Abstract
The chemical characteristics and antioxidant properties of Maillard reaction products formed in the Maillard reaction in a model system at 95°C for different lengths of time (0–6 h) were investigated at three mass ratios (1:1, 1:2, and 1:3) of porcine plasma protein hydrolysate to galactose. The results revealed that the pH value and free amino group content decreased (p < 0.05), whereas the browning index, intermediate products, and browning intensity, as well as reducing power and 2,2’-amino-di(2-ethyl-benzothiazoline sulfonic acid-6)ammonium salt radical scavenging activity of the Maillard reaction products increased as the reaction time increased (p < 0.05). Moreover, when the mass ratio of porcine plasma protein hydrolysate to galactose was 1:3, the Maillard reaction progressed easily, which rendered a higher degree of glycation and antioxidant activity (p < 0.05). These results indicated that the Maillard reaction can improve the antioxidant capacity of porcine plasma protein hydrolysate.
INTRODUCTION
The Maillard reaction (MR) is a complex series of chemical reactions that occurs naturally during food processing (e.g., baking, roasting, and frying) and storage at higher temperatures.[Citation1] The MR begins with the reaction between a carbonyl group of a reducing sugar, aldehyde, or ketone and an amine group of a free amino acid (such as is found in amino acids, peptides, and proteins) or any nitrogenous compound,[Citation2] which results in a complex network of reaction products known as Maillard reaction products (MRPs). This reaction is one of the so-called “non-enzymatic browning reactions,” which produce a variety of early, intermediate, and advanced compounds. The formation of protein-carbohydrate conjugates occurs during the early stage of the MR in which the free amino group reacts with the carbonyl group of carbohydrates to form Amadori products. During the advanced stages of the reaction, products resulting from the degradation of the Amadori products can undergo numerous transformations via various pathways, giving rise to peptide-bound adducts, protein crosslinking, and the formation of brown and polymeric materials.
Not only can MRPs alter important food properties, including color, flavor, and functionality,[Citation3,Citation4] but they may also be associated with the formation of compounds with pronounced antioxidant activities. Various mechanisms are involved in the antioxidant activity of MRPs, including radical chain-breaking activity,[Citation5] scavenging of reactive oxygen species,[Citation6] decomposition of hydrogen peroxide, and metal chelation.[Citation7] Antioxidant activity is commonly observed in peptides found in enzymatic hydrolysates from various plant and animal proteins. However, due to the complexity of peptides based on their molecular weight (Mw), structure, amino acid composition, and sequences, only a few studies have investigated the antioxidant activity of MRPs that are formed from protein hydrolysates.[Citation2] The MRPs that are produced from the peanut hydrolysate-sugar model system exhibit higher oxygen radical absorbance capacity (ORAC) values than peanut hydrolysate itself.[Citation8] Guérard and Sumaya-Martinez[Citation9] also reported that the antiradical scavenging effect was improved when casein peptone and cod viscera hydrolysates were heated in the presence of sugar. Additionally, MRPs derived from the hydrolysates of mechanically deboned chicken residue exhibited pronounced antioxidant activity, as measured by their reducing power, 1,1-diphenyl-2-pycryl hydrazyl (DPPH) radical scavenging activity and FeCitation2+ chelating activity.[Citation10]
Animal blood is an important by-product of the meat industry and is known to be a potential source of nutritional and functional protein. Previously, we reported that porcine plasma protein was hydrolyzed efficiently with Alcalase and that the 5 h hydrolysate exhibited the strongest inhibition of lipid oxidation, as indicated by thiobarbituric acid-reactive substance values in a liposome-oxidising system, as well as the free radical scavenging activities, which were evaluated by electron spin resonance spectrometry.[Citation11] In addition, we also investigated the chemical characteristics and antioxidant properties of MRPs that were prepared by the reaction of porcine plasma protein hydrolysate (PPPH) with three monosaccharides (glucose, fructose, and galactose) at 95°C for different lengths of time (0–6 h). The PPPH-galactose model system combination resulted in a higher browning intensity, more intermediate products and higher antioxidant activities than the PPPH-glucose or PPPH-fructose combinations, mainly due to the chemical structure of each type of sugar.[Citation12] Based on the above-mentioned findings, PPPH has specific antioxidant activities that can be significantly enhanced via the MR. Nevertheless, the MR is regarded as an extremely complicated reaction because it is influenced by numerous factors, such as pH, temperature, moisture, and heating time, and is reactant dependent. Concerning reactants, different carbohydrates, amino acids, and their reactant ratios result in different reaction kinetics. Hofmann and Schieberle[Citation13] evaluated the overall characteristics of conventional thermally treated ribose-cysteine reaction mixtures with ratios of ribose to cysteine ranging from 1:1 to 10:1 and found that the profile would be dominated by caramel-like compounds when the ratio of ribose to cysteine was increased to 10:1. Martinez-Alvarenga et al.[Citation14] found that a higher reactant ratio of reactants could contribute to a higher degree of glycation and color development in whey protein isolate conjugated with maltodextrins.
The objective of the present study was, therefore, aimed at investigating the effect of the mass ratio of reactants on the antioxidant activities (reducing power and radical scavenging activities) of MRPs derived from the reaction of PPPH with galactose. Additionally, the formation of non-fluorescent intermediate products, browning products, and changes in the pH and free amino group content due to non-enzymatic glycation were characterized to gain more insight into the antioxidant activity.
Materials
Porcine plasma protein was obtained from Baodi Meat Corporation (Zhaodong, Heilongjiang, China). The dry porcine plasma protein powder contained 70% protein, 15% ash, 13% moisture, and 1.5% lipids. Alcalase 2.4L (6×104 U/g) was obtained from Novo Nordisk (Bagsvaerd, Denmark). The 2,4,6-trinitrobenzenesulfonic acid (TNBS), 2,2’-azinobis(3-ethylbenzothiazoline-6-sulfonic acid; ABTS), L-leucine, galactose, and potassium ferricyanide were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals and reagents used were of analytical grade.
METHODS
Preparation of PPPHs
Porcine plasma protein was hydrolysed following the method of Liu et al.[Citation11] Porcine plasma protein solution (40 mg protein/mL) was preheated (90°C, 5 min) and then hydrolyzed with Alcalase at 55°C for 5 h. The enzyme to substrate ratio [E/S] was 2:100 (g/g). The pH of the porcine plasma protein solution was adjusted to the optimal value for Alcalase (pH 8.0) before hydrolysis was initiated, and it was readjusted to the optimal value with 1 M NaOH every 15 min during hydrolysis. After hydrolysis, the pH of the solution was brought to 7.0 using 1 M HCl, and the solution was then heated at 95°C for 5 min to inactivate the enzymes. The hydrolysates were freeze-dried (FD-2A Freeze-Dryer, Beijing, China), pulverized, placed in sealed bags, and stored at 4°C until use.
Preparation of MRPs
The MR model system consisted of PPPH (2.0 g) and galactose (2.0, 4.0, and 6.0 g) dissolved in distilled water to 100 mL, with final PPPH/galactose mass ratios of 1:1, 1:2, or 1:3. The mixture was transferred to screw-sealed tubes, tightly capped and heated in a water bath to 95°C. The samples were removed after heating for 0, 0.5, 1, 2, 3, 4, 5, or 6 h. After heating, the samples were immediately placed in an ice bath to cool. The obtained MRP samples were maintained at 4°C until they were analyzed. The control group (containing only PPPH) was heated under the same experimental conditions. All model systems were prepared in triplicate.
Measurement of pH Value and Color
The pH of MRPs produced for different times was measured using a DELTA 320 pH meter (Mettler-Toledo Instruments Co., Ltd, Shanghai, China). The color changes of the MRPs were determined using a ZE-6000 colorimeter (Nippon Denshoku, Kogyo Co., Tokyo, Japan). The results are displayed as L*-value (lightness), a*-value (redness), and b*-value (yellowness), which were used to calculate the browning index (BI; Eq. [2]). The instrument was set to the reflectance mode with the standard illuminant D65, which corresponds to natural daylight. A white standard plate (L* = 95.26, a* = –0.89, b* = 1.18) was used for calibration before measurement.
where, L*, a*, and b* are the values obtained in the colorimeter, BI, and x is the value obtained from Eq. (1).
Measurement of UV-Absorbance and Browning Intensity
Appropriate dilutions (10-fold) of the MRPs were made, and the absorbance was measured at 294 nm (early MRPs) and 420 nm (late MRPs) using a UV-1800 spectrophotometer (Pgeneral, Beijing, China) for the UV absorbance and browning intensity, respectively.
Measurement of Fluorescence Intensity
Appropriate dilutions (50-fold) of the MRPs were made, and the intrinsic emission fluorescence spectra of the MRPs were obtained using a fluorometer (F-4500 model, Hitachi, Tokyo, Japan) according to the method developed by Morales and Jimenez-Perez[Citation5] with a slight modification: The solutions were measured at an excitation wavelength of 347 nm and an emission wavelength of 415 nm.
Determination of Free Amino Group Content
The MRPs (10-fold dilution; 125 μL) were mixed with 2.0 mL of 0.2125 M phosphate buffer (pH 8.2) and 1.0 mL of 0.01% TNBS solution. The solutions were mixed thoroughly and placed in a temperature-controlled water bath (Taisite Instrument, Tianjin, China) at 50°C for 30 min in the dark. The reaction was terminated by adding 2.0 mL of 0.1 M sodium sulphite. The mixtures were cooled at room temperature for 15 min. The blank was prepared in the same manner as the samples, except that distilled water was used instead of 0.01% TNBS. The absorbance was measured at 420 nm. The free amino group content was expressed in terms of L-leucine.
Reducing Power
The reducing power of the MRPs was determined following the method of Oyaizu[Citation15] with some modifications. A total of 0.5 mL of MRPs (10-fold dilution) was mixed with 2.5 mL of sodium phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of 1% potassium ferricyanide. After incubation at 50°C for 20 min, 2.5 mL of 10% trichloroacetic acid (w/v) was added. The mixture was centrifuged at 3000 × g for 10 min. An aliquot (2.5 mL) of the supernatant was mixed with 2.5 mL of distilled water and 0.5 mL of 0.1% ferric chloride, and the absorbance at 700 nm was measured after reaction for 10 min. An increase in absorbance is indicative of an increase in reducing power.
ABTS Radical-Scavenging Activity
The method described by Ozgen et al.[Citation16] was used to measure the ABTS radical-scavenging activity. The ABTS radical was formed by adding K2S2O8 to ABTS. Briefly, 100 μL of MRPs (10-fold dilution) was mixed with 3 mL of ABTS•+ solution (absorbance of 0.70 ± 0.01 at 734 nm). The mixture was incubated in the dark for 6 min, and the absorbance was measured at 734 nm. The percentage of ABTS radical-scavenging activity was calculated as follows:
where, Ac is the absorbance of the control (ABTS•+ solution without MRPs), and As is the absorbance of the sample.
Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
SDS-PAGE was performed using a 3.0% stacking gel and 12.0% running gel lusing a Mini-Protean II cell electrophoresis system (Bio-Rad Laboratories, Richmond, CA, USA). The MRPs (1.0 mL at 4.0 mg of protein/mL) were dissolved in 1.0 mL of the sample buffer (4% SDS, 20% glycerol, 10% β-mercaptoethanol, and 0.125 M Tris-HCl, pH 6.8), heated to 100°C for 3 min, and centrifuged at 1800 × g to remove particulates. Aliquots of 20 μL of the supernatants were loaded into each well of the gel. A Mw standard, which was composed of a cocktail of proteins (6500–200,000; TaKaRa Biotechnology Co., Ltd. Dalian, China), was also run.
Statistical Analysis
All of the experiments were conducted in triplicate for each sample. Three independent experimental trials (replications) were conducted. The data were analyzed using the General Linear Models procedure of the Statistix 8.1 software package (Analytical Software, St. Paul, MN, USA) for microcomputers. An analysis of variance was performed to determine the significance of the main effects. Significant differences (p < 0.05) between the means were identified using Tukey’s procedures.
RESULTS AND DISCUSSION
Changes in pH
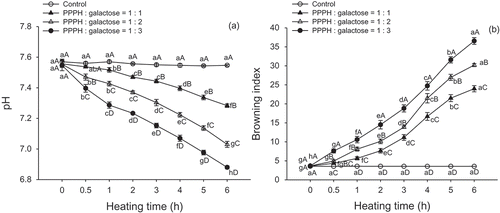
The pH values of the MRPs in the PPPH-galactose model system decreased gradually as the heating time increased (p < 0.05; ). In all model systems, the reaction with a 1:3 reactant mass ratio (PPPH/galactose) produced a lower pH value than the reactions with 1:1 or 1:2 reactant mass ratios (p < 0.05). There was little or no change in the pH of the control samples (p > 0.05). Benjakul et al.[Citation17] noted that the MRPs obtained from a porcine plasma protein-galactose model system caused the pH to decrease as the heating time increased, and a higher sugar concentration (2%) resulted in a greater decrease in pH values than did a 1% sugar concentration. Xu et al.[Citation18] also reported that the formation of acids (decrease in pH values) was generally positively correlated with the increasing reactant ratio of ribose to cysteine. These phenomena are most likely due to the production of formic and acetic acid from the reducing sugars, as reported by Rufian-Henares et al.,[Citation19] who demonstrated that the monosaccharide degradation products were 1-deoxyglucosone and 3-deoxyglucosone (compounds that were also generated from the MR), giving rise to the formation of formic and acetic acid. Chang et al.[Citation20] speculated that consumption of the amino group by the MR could shift systems to more acidic conditions and that formic acid is a major degradation product from the MR of galactose. Additionally, Liu et al.[Citation12] proposed that the decrease in pH is also related to the formation of reductones and melanoidins, leading to increased reducing power and radical scavenging activity. Therefore, it is assumed that greater reactant ratios of galactose to PPPH resulted in a greater amount of co-existing amino groups and carbonyl groups, which resulted in a lower pH value.
FIGURE 1 Changes in the pH. A: and browning index; B: of PPPH–galactose MRPs produced by heating to 95°C for different lengths of time. Bars indicate the standard deviation from triplicate determinations. Means within the same molar ratio of PPPH to galactose (line) with different lowercase letters (a–h) and means between different molar ratios of PPPH to galactose in the same reaction with different uppercase letters (A–D) differ significantly (p < 0.05).
Changes in Color Development
The BI, which represents the purity of the brown color, is considered an important parameter in the MR process. We calculated the BI starting from the primary color parameters (L*-, a*-, and b*-values). When the PPPH was heated alone (control), no change in the BI was detected (p > 0.05). The reaction of the PPPH with galactose was found to have significant increases in BI values (p < 0.05) with increased heating time (). The BI of the MRPs at 0 h was 3.62, 3.66, and 3.66, in the 1:1, 1:2, and 1:3 (PPPH/galactose) mass ratio model systems, respectively, which increased to 23.99, 30.19, and 36.55 after 6 h of heating. This phenomenon clearly indicated that the developed color of PPPH and galactose mixtures was due to the MR. Medrano et al.[Citation21] reported that the reactant ratio had a significant effect on the color development of MRPs obtained from β-lactoglobulin and glucose, which indicates that the carbohydrate concentration is an important factor. Renn and Sathe[Citation22] also noted that the reactant ratio had a positive effect on the color development in a monosaccharide-single amino acid system. The development of color in the peptide and sugar model system was mainly produced in the MR, which generated unsaturated, brown nitrogenous polymers and copolymers. The BI increased as the mass ratio of reactants and heating time increased, as it exhibited a greater shift to red (a*-value) and yellow (b*-value). Martinez-Alvarenga et al.[Citation14] also suggested that the generation of melanoidins, which contributed to the increasing darkness of color, was maximal at the highest molar concentration of sugar.
Changes in Absorbance At 294 nm and Browning Intensity
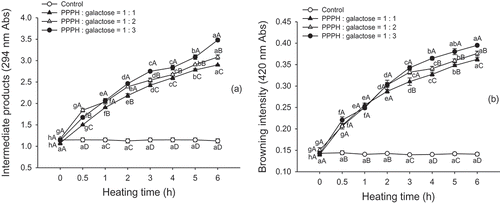
The MR is associated with the development of UV-absorbing intermediate compounds prior to the generation of brown pigments. The intermediate products and browning intensity were detected by UV-Vis absorbance at 294 and 420 nm, respectively. As illustrated in , there was a sharp increase in the UV absorbance of the PPPH-galactose solutions at 294 and 420 nm as the heating time increased (p < 0.05). However, in the case where the PPPH was heated alone (control), the increases in UV absorbance and browning intensity were not significant (p > 0.05). Moreover, we found that the 1:1 mass ratio of the PPPH to galactose treatment had a lower UV absorbance and browning intensity, indicating less extensive browning than the 1:2 and 1:3 treatments (p < 0.05). The UV absorbance levels of the MRPs at 294 nm at 0 h were 1.04, 1.07, and 1.11 for the 1:1, 1:2, and 1:3 mass ratio (PPPH/galactose) model systems, respectively, and there were 2.75-, 2.96- and 3.29-fold increases when the heating time was increased to 6 h. The browning intensity (absorbance at 420 nm) had a similar trend. These results suggest that the mass ratio of reactants involved in the reaction significantly influenced the production of both intermediate and final MR products (brown pigments). Luo et al.[Citation23] noted that the UV absorbance of the MRPs from the reaction of chitosan and xylan in a 1:3 mass ratio displayed the sharpest increase at 6 h of heating. Joubran et al.[Citation24] also reported that UV absorbance data of the MRPs from the reaction of lactoferrin and glucose significantly increased with increasing duration of thermal treatment, which is attributable to an increasing reactant ratio of protein to monosaccharide. In the present study, it is also important to note that the nucleophilicity of PPPH was expected to rise at elevated galactose concentrations and to thus, affect the rate and extent of the reaction.
FIGURE 2 Intermediate products (absorbance at 294 nm). A: and browning intensity (absorbance at 420 nm); B: of PPPH–galactose MRPs produced by heating to 95°C for different lengths of time. Bars indicate the standard deviation from triplicate determinations. Means within the same molar ratio of PPPH to galactose (line) with different lowercase letters (a–h) and means between different molar ratios of PPPH to galactose in the same reaction with different uppercase letters (A–D) differ significantly (p < 0.05).
Changes in Free Amino Group Content
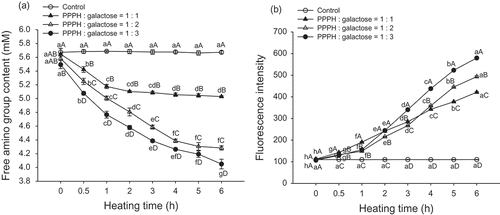
The changes in the free amino group content in PPPH-galactose mixtures subjected to the experimental conditions studied are depicted in . The free amino group contents of the MRPs at 0 h were 5.59, 5.58, and 5.54 mM in the 1:1, 1:2, and 1:3 mass ratio (PPPH/galactose) model systems, respectively, and they decreased by 10.97, 23.30, and 26.49%, respectively, when heated for 6 h (p < 0.05). Moreover, the changes in the control samples were not significant (p > 0.05), which demonstrated that this decrease could not be attributed to the liberation of NH2 groups from the PPPH substrate. These results indicate that the α- or ε-NH2 groups in the PPPH covalently attached to the galactose to form advanced glycation end-products to a greater extent with increased reaction time. In addition, an excess of carbonyl groups in the reactants promotes the development of the MR. Furthermore, results reported by Seo et al.[Citation25] confirm that carbohydrates with shorter chains, present in a high proportion in model systems, had higher reactivity. Martinez-Alvarenga et al.[Citation14] noted that a sharp decrease in the free amino acid content when going from a ratio of 1:3 to 1:15 in conjugates formed between bovine serum albumin and galactomannan resulted in the binding of 2.5 to 6.7 molecules of polysaccharide to protein. ter Haar et al.[Citation26] highlighted that saccharide concentration and reactivity, as well as peptide accessibility are the most important factors for determining the rate of the MR. Wooster and Augustin[Citation27] also evaluated the degree of glycation of conjugates created between β-lactoglobulin and maltodextrin when reacted in the following molar ratios: 1:0.09, 1:0.17, 1:0.25, 1:0.33, 1:0.66, and 1:1.7, obtaining 10.4, 12.9, 16.0, 25.5, 35.0, and 47.0% decreases in the free amino group content, respectively. Our results also indicate that the reactant ratio of galactose obviously influences the protein polymerization and browning associated with the advanced MR. Furthermore, a rapid decrease in the free amino group content of the MRPs was observed within the first 4 h of heating, followed by a slight decrease at later time points, suggesting that not all of the free amino groups can be conjugated with galactose.
FIGURE 3 Changes in the free amino group content. A: and fluorescence intensity; B: of PPPH–galactose MRPs produced by heating to 95°C for different lengths of time. Bars indicate the standard deviation from triplicate determinations. Means within the same molar ratio of PPPH to galactose (line) with different lowercase letters (a–h) and means between different molar ratios of PPPH to galactose in the same reaction with different uppercase letters (A–D) differ significantly (p < 0.05).
Changes in Fluorescence Intensity
The MR is also associated with the development of fluorescent compounds formed prior to the generation of brown pigments, which are not only precursors but also final products of the reaction. The fluorescence intensities of the intact PPPH and PPPH glycated with galactose are presented in . There were nearly no changes in fluorescence intensity when PPPH was heated alone for different lengths of time (p > 0.05). All of the MRPs exhibited higher fluorescence intensity than that observed for intact PPPH with increasing heating time (p < 0.05). Furthermore, the 1:3 mass ratio (PPPH/galactose) model system rendered the highest fluorescence intensity during 2 to 6 h of heating time (p < 0.05). We speculate that the higher sugar concentration may be due to a relatively decreased exposure of tryptophan (Trp) toward hydrophobic surroundings in the conjugates obtained after longer heating times due to heat-induced denaturation, which indicates a more hydrophobic microenvironment surrounding Trp. Seo et al.[Citation25] reported that the MRPs formed from potato proteins and galactose at the reactant ratio of 1:9 exhibited a significant red-shift of the Trp emission maximum to 340 nm, which indicates a higher concentration of galactose, leading to an increased exposure of Trp to more hydrophilic surroundings. A similar tendency, which was confirmed by the shift in the maximum emission wavelength to higher values with higher sugar concentrations, was observed in the studies by Luo et al.[Citation23] This phenomenon also agrees with the observed increase in the UV absorbance and browning intensity (as shown in ).
Reducing Power
The reducing power of the MRPs was determined by the potassium ferricyanide reduction method. From , it is clear that the reducing powers of all MRPs were enhanced with increased heating time (p < 0.05), and the 1:3 mass ratio (PPPH/galactose) model system had the highest (p < 0.05) reducing power, which indicated that the higher sugar concentration had a relatively stronger reducing power. Additionally, no enhancement of reducing power was observed in the control (p > 0.05). Benjakul et al.[Citation17] reported that MRPs from a porcine plasma protein-galactose model system had a greater reducing power than those prepared with fructose and glucose, while the MRP samples containing a higher level of galactose had a greater reducing power than those with a lower level. The reducing power might be due to the hydrogen-donating ability, which can exert antioxidant activity by breaking the free radical chain and donating a hydrogen atom.[Citation12] Hwang et al.[Citation28] suggested that compounds responsible for reducing activity are formed during the thermolysis of Amadori products in the primary phase of the MR, but heterocyclic compounds with reducing capacity may also be produced. It was also suggested that intermediate reductone compounds of MRPs can break the radical chain by donating a hydrogen atom,[Citation29] and that the hydroxyl groups of the MRPs play an important role in the reducing activity. Furthermore, we speculated that the different molar ratios of reactants obviously induced structural changes in the PPH-galactose systems during the MR, which resulted in more exposed hydroxyl groups that contribute to a stronger hydrogen donating ability. The reducing power of a compound may serve as a significant indicator of its potential antioxidant activity.
FIGURE 4 Reducing power. A: and ABTS radical scavenging activity; B: of PPPH–galactose MRPs produced by heating to 95°C for different lengths of time. Bars indicate the standard deviation from triplicate determinations. Means within the same molar ratio of PPPH to galactose (line) with different lowercase letters (a–h) and means between different molar ratios of PPPH to galactose in the same reaction with different uppercase letters (A–D) differ significantly (p < 0.05).
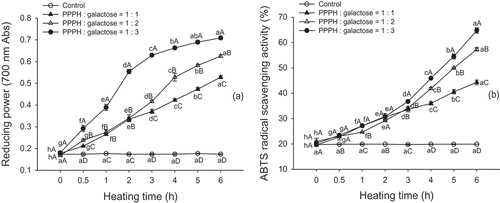
ABTS Radical-Scavenging Activity
When PPPH was heated alone, there was no change detected in the ABTS radical-scavenging activity (p > 0.05; ). However, the ABTS radical-scavenging activity increased significantly with increased heating time (p < 0.05), from 19.53, 20.98, and 20.21% (0 h) to 42.29, 57.33, and 64.76% (6 h) at the 1:1, 1:2, and 1:3 mass ratio (PPPH/galactose) model systems, respectively. Hayase et al.[Citation30] reported that MRPs derived from a D-xylose-glycine model system exhibited stronger ABTS radical-scavenging activity as the reaction time increased. Meanwhile, in agreement with observations of reducing power, a higher galactose concentration rendered a higher ABTS radical-scavenging activity (p < 0.05). Joubran et al.[Citation24] noted that the MRPs formed with a 1:3 reactant ratio (bovine lactoferrin:monosaccharide ratio) presented a much higher radical scavenging activity than MRPs formed with a 1:1 reactant ratio. From the results presented here, we found that there was a strong link between the amount of galactose used in the reaction and the radical-scavenging activity of the corresponding conjugates. Therefore, the formation of compounds that can function as hydrogen donors, including intermediates and the final brown polymers, contributes to the antiradical activity as measured by ABTS and hydroxyl-scavenging determination. In general, as with the reducing power, the ABTS radical-scavenging activity of the MRPs correlated well with the UV absorbance at 294 and 420 nm, which measures the brown pigmented end products of the MR.
Changes in SDS-PAGE Pattern
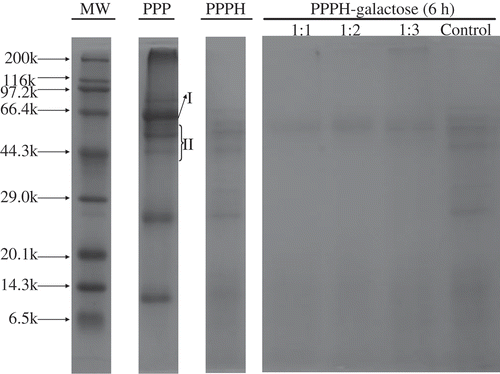
Changes in the molar mass distribution of the PPPH alone and PPPH with galactose were verified by SDS-PAGE patterns. depicts the electrophoretic patterns of PPPH alone and conjugated PPPH samples with different initial reaction ratios after heating at 95°C for 6 h. Non-hydrolyzed porcine plasma protein was composed largely of two proteins: albumin (MW 65,000) for band І, and α-globulin and β-globulin (MW 43,000–60,000) for band II. With the action of Alcalase, these major protein subunits were readily hydrolyzed. The MW of most peptides after 5 h was less than 6500, as demonstrated in our previous study.[Citation31] Additionally, the PPPH alone exhibited a characteristic electrophoretic pattern with no obvious changes after 6 h heating, which indicated no obvious protein polymerization or decomposition. However, during the MR with different initial reaction molar ratios, the gradual disappearance of a band (6.5–29.0 kDa MW) in the PPPH was observed. Furthermore, a broad band near the top of the running gel of the SDS-PAGE was observed in the MRPs from the 1:3 mass ratio (PPPH/galactose) model system, which suggested that a higher level of galactose formed conjugates more easily with PPPH than in reactions with a lower level of galactose. These analyses also revealed that the rate and extent of formation of higher MW species was more rapid and pronounced with a higher monosaccharide reactant ratio, as demonstrated previously.[Citation14,Citation24] Medrano et al.[Citation21] noted that a higher reactant ratio of lactose binding to β-lactoglobulin seemed to provoke a higher degree of protein aggregation, as revealed by a decrease in the exposed hydrophobicity. Jing and Kitts[Citation2] also reported that the formation of high Mw polymers was a major factor that contributed to higher antioxidant activities.
CONCLUSION
In conclusion, the modification of PPPH with galactose in the tested model systems resulted in products that exhibited strong antioxidant activities via electron donating and radical scavenging, which were dependent not only on the MR heating times but also on the molar ratio of reactants. MRPs prepared by heating PPPH with galactose at a mass ratio of 1:3 yielded the greatest antioxidant activity. Moreover, the increase in antioxidant activity coincided with an increase in the BI, UV absorbance, and browning intensity of the MRPs. Altogether, future research should be directed at investigating the protein functionality with changes in its structure, biological activity, and digestive fate of the active compounds in the MRPs.
FUNDING
This study was supported by the Nation Natural Science Foundation for Young Scholars of China (Grant No. 31301450), Foundation for University Key Teacher by the Heilongjiang Educational Committee (Grant No. 1253G007), and the Research Fund of Young Scholars for the Doctoral Program of Higher Education of China (Grant No. 20122325120018).
Additional information
Funding
REFERENCES
- Langner, E.; Rzeski, W. Biological Properties of Melanoidins: A Review. International Journal of Food Properties 2014, 17, 344–353.
- Jing, H.; Kitts, D.D. Antioxidant Activity of Sugar-Lysine Maillard Reaction Products in Cell Free and Cell Culture Systems. Archives of Biochemistry and Biophysics 2004, 429, 154–163.
- Golkar, A.; Nasirpour, A.; Keramat, J.; Desobry, S. Emulsifying Properties of Angum Gum (Amygdalus scoparia Spach) Conjugated to β-lactoglobulin Through Maillard-Type Reaction. International Journal of Food Properties 2015, 18, 2042–2055. DOI:10.1080/10942912.2014.962040.
- Ee, K.Y.; Zhao, J.; Rehman, A.U.; Agboola, S. Effects of Roasting on the Characteristics of Australian Wattle (Acacia victoriae Bentham) Seed and Extracts. International Journal of Food Properties 2013, 16, 1135–1147.
- Morales, F.J.; Jimenez-Perez, S. Free Radical Scavenging Capacity of Maillard Reaction Products As Related to Colour and Fluorescence. Food Chemistry 2001, 72, 119–125.
- Bersuder, P.; Hole, M.; Smith, G. Antioxidants from a Heated Histidine-Glucose Model System. Investigation of the Copper (II) Binding Ability. Journal of the American Oil Chemists’ Society 2001, 78, 1079–1082.
- Wijewickreme, A.N.; Kitts, D.D.; Durance, T.D. Reaction Conditions Influence the Elementary Composition and Metal Chelating Affinity of Nondialyzable Model Maillard Reaction Products. Journal of Agricultural and Food Chemistry 1997, 45, 4577–4583.
- Su, G.W.; Zheng, L.; Cui, C.; Yang, B.; Ren, J.Y.; Zhao, M.M. Characterization of Antioxidant Activity and Volatile Compounds of Maillard Reaction Products Derived from Different Peptide Fractions of Peanut Hydrolysate. Food Research International 2011, 44, 3250–3258.
- Guérard, F.; Sumaya-Martinez, M.T. Antioxidant Effects of Protein Hydrolysates in the Reaction with Glucose. Journal of the American Oil Chemists’ Society 2003, 80, 467–470.
- Sun, W.Z.; Zhao, M.M.; Cui, C.; Zhao, Q.Z.; Yang, B. Effect of Maillard Reaction Products Derived from the Hydrolysate of Mechanically Deboned Chicken Residue on the Antioxidant, Textural, and Sensory Properties of Cantonese Sausages. Meat Science 2010, 86, 276–282.
- Liu, Q.; Kong, B.H.; Jiang, L.Z.; Cui, X.H.; Liu, J. Free Radical Scavenging Activity of Porcine Plasma Protein Hydrolysates Determined by Electron Spin Resonance Spectrometer. LWT-Food Science and Technology 2009, 42, 956–962.
- Liu, Q.; Li, J.; Kong, B.H.; Jia, N.; Li, P.J. The Antioxidant Capacity of Maillard Reaction Products Formed by a Porcine Plasma Protein Hydrolysate-Sugar Model System As Related to Chemical Characteristics. Food Science and Biotechnology 2014, 23, 33–41.
- Hofmann, T.; Schieberle, P. Evaluation of the Key Odorants in a Thermally Treated Solution of Ribose and Cysteine by Aroma Extract Dilution Techniques. Journal of Agricultural and Food Chemistry 1995, 43, 2187–2194.
- Martinez-Alvarenga, M.S.; Martinez-Rodriguez, E.Y.; Garcia-Amezquita, L.E.; Olivas, G.I.; Zamudio-Flores, P.B.; Acosta-Muniz, C.H.; Sepulveda, D.R. Effect of Maillard Reaction Conditions on the Degree of Glycation and Functional Properties of Whey Protein Isolate-Maltodextrin Conjugates. Food Hydrocolloids 2014, 38, 110–118.
- Oyaizu, M. Antioxidant Activity of Browning Products of Glucosamine Fractionated by Organic Solvent and Thin-Layer Chromatography. Nippon Shokuhin Kogyo Gakkaishi 1986, 35, 771–775.
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2’-Azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) Method to Measure Antioxidant Capacity of Selected Small Fruits and Comparison to Ferric Reducing Antioxidant Power (FRAP) and 2,2’-Diphenyl-1-picrylhydrazyl (DPPH) Methods. Journal of Agricultural and Food Chemistry 2006, 54, 1151–1157.
- Benjakul, S.; Lertittikul, W.; Bauer, F. Antioxidant Activity of Maillard Reaction Products from a Porcine Plasma Protein-Sugar Model System. Food Chemistry 2005, 93, 189–196.
- Xu, H.G.; Liu, X.; Zhao, J.; Gao, Y.X. Effects of Ribose to Cysteine Ratios on the Formation of Volatile Compounds from the Maillard Reaction in Supercritical Carbon Dioxide. Food Research International 2008, 41, 730–737.
- Rufian-Henares, J.A.; Delgado-Andrade, C.; Morales, F.J. Occurrence of Acetic Acid and Formic Acid in Breakfast Cereals. Journal of the Science of Food and Agriculture 2006, 86, 1321–1327.
- Chang, H.L.; Chen, Y.C.; Tan, F.J. Antioxidative Properties of a Chitosan-Glucose Maillard Reaction Product and Its Effect on Pork Qualities during Refrigerated Storage. Food Chemistry 2011, 124, 589–595.
- Medrano, A.; Abirached, C.; Panizzolo, L.; Moyna, P.; Añón, M.C. The Effect of Glycation on Foam and Structural Properties of β-Lactoglobulin. Food Chemistry 2009, 113, 127–133.
- Renn, P.T.; Sathe, S.K. Effects of pH, Temperature, and Reactant Molar Ratio on l-Leucine and d-Glucose Maillard Browning Reaction in An Aqueous System. Journal of Agricultural and Food Chemistry 1997, 45, 3782–3787.
- Luo, Y.Q.; Ling, Y.Z.; Wang, X.Y.; Han, Y.; Zeng, X.J.; Sun, R.C. Maillard Reaction Products From Chitosan-Xylan Ionic Liquid Solution. Carbohydrate Polymers 2013, 98, 835–841.
- Joubran, Y.; Mackie, A.; Lesmes, U. Impact of the Maillard reaction on the antioxidant capacity of bovine lactoferrin. Food Chemistry 2013, 141, 3796–3802.
- Seo, S.Y.; Karboune, S.; Archelas, A. Production and Characterisation of Potato Patatin-Galactose, Galactooligosaccharides, and Galactan Conjugates of Great Potential As Functional Ingredients. Food Chemistry 2014, 158, 480–489.
- ter Haar, R.; Schols, H.A.; Gruppen, H. Effect of Saccharide Structure and Size on the Degree of Substitution and Product Diversity of α-Lactalbumin Glycated via the Maillard Reaction. Journal of Agricultural and Food Chemistry 2011, 59, 9378–9385.
- Wooster, T.J.; Augustin, M.A. The Emulsion Flocculation Stability of Protein-Carbohydrate Diblock Copolymers. Journal of Colloid and Interface Science 2007, 313, 665–675.
- Hwang, J.Y.; Shue, Y.S.; Chang, H.M. Antioxidative Activity of Roasted and Defatted Peanut Kernels. Food Research International 2001, 34, 639–647.
- Eichner, K. Antioxidative Effect of Maillard Reaction Intermediates. Progress in Food Nutrition Science 1981, 5, 441–451.
- Hayase, F.; Usui, T.; Watanabe, H. Chemistry and Some Biological Effects of Model Melanoidins and Pigments As Maillard Intermediates. Molecular Nutrition and Food Research 2006, 50, 1171–1179.
- Liu, Q.; Kong, B.H.; Xiong, Y.L.; Xia, X.F. Antioxidant Activity and Functional Properties of Porcine Plasma Protein Hydrolysate As Influenced by the Degree of Hydrolysis. Food Chemistry 2010, 118, 403–410.