Abstract
Fat mimicking properties of citric acid treated sweet potato starch were investigated in this present study. Citric acid treated sweet potato starch was prepared by treating the native sweet potato starch with 3% citric acid for 6 h at a temperature of 45°C. Dextrose equivalent value of citric acid treated sweet potato starch was 2.05%. A significant increase in amylose content was noticed in citric acid treated sweet potato starch possibly due to the lyses of amylopectin fractions. The melting temperature of citric acid treated sweet potato starch was 51.44°C, which was close to the melting point of fat. Citric acid treated sweet potato starch exhibited superior water holding capacity and in vitro digestibility. Gel strength and enthalpy (∆H) of citric acid treated sweet potato starch were comparatively lower than native sweet potato starch; correspondingly, citric acid treated sweet potato starch confirmed a low pasting profile. Native sweet potato starch and citric acid treated sweet potato starch exhibited a shear-thinning behavior. Acid treatment did not alter the granule size of native sweet potato starch (≈8 µm). Hence, this study concluded that citric acid treated sweet potato starch would be used as a potential fat replacer in food preparations due to its fat mimicking properties.
Introduction
Currently, consumers have a deep concern for their health and so they would like to limit the quantity of fat in their diet. Therefore, many types of fat replacers are being used to improve the quality of food and this affects the appearance and taste of the food.[Citation1] Fat replacers are usually characterized into two groups: fat substitutes and fat mimetics. Fat substitutes are components which have a chemical structure fairly close to fats and comprise physiochemical properties similar to fats.[Citation2,Citation3] Usually they are either indigestible or contribute lower calories. On the other hand, fat mimetics are ingredients that have markedly different chemical structures from fat. They are typically carbohydrate and/or protein-based.[Citation4,Citation5]
Carbohydrate based fat replacer includes fiber, hydrocolloids, gums, and modified starch which gives more benefits to product developers and at last consumers in a reduced fat food. In nature, they are hydrophilic, creating a carbohydrate water network when binding with water. That gives the original texture of fat and also is completely digestible in a normal metabolic process due to their all-time availability it is recognized as safe (Generally Regarded as Safe).[Citation6] Since carbohydrate-based fat replacers can bind to considerable amounts of water, their unique functionalities, such as emulsifying, gelling, and thickening properties, permit them to mimic the mouthfeel and flow properties similar to those of fat in aqueous systems.[Citation7] An ideal fat replacer ought to be absolutely safe and physiologically inert, i.e., have no calories or a reduced caloric value, nutritionally equivalent, and creating the illusion of fat.[Citation8] Even though a variety of fat replacers have been developed, there are unfortunately no ideal fat replacers which completely function like conventional fat.[Citation7]
Native starch can sometimes be used to replace fat. Nevertheless, starch modified by acid or enzymatic hydrolysis, oxidation, dextrinization, crosslinking, or mono-substitution is more commonly used to achieve desired functional and sensory properties.[Citation9] Acid cause scission of the glucosidic linkages, thereby altering the structure and properties of the native starch. The amorphous regions of the starch granules are more susceptible to acid hydrolysis than the crystalline regions.[Citation10] Acid hydrolysis reduces the molar mass, and consequently it increases the free aldehyde group content. It also decreases viscosity, increases the solubility of the granules, minimizes syneresis, and causes gel thermo-reversibility when subjected to cooling after melting[Citation11] creating a potential fat mimetic for the food industries. In a study conducted by Amaya-Llano et al.[Citation12] acid hydrolyzed jicama starch was prepared and used as a fat substitute in yogurt. Gels of hydrolyzed jicama starch showed thermo-reversibility. Acid treated jicama starch exhibited higher water solubility index and water absorption index and lower gel strength. The addition of hydrolyzed jicama starch (2.03 g/100 g) as a fat substitute in the preparation of stirred yogurt had good functional and sensory properties. Thys et al.[Citation13] investigated the technological, functional properties of acid-thinned pinhao starch. Acid-thinned pinhao starch showed high solubility, thermo reversibility and showed melting point similar to fats. They concluded that the acid treatment was efficient in producing a potential fat substitute from pinhao starch. Ma et al.[Citation14] reported that enzymatic hydrolysis of corn starch could be used as fat replacers.
The following are the criteria for a starch based fat mimetic:
Starch should contain an amylose content of ~20%.[Citation15]
Starch ought to require a granule size of 2 µm or in similar size to liquid micelle to act as fat mimetic.[Citation16]
According to the Food and Drug Administation (FDA),[Citation17] a starch-based fat mimetic is supposed to be partially or completely digestible.
Starch must possess a dextrose equivalent (DE) of ≤5.0.[Citation18]
Starch gel with a melting point close to that of the conventional fats (37–45°C) could be used as a fat substitute.[Citation19]
Starch must possess high water-holding capacity[Citation15] and better emulsifying properties.[Citation7]
Starch should display shear thinning characteristic.[Citation20]
Sweet potato (Ipomoea batatas (L.)) belongs to the Convolvulaceae family[Citation21] and is considered as the world’s most important and under-exploited crop.[Citation22] Low industrial application of sweet potato flour and starch caused a negative growth in its production. The intended use of sweet potato starch for industrial purpose will depend on advanced processing technologies to prepare sweet potato starch with desirable functional properties, and thorough indulgent of the effect of processing conditions on their properties. A better knowledge and understanding of sweet potato starch properties and how they compare with those of other starch source, is necessary to enhance the potential value of sweet potato starch in existing and novel uses.[Citation23] Based on this background, the research was aimed to evaluate the properties of acid-modified sweet potato starch as fat mimetic.
Materials and methods
Materials
Sweet potato was purchased from a local supermarket (Palzhamuthirsollai) in Salem, Tamil Nadu, India. A glucose oxidase-peroxidase (GOD-POD) kit was obtained from Beacon Diagnostics, Navasari, India. Amyloglucosidase from Aspergillusniger (30 U/mL) and all reagents were purchased from Sigma-Aldrich.
Isolation and Preparation of Acid-Thinned Sweet Potato Starch
Starch was isolated from sweet potato by the method of Wickramasinghe et al.[Citation24] An edible portion of sweet potato was cut into small pieces and homogenized with distilled water. The slurry was then passed through double-layered cheesecloth and the filtrate was allowed to settle for a minimum of 3 h at ambient temperature. The precipitated starch was washed three times with distilled water, dried at ambient temperature for 48 h and then the dried starch was kept in an oven at 50°C for 3 h and ground into fine powder and named as native sweet potato starch (NSPS). Citric acid treated sweet potato starch (CTSPS) was prepared by the method of Zambrano and Camargo.[Citation25] Starch slurry was prepared by dispersing NSPS (40 g dry basis) in 3% citric acid solution (0.15 M) and kept in a water bath at 45°C for 6 h with constant stirring. After hydrolysis, pH was adjusted to 5.5 ± 0.2 by slowly adding aqueous sodium hydroxide (5 g/100 mL). The starch was washed three times with two-fold volume of deionized water prior to filtration and dried in a convection oven at 45°C for 48 h. The dried starch was made into powder and packed in airtight containers for further use
Physiochemical Properties
Reducing sugar value of NSPS and CTSPS was measured using the dinitrosalicylic acid method of Miller[Citation26] to determine its DE. The starch sample (1 g) was diluted with 50 mL distilled water and filtered. Then the filtrate (0.5 mL) was mixed with 3,5-dinitrosalicylic acid to measure the reducing sugar content with dextrose as standard. The DE value was calculated as follows:
Amylose content determination was carried out by colorimetric iodine affinity procedure[Citation27] A mixture of 0.1 g of the starch sample, 1 mL of ethanol and 9 mL 1N sodium hydroxide was boiled for 10 min in a boiling water bath and allowed to cool. To that portion (5 mL) of the mixture, 1 mL of 1N acetic acid and 2 mL of iodine solution were added and Absorbance (A) was read in Spectrophotometer at 620 nm. The apparent amylose content was calculated as follows:
Moisture content and dry matter were determined by the method of Adebayo et al.[Citation28] Two milligrams (2 mg) of starch sample was measured into a previously weighed crucible. The crucible plus sample was then placed in an oven set at 100°C. Drying was carried out until a constant weight was observed. Later the crucible-containing sample was removed from the oven and transfer to a dessicator, cooled for 10 min, and weighed. The moisture content and dry matter were determined and expressed in percentage.
Melting Point, Clear Point, and Thermo-Reversibility of the Starch Gel
Starch gel was prepared by the method described by the National Starch and Chemical Corporation[Citation18] with modification. Sweet potato starch suspension (5%) was prepared with 0.02% (w/v) sodium metabisulphite in a beaker at 80°C for 10 min and then autoclaved at 121°C for 15 min. Subsequently, the beaker was cooled, hermetically sealed, and stored at 4°C for 24 h. The obtained gel was melted in a water bath at 80°C under agitation. The change in consistency was visually observed, and melting point was considered as the temperature at which the liquid phase was formed and mixing of the gel was possible. The clear point was considered as the temperature at which the sol appears optically clear. For the gel thermo-reversibility, the gel was melted in a water bath with constant stirring and allowed to cool down to room temperature, followed by refrigeration at 4°C for 18 h and the gel formation was observed.[Citation12]
In Vitro Digestibility of Starch Samples
In vitro digestibility of starch was analyzed according to the method of Noda et al.[Citation29] with some modifications. A mixture consisting of 4% (w/v) starch suspension in tubes was placed in a water bath at 100°C for 10 min to obtain the starch suspension. A 0.5 mL of starch suspension, 0.25 mL of 100 mM acetate buffer (pH 5.0), and 0.25 ml of glucoamylase solution were incubated at 40°C for 2 h with stirring. After digestion, surplus starch was removed by centrifuge (4350 tour/min during 10 min), and glucose in the filtrate was analyzed using the GOD-POD reagent.
Functional Properties
Water holding capacity (WHC) was determined by the method of Niba et al.[Citation30] Starch sample (1 g) was suspended in 5 mL water in a centrifuge tube. The slurry was shaken on a wrist-action shaker for 1 min at ambient temperature and centrifuged at 3000 × g for 10 min. The supernatant was poured carefully into a tared evaporating dish. WHC was calculated as follows (g/g):
Emulsifying activity and stability of starch samples were determined by the method of Neto et al.[Citation31] A starch dispersion was prepared (10 mg/mL) and was homogenized for 1 min with 5 mL of refined sunflower oil. The emulsion was centrifuged (1100 × g, 5 min) and the emulsifying activity was calculated as follows.
Emulsion stability was determined by heating the emulsion at 80°C for 30 min before centrifuging (1100 × g, 5 min)
Textural Properties of the Starch Gel
Gel textural properties were determined using a texture analyzer (Texture analyzer HD plus, Stable Microsystems, Godalming, UK). A starch suspension of 10% in a 50 mL beaker was heated to 95°C for 15 min and cooled to ambient temperature, then stored at 4°C for 24 h. The gel formed in the beaker was directly used for the texture analysis, and each gel was penetrated 4 mm by a P25/L (25 mm diameter) cylindrical probe. Two strength-time curves were obtained with a 1.0 mm/s speed during the penetration cycles. The texture profile curves were used to calculate gel strength, cohesiveness, adhesiveness, springiness, gumminess, and chewiness.
Pasting Properties
The pasting profile of starch was recorded using a Rapid Visco Analyser (RVA Tech Master, Perten Instruments, and Japan). The viscosity profiles were recorded using starch suspensions (12% w/v). The Std1 profile of the Perten Instruments was used, where the samples were held at 50°C for 1 min, heated from 50 to 95°C at 12.16°C/min, held at 95°C for 2.30 min, cooled from 95 to 50°C at 11.84°C/min, and held at 50°C for 2 min. The peak viscosity (PV), breakdown (BD), trough viscosity (TV), set back (SB), final viscosity (FV), pasting time (Pt), and pasting temperature (PT) were recorded.
Thermal Analysis
A differential scanning calorimeter (DSC; TA Instrument, Q2000, New Castle, NJ, USA) was used to determine the gelatinization characteristics of starch samples. A 4.5 mg sample (dry basis) was weighed in an aluminum pan and 10 µL of deionized water was added. The pan was sealed tightly and then it was allowed to stand for 1 h before carrying out the analysis. An empty aluminum pan was used as reference. The sample was subjected to a heating program over a range of temperature from 10 to 125°C and a heating rate of 5°C/min. The onset, peak, and final temperatures (To, Tp, and Tc, respectively) and transition enthalpy (∆H) were determined.
where, ∆H is the enthalpy change of the reaction, m is the mass of the sample at the beginning of the experiment, K is the calibration coefficient, and A is the area under the peak.[Citation32]
Rheological Properties
Dynamic rheological measurements of native and acid modified sweet potato starch-water suspensions (10%, dry sample to distilled water) were performed at 85°C with a CVOR Rheometer (Bohlin, Malvern, Worcestershire, United Kingdom), using parallel plate geometry (20 mm diameter, 1 mm gap). For each measurement, 1 mL of sample was carefully deposited over the plateau of the rheometer. After the plateau comes into contact with the plate, the exposed surface of the sample was covered with a thin layer of low-density silicone oil to prevent evaporation during the measurement. In order to describe the variation in the rheological properties of samples under steady shear, the data were fitted to the well-known power law model (Eq. 1), which was used extensively to describe the flow properties of non-Newtonian liquid engineering applications. A linear regression of shear rate versus shear stress was plotted to obtain statistically best values of R2, K, and n.
where, σ = shear stress (Pa), γ = shear rate (s-1), K = consistency index (Pa sn), n = the flow behavior index (dimensionless).
Structural Properties
Powder X-ray diffraction (XRD)
XRD pattern of starch samples was obtained using a Powder X-ray diffractometer (Rigaku Mini Hex-II, Japan). The graphs were plotted between the 2θ angles of 10 and 60 and smoothed with the software PowderX (Chinese Academy of Sciences, Beijing). The degree of crystallinity of samples was quantitatively estimated following the method of Nara and Komiya[Citation33] with the Origin–version 6.0 software (Microcal Software Inc., Northampton, MA, USA).
Scanning electron microscope (SEM)
Starch granules were observed using a SEM (JEOL-Model 6390, Japan). Granule size was determined by using ImageJ 1.46r (National Institute of Health, USA) software.
Statistical Analysis
The data of physiochemical and functional properties of the CTSPS were nine replications while other properties are triplicates. All data obtained were subjected to student’s t-test using MS Excel 2007. Differences at p < 0.05 were considered to be significant.
RESULTS AND DISCUSSION
Physiochemical Properties
Recovery yield
The recovery yield of CTSPS was 90% () and it was in the range as reported by Dutta et al.[Citation34] and Babu et al.[Citation35] Starch yield was reduced after acid treatment as starch might be hydrolyzed by citric acid during the acid starch reaction.
TABLE 1 Physicochemical properties
DE
DE value is an indicator of degree of acid hydrolysis. The DE value of CTSPS was 2.05%. Thys and Aires[Citation13] reported a DE value of 6.5 for pinhao starch treated with HCl, nevertheless DE values obtained in the present study was much lower as citric acid used for acid treatment was a weak organic acid, hence the degree of hydrolysis of the starch seems to be lower. On the other hand, sweet potato starch was treated with low concentration of citric acid (3%), and hence, resulted in the lower DE value. Since the DE value of acid treated starches were within the range referred by National Starch and Chemical Corporation[Citation18] indicates a potential applicability of the CTSPS starch as a fat mimetic.
Apparent amylose
The amylose content of NSPS was 18.56%, this result is agreed with the data reported by Tsakama et al.[Citation36] Acid treated starch displayed significantly higher portion of apparent amylose (25.81%) which might be due to the de-polymerization of amylopectin fractions during acid hydrolysis. Acid hydrolysis increases the amylose content of starch.[Citation37] Yackel and Cox[Citation38] stated that starches with a higher linear fraction (amylose content) bind strongly and orient water to endow with a sensation comparable to the rheology of fat in the oral cavity. Vanderveen and Glinsmann[Citation15] suggested an amylose of 20% was required for a carbohydrate-based fat replacer. Hence, CTSPS could mimic the functionality of fat when used as a fat replacer.
Moisture and dry matter
Moisture content and dry matter of NSPS was 14.11 and 85.89%, respectively, and in contrast CTSPS showed lower moisture content (9.50%) and higher dry matter (90.50%). A similar trend was observed by Omojola et al.[Citation39] during acid hydrolysis of cola starch.
Melting point, clear point, and thermo-reversibility
Melting point, clear point and thermo-reversibility of NSPS and CTSPS are shown in . Both NSPS and CTSPS illustrated a perfect gel formation when gelatinized and stored under refrigeration at 4°C. Melting point of NSPS was observed at 67.00°C while the CTSPS melted at 51.44°C that was lower than the native starch and it was closer to the melting point of conventional fats (37–45°C), this indicated that CTSPS would be used as a fat substitute. Amylopectin plays a major role in starch granule crystallinity and the presence of amylose indirectly lowers the melting point of the starch granule.[Citation40] The higher amylose content of CTSPS might be responsible for its lower melting point and clear point. Shorter amylopectin chains with less stable crystalline structure might be formed due to acid treatment of starch there by decreasing the melting point and clear point of starch gel.[Citation41] Thys et al.[Citation13] noticed a melting point of 46°C for acid treated pinhao starch. CTSPS had shown a clear point at 61.33°C however, the clear point was not displayed by native starch up to 80°C. Therefore, it might occur at a temperature above 80°C. NSPS resisted gel thermo-reversibility. Conversely, CTSPS displayed gel thermo-reversibility which implies that the degree of hydrolysis of the starch molecules was not very extensive, and thereby retained its gel structure. Acid treatment of starch causes partial hydrolysis of starch chains, resulting in much lower paste viscosity. However, when the paste cools down, acid-thinned starch chains tend to associate with each other more easily, forming a thermo-reversible gel.[Citation42] Similar fashion of gel thermo-reversibility was registered in the previous study[Citation13] for pinhao and corn starches.
In vitro digestibility
In vitro digestibility of NSPS and CTSPS was measured by glucoamylase and it is shown in . The digestibility of NSPS was 63.27% and this result is in comparable with the previous report on sweet potato starch (28.3–67.2%).[Citation43] The digestibility rate had been increased by citric acid treatment. The acid hydrolysis might take place in the amorphous area of the starch granule, and thereby crystallites were decoupled from the amorphous parts. Consequently, unlocked amorphous regions would be more sensitive to the enzyme attack and prone to rapid hydrolysis on the external glucose residues of amylose or amylopectin and it could be the reason for the increase in digestibility of acid modified starch. Hence CTSPS was confirmed as a fat replacer since the FDA[Citation17] recommended that a fat replacer might be partially or completely digestible.
Functional Properties
WHC
The result of WHC of NSPS and CTSPS is shown in . A significant difference was observed in the WHC between NSPS and CTSPS. Citric acid treatment probably increased the low molecular weight starch fraction with hydroxyl groups that hold water molecules to form hydrogen bonds consequently increasing the WHC. Thus, high WHC of CTSPS may mimic the property of fat when used in reduced fat foods.
TABLE 2 Functional properties of sweet potato starches
Emulsion activity and emulsion stability
CTSPS exhibited a higher emulsion activity and emulsion stability compared to NSPS (). CTSPS with higher linear amylose was found to exhibit emulsion property for preventing the separation of oil and water, the CTSPS evenly distributed to stabilize the emulsion system. The linear amylose fractions might be capable of film formation which would enhance the emulsion capacity and stability of the starch.[Citation44]
Instrumental Texture Analysis
Texture profile analysis (TPA) of NSPS and CTSPS gels is shown in . The gel strength of NSPS was 1.644 Kg and CTSPS was 0.392 Kg. The gel formed from NSPS was harder than CTSPS possibly due to the presence of long chains in sweet potato native starch that contribute to its firmer gel. On the other hand, the low gel strength of acid-thinned sweet potato starch might be attributed to a higher degree of short chains due to acid hydrolysis.[Citation10] A similar result was also noted in the study conducted by Sodh et al.[Citation45] Wang and Wang[Citation10] reported gel strength of 0.306, 0.484, and 0.094 Kg for acid thinned corn, potato, and rice starches, respectively. Springiness represents the ability of a gel to recover its original shape/height after a deforming force is removed.[Citation46] No significant change in the springiness was noticed due to acid treatment. Adhesiveness is the ability of the gel sample to become sticky.[Citation47] It is a surface characteristic which depends on a combined effect of adhesive and cohesive forces, viscosity, and viscoelasticity of the sample.[Citation48] Adhesiveness of native and acid treated sweet potato starch was 0.05 and 0.01 g/s, respectively. Starch with high amylose content (CTSPS) was observed to have lower adhesiveness.[Citation49] Cohesiveness is how well a sample with stands a second deformation relative to how it behaved under the first deformation.[Citation50] Cohesiveness of starch samples was ranged from 0.49 to 0.62. Gumminess is the product of hardness and cohesiveness, a characteristic of semi-solid foods which have a low degree of hardness and a high degree of cohesiveness.[Citation50] NSPS displayed a higher chewiness and gumminess compared to the CTSPS while CTSPS showed a higher cohesiveness than NSPS. The difference in textural properties of all sample gels was influenced by rigidity in gelatinized starch, amylose content as well as interaction between the dispersed and continuous phase of the gel which in turn is dependent on the amylose and amylopectin structure.[Citation51]
TABLE 3 Textural profile of native and citric acid treated sweet potato starch
Pasting Properties
reveals a significant influence of citric acid on viscosity of the sweet potato starch. RVA results of the CTSPS confirmed a low PV compared to native starch. A similar fashion of change was reported in the literature by Sandhu et al.[Citation52] and Dutta et al.[Citation34] The lower PV of CTSPS may be due to considerable BD of amorphous regions and the production of low molecular weight dextrins.[Citation53] TV is the viscosity that develops after holding the paste at 95°C and it measures the ability of the paste to withstand BD during cooling.[Citation54] TV and BD viscosity values of acid treated starch displayed the same decreasing trend compared to native starch. The increased degree of amylose recrystallization by acid thinning might be attributed to the change in break down viscosity.[Citation55] CTSPS displayed a lower FV compared to NSPS. Han et al.[Citation56] reported that acid hydrolysis results in considerable lyses of glycoside linkages of the long amylopectin chains, which apparently causes the fall in FV. The lower FV of acid treated starch implies that it undergo mild re-association and formed a weak network structure than native starch. SB viscosity is a measure of recrystallization of gelatinized starch. CTSPS registered a higher setback than native starch indicating that these starches got higher retrogradation tendency than native starch. Native starch took more time (4 min) to reach its PV than acid thinned starches. Overall pasting characteristics of sweet potato starch were found to be modified because of citric acid treatment. A similar decrease in pasting profile was noticed up on acid treatment of corn by Singh et al.[Citation53] in acid thinned sorghum starch.
TABLE 4 Pasting properties of native and citric acid treated sweet potato starch
Thermal Analysis
Thermal properties of starches determined by the DSC are represented in . Results showed variations in onset (To), peak (Tp), conclusion (Tc) temperatures and enthalphy (ΔH) between NSPS and CTSPS. NSPS displayed higher onset temperature than CTSPS. This is in agreement with the result reported that decreased To value was observed for acid modified maize starch[Citation57] CTSPS displayed a higher Tp and Tc temperature. This specifies that an acid hydrolysis might occur at the amorphous region of starch, which results in an increase in relative crystallinity and subsequently demonstrating an increased gelatinization temperature. Similar results were reported for acid modified potato starch[Citation10] and sweet potato starch.[Citation58] The ∆H of NSPS was 12.96 J/g on the other hand ∆H of CTSPS was lower than their counterpart native starch. The enthalphy of citric acid treated starch was 12.57 J/g. ΔH gives an overall measure of crystallinity and is an indicator of the loss of molecular order within the granule during gelatinization.[Citation59] CTSPS had a greater loss of ordered structure due to citric acid treatment.
TABLE 5 Thermal properties of native and citric acid treated sweet potato starch
Rheological Analysis
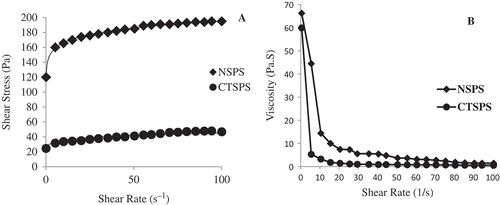
Shear rate versus shear stress plot of both 10% NSPS and CTSPS at 85°C was well fitted to the power law model (Eq. 1) with determination coefficients (R2) of 0.97 and 0.92 respectively, represented in . NSPS and CTSPS confirmed the values of flow behavior indexes (n) less than 1 indicating a shear-thinning behavior. NSPS showed a higher flow behavior index while CTSPS exhibited a lower flow behavior index suggesting that it possess a greater shear thinning behavior than its counter NSPS studied. This shear-thinning behavior might be attributed to the higher amount of breakage of the intra- and inter-molecular associative bonding system in the starch network micelles due to shearing at higher shear rates, as noted by Bhandari et al.[Citation60] It is evident that n was higher for NSPS which contains lower amylose content. The decrease in the power law index (n) with increasing amylose content (CTSPS) was generally attributed to an increase in the entanglements between amylose chains, since the highly branched amylopectin was not expected to form effective entanglements.[Citation61] Same is the case with the consistency index where NSPS exhibited a higher K value while CTSPS showed a lower K value. Both starches (NSPS and CTSPS) showed a non-Newtonian behavior, where the viscosity decreased when shear rate was increased (). This pattern is defined as shear thinning and is produced when the stress disorganized the arrangement of the macromolecules inside of the matrix.[Citation62] When shear force is applied, the particles may rearrange themselves into a parallel direction with shear force and big particles may break into small particles. The particles can flow easily as a result of resistance arising from particle-particle interaction which results in decreasing of viscosity.[Citation63]
TABLE 6 Rheological properties of acid treated sweet potato starches (10% w/v) at 85°C
Structural Properties
XRD
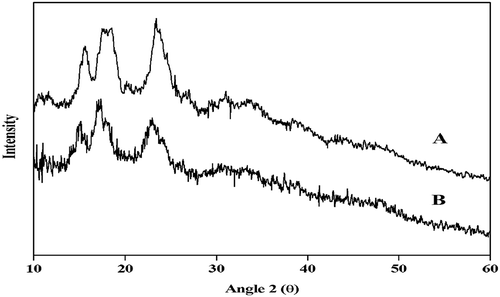
X-ray diffractogram of NSPS and CTSPS samples are presented in . NSPS and CTSPS exhibited a C-type X-ray diffractogram pattern. Both the starches exhibited peaks at the 2θ diffraction angles around 10, 11, 15, 17, 20, 23, and 26. The degree of crystallinity was higher in CTSPS than NSPS. The results of the present study showed that the crystallinity of sweet potato starch was increased with acid treatment () as suggested by Atichokudomchai, Shobsngob, and Varavinit.[Citation64] Similar pattern was reported by Babu et al.[Citation35] The increase in crystallinity of CTSPS has been attributed to preferential hydrolysis of the amorphous regions of the starch granule.[Citation65]
SEM
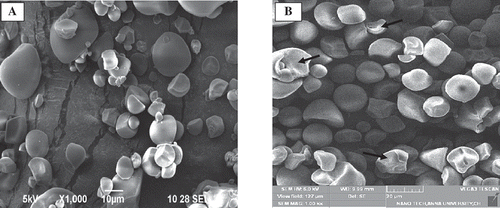
The SEM of the NSPS was illustrated in . Illustration of starch sample showed the presence of starch granules from small to large sizes. The granule surface of starches appeared to be smooth with no sign of any fissure. Zhu et al.[Citation66] also observed a smooth granule surface of sweet potato starches without cracks. Most of the sweet potato starch granules were polygonal in shape, however, round and irregular shapes were also noted. The surface of CTSPS granule was found to have a crack (depicted by arrows) as acid might have attacked the surface of the granule. Acid thinning resulted in a higher degree of surface erosion similar result was noticed in the literature.[Citation35,Citation67] Nevertheless a remarkable cleavage of the external surface of starch granule was observed in CTSPS starch as acid tends to attack the outer crystalline region subsequently assaulting the inner amorphous region. Acid treatment had no significant effect was noticed on the granule size of sweet potato starches. Granule size of NSPS and CTSPS () was 8.61 and 8.30 µm, respectively. Granule size of CTSPS was greater than the required size for a fat replacer mentioned in the literature.
Conclusion
The NSPS exhibited a recovery yield of 90% after treatment with 3% citric acid up to 6 h. The CTSPS exhibited a lower DE value, greater amylose content, and a melting point close to fat, which reveals that CTSPS exhibited the fat mimicking properties. Acid hydrolysis caused gel thermo-reversibility and it had superior WHC and emulsion properties. CTSPS demonstrated a reduced gel strength, which attributed to a higher degree of short chains. A marked decline in viscosity was observed for acid treated starch compared to NSPS as proved by RVA results. Higher gelatinization temperature (Tp and Tc) and crystallinity displayed by CTSPS specifies that acid hydrolysis might occur at amorphous region. The power-law model showed the best shear stress versus shear rate data with R2 values between 0.97 and 0.92 for NSPS and CTSPS, respectively, with an n-value less than 1. A visible degradation was observed when the granules were subjected to citric acid treatment with no significant change in granule size. The study led to infer that CTSPS possess fat mimicking properties and can be useful as a fat replacer.
REFERENCES
- Calorie Control Council. Fat replacers: Food Ingredients for Healthy Eating. http://www. caloriecontrol.org/fatreprint.html ( accessed May 22, 2013).
- Lipp, M.; Anklam, E. Review of Cocoa Butter and Alternative Fats for Use in Chocolate—Part A. Compositional Data. Food Chemistry 1998, 62, 73–97.
- Peters, J.C.; Lawson, K.D. Middleton S.J.; Triebwasser K.C. Assessment of the Nutritional Effects of Olestra, A Nonabsorbed Fat Replacement: Introduction and Overview. Journal of Nutrition 1997, 127, 1539S–1546S.
- Duflot, P. Starches and Sugars Glucose Polymers As Sugar/Fat Substitutes. Trends in Food Science & Technology 1996, 7, 206.
- Jonson, B.R. Whey Protein Concentrates in Low-Fat Applications; published by U.S. Dairy Export Council: Arlington, VA, 2002.
- Nonaka, H.H. Plant Carbohydrate Derived Products As Fat Replacers and Calorie Reducers. Cereal Foods World 1997, 42, 377–378.
- Lim, J.; Inglett, G.E.; Lee, S. Response to Consumer Demand for Reduced-Fat Foods: Multi-Functional Fat Replacers. Japan Journal of Food Engineering 2010, 11, 147–152.
- Grossklaus, R. Fat Replacers—Requirements from a Nutritional Physiological Point of View, European Journal of Lipid Science and Technology 1996, 98, 136–141.
- Akoh, C.C. “Fat Replacers.” Food Technology 1998, 52, 47–53.
- Wang, L.; Wang, Y.J. Structures and Physicochemical Properties of Acid-Thinned Corn, Potato, and Rice Starches. Starch-Stärke 2001, 53,570–576.
- Whistler, R.; Daniel, R. Function of Polysaccharides; Dekker: New York, NY, 1990; 256 pp.
- Amaya-Llano, S.L.; Martínez-Alegría, A.L.; Zazueta-Morales, J.J.; Martínez-Bustos, F. Acid Thinned Jicama and Maize Starches As Fat Substitute in Stirred Yogurt. LWT–Food Science and Technology 2008, 41, 1274–1281.
- Thys, R.C.S.; Aires, A.G. The Effect of Acid Hydrolysis on the Technological Functional Properties of Pinhão (Araucaria brasiliensis) Starch, Ciênncia e Tecnologia de Alimentos, Campinas 2013, 33, 89–94.
- Ma, Y.; Cai, C.; Wang, J.; Sun, D. Enzymatic Hydrolysis of Corn Starch for Producing Fat Mimetics. Journal of Food Engineering 2006, 73, 297–303.
- Vanderveen, J.E.; Glinsmann, W.H. Fat Substitutes: A Regulatory Perspective. Annual Review in Nutrition 1992, 12, 473–487.
- Daniel, J.R.; Whistler, R.L. Fatty Sensory Qualities of Polysaccharides. Cereal Foods World 1990, 35, 825.
- Food and Drug Administration. Toxicological Principles for the Safety Assessment of Direct Food Additives and Color Additives Used in Food; Bureau of Foods: Washington, DC, 1982.
- National Starch and Chemical Corporation. Converted Starches for Use As a Fat Or Oil Replacement in Food Stuffs. US Patent no. US4510166 A, 1985.
- Radley, J.A. Industrial Uses of Starch and Its Derivates; Applied Science Publishers Ltd.: London, 1976; 58 pp.
- Clark, D. Fat Replacers and Fat Substitutes. Food Technology 1994, 48, 86–87.
- Sweet Potato Starch Production- TM22-3e, International Starch Institute 1999-2006, Science Park Aarhus, Denmark. Www.Starch.Dk
- Sathe, S.K.; Salunkhe, D.K.; Functional Properties of the Great Northern Bean Protein: Emulsion, Foaming, Viscosity, and Gelation Properties. Journal of Food Science 1999, 46, 71–76.
- Woolfe, J.A. Sweet Potato—An Untapped Food Resource; Published in collaboration with the International Potato Centre, Peru. Cambridge University Press: Cambridge, UK, 1992; 643 pp.
- Wickramasinghe, H.A.M.; Takigawa, S.; Matsura-Endo, G.; Yamauchi, H.; Noda, T. Comparative Analysis of Starch Properties of Different Root and Tuber Crops Sri Lanka. Food Chemistry 2009, 112, 98–103.
- Zambrano, F.; Camargo, C.R.O. Otimizacao das condicoesdehidroliseacida do amido de mandioca para obtencao de substitutodegordura (English translation: Optimization of the conditions for the acid hydrolysis of cassava starch to obtain a fat replacer.). Brazilian Journal of Food Technology 2002, 4, 147–154.
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Analytical Chemistry 1959, 31, 426–428.
- Williams, V.R.; Wu, W.; Tsai, H.Y.; Bates, H.G. Varietal Differences in Amylose Content of Rice Starch. Journal of Agriculture and Food Chemistry 1958, 6, 47–48.
- Adebayo, A.O.; Lateef, S.O.; Elizabeth, A.B. Physicochemical, Rheological, and Consumer Acceptability of Cassava Starch Salad Cream. Journal of American Science, 2010, 6, 65–72.
- Noda, T.; Takigawa, S.; Matsuura-Endo, C.; Saito, K.; Takata, K.; Tabiki, T.; Wickramasinghe, H.A.M.; Yamauchi, H. The Physicochemical Properties of Partially Digested Starch from Sprouted Wheat Grain. Carbohydrate Polymers 2004, 56, 271–277.
- Niba, L.L.; Bokanga, M.M.; Jackson, F.L.; Schlimme, D.S.; Li, B.W. Physicochemical Properties and Starch Granular Characteristics of Flour from Various Manihot Esculenta (Cassava) Genotypes. Journal Food Science 2001, 67, 1701–1705.
- Neto, V.Q.; Narain, N.; Silvia, J.B.; Bora, P.S. Functional Properties of Raw and Heat-Processed Cashew Nut (Anacardiumoccidentale L.) Kernel Protein Isolate. Nahrung 2001, 45, 258–262.
- Artiaga, R.; Nayab, S.; Garcia, A.; Barbadilloa, F.; Garcia, L. Subtracting the Water Effect from DSC Curves by Using Simultaneous TGA Data. Thermochimica Acta 2005, 428, 137–139.
- Nara, S.; Komiya, T. Studies on the Relationship Between Water-Saturated State and Crystallinity by the Diffraction Method for Moistened Potato Starch. Starch/Starke 1983, 35, 407–410.
- Dutta, H.; Paul, S.K.; Kalita, D.; Mahanta, C.L.; Effect of Acid Concentration and Treatment Time on Acid–Alcohol Modified Jackfruit Seed Starch Properties. Food Chemistry, 2011, 128, 284–291.
- Babu, A.S.; Parimalavalli, R.; Jagannadham, K.; Rao, J.S. Chemical and Structural Properties of Sweet Potato Starch Treated with Organic and Inorganic Acid. Journal of Food Science and Technology 2014. DOI:10.1007/s13197-014-1650-x.
- Tsakama, M.; Mwangwela, A.M.; Manani, T.A.; Mahungu, N.M. Effect of Heat Moisture Treatment on Physicochemical and Pasting Properties of Starch Extracted from Eleven Sweet Potato Varieties. International Research Journal of Agriculture Science and Soil Science 2011, 1, 254–260.
- Betancourt, A.D.; Chel, G. Acid Hydrolysis and Characterization of Canavalia Ensiformis Starch. Journal of Agricultural and Food Chemistry 1997, 45, 4237–4241.
- Yackel, W.C.; Cox, C. Application of Starch-Based Fat Replacers. Food Technology 1992, 46, 146–148.
- Omojola, M.O.; Manu, N.; Thomas, S.A. Effect of Acid Hydrolysis on the Physicochemical Properties of Cola Starch. African Journal of Pure and Applied Chemistry 2011, 5, 307–315.
- Flipse, E.; Keetels, C.J.A.M.; Jacobson, E.; Visser, R.G.F. The Dosage Effect of the Wildtype GBSS Allele Is Linear for GBSS Activity But Not for Amylose Content: Absence of Amylose Has a Distinct Influence on the Physico-Chemical Properties of Starch. Theoretical and Applied Genetics 1996, 92, 121–127.
- van Soest, J.J.G.; Bezemer, R.C.; de Wit, D.; Vliegenthart, J.F.G. Influence of Glycerol on the Melting of Potato Starch. Industrial Crops and Products 1996, 5, 1–9.
- Hoseney, R.C. Principles of Cereal Science and Technology. American Association of Cereal Chemists, Inc.: St. Paul, MN, 1994; 52–54 pp.
- Zhang, Z.T. Nutritional Quality and Starch Physicochemical Properties in Sweet Potato, Ph.D. thesis. The University of Hong Kong, Hong Kong 2001.
- Han, J.-A.; Lim, S.-T. Effect of γ-Irradiation on Pasting and Emulsification Properties of Octenylsuccinylated Rice Starches. Carbohydrate Polymers 2012, 90, 1480–1485.
- Sodhi, N.S.; Chang, Y.-H.; Midha, S.; Kohyama, K. Molecular Structure and Physicochemical Properties of Acid-Methanol-Treated Chickpea Starch. International Journal of Food Properties, 2013, 16, 125–138.
- Park, E.Y.; Baik, B.; Lim, S. Influences of Temperature-Cycled Storage on Retrogradation and Invitro Digestibility of Waxy Maize Starch Gel. Journal of Cereal Science 2009, 50, 43–48.
- Sariçoban, C.; Yilmaz, M.T.; Karakaya, M. Response Surface Methodology Study on the Optimisation of Effects of Fat, Wheat Bran, and Salt on Chemical, Textural, and Sensory Properties of Patties. Meat Science 2009, 83, 610–619.
- Huang, M.; Kennedy, J.F.; Li, B.; Xu, X.; Xie, B.J. Characters of Rice Starch Gel Modified by Gellan, Carrageenan, and Glucomannan: A Texture Profile Analysis Study. Carbohydrate Polymers 2007, 69, 411–418.
- Yu, S.; Ying, M.; Da-Wen, S. Impact of Amylose Content on Starch Retrogradation and Texture of Cooked Milled Rice During Storage. Journal of Cereal Science 2009, 50, 139–144.
- Bourne, M.C. Food Texture and Viscosity Concept and Measurement, 2nd Ed; Food Science and Technology International Series. Academic Press: San Diego, CA, 2002, 427 pp.
- Yamin, F.F.; Lee, M.; Pollak, L.M.; White, P.J. Thermal Properties of Starch in Cornvariants Isolated after Chemical Mutagenesis of Inbred Line B73. Cereal Chemistry 1999, 76, 175–181.
- Sandhu, K.S.; Singh, N.; Lim, S.T. A Comparison of Native and Acid Thinned Normal and Waxy Corn Starches: Physicochemical, Thermal, Morphological, and Pasting Properties. LWT: Food Science and Technology 2007, 40, 1527–1536.
- Singh, H.; Sodhi, N.S.; Singh, N. Structure and Functional Properties of Acid Thinned Sorghum Starch. International Journal of Food Properties 2009, 12, 713–725.
- Ikegwu, O.J.; Okechukwu, P.E. Physicochemical and Pasting Characteristics of Flours and Starch from Achi Brachystegia Eurtcoma Seed. Journal of Food Technology 2010, 8, 58–66.
- Chung, H.-J.; Jeong, H.-Y.; Lim, S.-T. Effects of Acid Hydrolysis and Defatting on Crystallinity and Pasting Properties of Freeze-Thawed High Amylose Corn Starch. Carbohydrate Polymers 2003, 54, 449–455.
- Han, X.Z.; Campanella, O.H.; Mix, N.C.; Hamaker, B.R. Consequence of Starch Damage on Rheological Properties of Maize Starch Pastes. Cereal Chemistry 2002, 79, 897–901.
- Lin, J.H.; Lee, S.Y.; Chang, Y.H. Effect of Acid—Alcohol Treatment on the Molecular Structure and Physicochemical Properties of Maize and Potato Starches. Carbohydrate Polymers 2003, 53, 475–482.
- Singh, S.; Raina, C.S.; Bawa, A.S.; Saxena, D.C. Effect of Heat-Moisture Treatment and Acid Modification on Rheological, Textural, and Differential Scanning Calorimetry Characteristics of Sweetpotato Starch. Journal of Food Science 2005, 70, E373–E378.
- Hoover, R.A.; Vasanthan, T. Effect of Heat Moisture Treatment on Structure and Physicochemical Properties of Cereal, Legume, and Tuber Starches. Carbohydrate Research 1994, 252, 33–53.
- Bhandari, P.N.; Singhal, R.S.; Kale, D.D. Effect of Succinylation on the Rheological Profile of Starch Pastes. Carbohydrate Polymers 2002, 47, 365–371.
- Yu, L.; Christie, G. Microstructure and Mechanical Properties of Orientated Thermoplastic Starches. Journal of Materials Science 2005, 40, 111–116.
- Guerra-DellaValle, D.; Sánchez-Rivera, M.M.; Zamudio-Flores, P.B.; Méndez-Montealvo, G.; Bello-Pérez, L.A. Effect of Chemical Modification Type on Physicochemical and Rheological Characteristics of Banana Starch. Revista Mexicana de Ingeniería Química 2009, 8, 197–203.
- Charm, S.E. The Nature and Role of Fluid Consistency in Food Engineering Applications. Advances in Food Research 1962, 11, 356–435.
- Atichokudomchai, N.; Shobsngob, S.; Varavinit, S. Morphological Properties of Acid Modified Tapioca Starch. Starch/Stärke 2000, 52, 283–281.
- Hoover, R. Acid-Treated Starches, Food Reviews International 2000, 16, 369–392.
- Zhu, F.; Yang, X.; Cai, Y.; Bertoft, E.; Corke, H. Physicochemical Properties of Sweet Potato Starch, Starch/Starke 2011, 63, 249–259.
- Ali, T.M.; Hasnain, A. Morphological, Physicochemical, and Pasting Properties of Modified White Sorghum (Sorghum Bicolor) Starch. International Journal of Food Properties 2014, 17, 523–535.