of Processing Conditions of Gorgon Nut (Euryale ferox). Journal of Agricultural Engineering Research 1996, 63, 103–111.
- Nath, B.K.; Chakraborty, A.K. Studies on the Physico-Chemical Properties of the Starch of Euryale ferox. Starch 1985, 37, 361–363.
- Saikia, J.P.; Konwar, B.K. Physicochemical Properties of Starch From Aroids of North East India. International Journal of Food Properties 2012, 15, 1247–1261.
- Sodhi, N.S.; Chang, Y.H.; Midha, S.; Kohyama, K. Molecular Structure and Physicochemical Properties of Acid-Methanol-Treated Chickpea Starch. International Journal of Food Properties 2013, 16, 125–138.
- Gujral, H.S.; Sharma, P.; Kaur, H.; Singh, J. Physiochemical, Pasting, and Thermal Properties of Starch Isolated from Different Barley Cultivars. International Journal of Food Properties 2013, 16, 1494–1506.
- Phrukwiwattanakul, P.; Wichienchotand, S.; Sirivongpaisal, P. Comparative Studies on Physico-Chemical Properties of Starches from Jackfruit Seed and Mung Bean. International Journal of Food Properties 2014, 17, 1965–1976.
- Wu, Y.; Lin, Q.L.; Cui, T.; Xiao, H.X. Structural and Physical Properties of Starches Isolated from Six Varieties of Millet Grown in China. International Journal of Food Properties 2014, 17, 2344–2360.
- Gao, H.M.; Cai, J.W.; Han, W.L.; Huai, H.Y.; Chen, Y.F.; Wei, C.X. Comparison of Starches Isolated from Three Different Trapa Species. Food Hydrocolloids 2014, 37, 174–181.
- Man, J.M.; Yang, Y.; Zhang, C.Q.; Zhou, X.H.; Dong, Y.; Zhang, F.M.; Liu, Q.Q.; Wei, C.X. Structural Changes of High-Amylose Rice Starch Residues Following in Vitro and in Vivo Digestion. Journal of Agricultural and Food Chemistry 2012, 60, 9331–9341.
- Tran, T.T.B.; Shelat, K.J.; Tang, D.; Li, E.; Gilbert, R.G.; Hasjim, J. Milling of Rice Grains. The Degradation on Three Structural Levels of Starch in Rice Flour Can Be Independently Controlled during Grinding. Journal of Agricultural and Food Chemistry 2011, 59, 3964–3973.
- Li, E.; Hasjim, J.; Dhital, S.; Godwin, I.D.; Gilbert, R.G. Effect of a Gibberellin-Biosynthesis Inhibitor Treatment on the Physicochemical Properties of Sorghum Starch. Journal of Cereal Science 2011, 53, 328–334.
- Cai, J.W.; Cai, C.H.; Man, J.M.; Zhou, W.D.; Wei, C.X. Structural and Functional Properties of C-Type Starches. Carbohydrate Polymers 2014, 101, 289–300.
- Wei, C.X.; Qin, F.L.; Zhou, W.D.; Xu, B.; Chen, C.; Chen, Y.F.; Wang, Y.P.; Gu, M.H.; Liu, Q.Q. Comparison of the Crystalline Properties and Structural Changes of Starches from High-Amylose Transgenic Rice and Its Wild Type during Heating. Food Chemistry 2011, 128, 645–652.
- Li, J.H.; Vasanthan, T.; Hoover, R.; Rossnagel, B.G. Starch from Hull-Less Barley: V. in-Vitro Susceptibility of Waxy, Normal, and High-Amylose Starches Towards Hydrolysis by Alpha-Amylases and Amyloglucosidase. Food Chemistry 2004, 84, 621–632.
- Singh, N.; Singh, J.; Kaur, L.; Sodhi, N.S.; Gill, B.S. Morphological, Thermal, and Rheological Properties of Starches from Different Botanical Sources. Food Chemistry 2003, 81, 219–231.
- Song, Y.; Jane, J. Characterization of Barley Starches of Waxy, Normal, and High Amylose Varieties. Carbohydrate Polymers 2000, 41, 365–377.
- Shi, Y.C.; Capitani, T.; Trzasko, P.; Jeffcoat, R. Molecular Structure of a Low-Amylopectin Starch and Other High-Amylose Maize Starches. Journal of Cereal Science 1998, 27, 289–299.
- Cheetham, N.W.H.; Tao, L. Variation in Crystalline Type with Amylose Content in Maize Starch Granules: An X-Ray Powder Diffraction Study. Carbohydrate Polymers 1998, 36, 277–284.
- Sevenou, O.; Hill, S.E.; Farhat, I.A.; Mitchell, J.R. Organisation of the External Region of the Starch Granule as Determined by Infrared Spectroscopy. International Journal of Biological Macromolecules 2002, 31, 79–85.
- Carcea, M.; Acquistucci, R. Isolation and Physicochemical Characterization of Fonio (Digitaria exilis Stapf) Starch. Starch 1997, 49, 131–135.
- Tawil, G.; Viksø-Nielsen, A.; Rolland-Sabaté, A.; Colonna, P.; Buléon, A. In Depth Study of a New Highly Efficient Raw Starch Hydrolyzing α-Amylase from Rhizomucor sp. Biomacromolecules 2011, 12, 34–42.
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and Measurement of Nutritionally Important Starch Fractions. European Journal of Clinical Nutrition 1992, 45, 33–50.
- Noda, T.; Takigawa, S.; Matsuura-Endo, C.; Suzuke, T.; Hashimoto, N.; Kottearachchi, N.S.; Yamauchi, H.; Zaidul, I.S.M. Factors Affecting the Digestibility of Raw and Gelatinized Potato Starches. Food Chemistry 2008, 110, 465–470.
- Chung, H.J.; Lim, H.S.; Lim, S.T. Effect of Partial Gelatinization and Retrogradation on the Enzymatic Digestion of Waxy Rice Starch. Journal of Cereal Science 2006, 43, 353–359.