Abstract
Palynological, physicochemical, and sensory methods were applied to ascertain the most discriminant variables for honey characterization. Fifteen physicochemical parameters, six indicators of antioxidant capacity and eight sensory attributes were considered. Clover and eucalyptus honeys were differentiated when the linear discriminant analysis was applied. Electrical conductivity, sugars, ferric ion reducing, and Trolox equivalent antioxidant capacity were the most discriminant variables. Odor and color intensities were the sensory attributes scoring the major differences between honeys. The canonical correlations technique pointed out that differences were related primarily to the content of pollen from Trifolium, Medicago sativa, and Lotus. These standards of quality provide a differentiating tool to classify unifloral honeys.
INTRODUCTION
Multifloral and unifloral commercial samples of honey differ in their chemical composition and sensory quality on account of various factors like plant source, season, geographical origin, packaging conditions, and storage period.[Citation1,Citation2] The current international market demands the setup of quality control protocols according to the palynological, physicochemical, and sensory characteristics. This trend becomes explicit in the European Standard Directive 110/01,[Citation3] which defines honey as unifloral when it is from a completely or partially botanical origin including its pollen, physicochemical, and sensory characteristics corresponding to their origin. Consequently, the three analytical systems provide complementary tests to characterize a honey.[Citation4] However, little general information is available about typified unifloral honeys, which are simultaneously subjected to sensory and physicochemical analysis.[Citation5–Citation8]
As honey is a complex natural food, distinct characterization of honey samples requires the use of several parameters in order to obtain a good authentication procedure. Honey characterization is essential when its commercial quality must be assessed. To establish the significance of parameters closely related to the origin of honey, quality control methods, and multivariate statistical analysis need to be used.[Citation9] These methods will help to give precise classifications for honey samples.[Citation10–Citation14]
Argentina is one of the largest world producers of honey, as stated by Food Agricultural Organization of the United Nations.[Citation15] Likewise, the central region of Argentina has the greatest production of honey, with abundant unifloral honeys corresponding to adventitious or cultivated exotic plants such as Eucalyptus spp, Brassicaceae, and Melilotus sp.[Citation16–Citation18] Although physicochemical and palynological separate studies have been carried out on Argentine honeys[Citation19–Citation21] and Uruguayan ones,[Citation22] there are few reports on the relationship between their botanical origin and their physicochemical[Citation22,Citation23] or sensory properties.[Citation24] Furthermore, the relationships among the tree set of measures remain unexplored.
The objective of this work was to find the variables that differentiate Argentine eucalyptus and clover honeys, which could be used for a successful authentication of each floral origin for commercial purposes, in response to consumer demands. This was performed using data from melissopalynological, physicochemical, and sensory analysis to attempt the classification of honey samples according to their botanical origin. In order to achieve a precise characterization for these honey samples, several multivariate statistical techniques were applied.
MATERIALS AND METHODS
Samples
There were 148 samples of honey collected in accordance with the Codex Stan 12,[Citation25] in the Argentine phytogeographical region known as Pampeana Region. This is characterized by grass pasture which has been highly altered by stocking, the development of many crops (Triticum aestivum L., Zea mays L., Glycine max (L.) Merr., Oryza sativa), and animal feed harvests (Lotus sp., Medicago sativa L., Trifolium repens L., Trifolium pratense L. and Melilotus albus), with predominance of non-native species and poor pollen diversity. The study was performed over four successive years. Honey samples were collected from bee colonies and were stored at 18 ± 2ºC in the dark, until the experiment.
The 54.7% of original set of 148 samples were unifloral honeys; 35.8% (n = 53) of which were clover honey (Trifolium sp.) and 18.9% (n = 28) came from eucalyptus (Eucalyptus spp.). Remaining honeys were multifloral or another unifloral honeys. The set of 81 samples of eucalyptus and clover honeys underwent palynological, physicochemical, and sensory analysis.
Palynological Analysis
Qualitative palynological analysis was performed by counting to 500 pollen grains per sample.[Citation26] Microscopical observation were carried out in an Olympus B×40 light microscopic at 200×. Pollen was identified by using previously published data and pollen collection of well-known plants.[Citation27,Citation28] The frequency of pollen grains was determined from the number of honey samples in which the different pollen types were present: very frequent (MF > 50 %), frequent (F: between 20–50%), uncommon (PF, between 10–20%), and rare (R < 10%).[Citation29] Honey was considered as coming of eucalyptus if the relative frequency of Eucalyptus pollen exceeded 70% and it was considered as clover honey if the relative frequency of the pollen from Trifolium, Lotus, Medicago sativa, and Mellilotus exceeded 45%.[Citation30] For declaration of a honey as a uniforal also the physical–chemical data and sensory testing were taken into consideration.
Physicochemical Characterization
Several physicochemical parameters were examined for all samples: moisture, by refractometric Association of Official Analytical Chemists (AOAC) Official Meth. 969.38 B;[Citation31] free acidity by acid-base titration, according to AOAC Official Meth. 962.19;[Citation31] ash, gravimetrically according to AOAC Official Meth. 920.181;[Citation31] electrical conductivity using a conductivity Horiba Model D24E; diastase index by spectrophotometry AOAC Official Meth. 958.09,[Citation31] using a Varian Cary 50 spectrophotometer; 5-hydroxy methyl-2-furfural (HMF) by high performance liquid chromatography (HPLC), according AOAC Official Meth. 980.23;[Citation31] sugar spectrum by HPLC, according AOAC Official Meth. 977.20;[Citation31] reducing sugars and apparent sucrose by titration with Fehling’s solution, according AOAC Official Meth. 920.183 and 920.184;[Citation31] specific rotation using a Scheitler Polarimeter WXG-4 according AOAC Official Meth. 920.182.[Citation31] Color was determined according AOAC Official Meth. 960.44,[Citation31] using a Pfund Koheler colorimeter. Each value was expressed as means ± standard deviation of two independent samples with two measurements per sample (n = 4).
In Vitro Assays for Determining Antioxidant Capacity
The total flavonoids content was determined spectrophotometrically[Citation32] and the hydroxyl radical (OH) scavenging activity was measured by the ability to inhibit the degradation of deoxyribose.[Citation33] Results were expressed as milligrams of quercetin equivalent in 100 g of honey (mg QE/100 g). Total phenolic constituents were determined by employing Folin Ciocalteau reagent and gallic acid as standard.[Citation34] The total phenolic content was determined as milligrams of gallic acid equivalent per 100 g of honey (mg GAE/100 g).
The effect of aqueous solution of honey on phenyl picryl hydrazyl radical (DPPH) was estimated according to.[Citation35] Trolox equivalent antioxidant capacity (TEAC) method relies on the ability of the radical from 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid; ABTS•+), to react with the hydrogen assigned by antioxidants, and transformed into a compound of lower intensity of absorbance at a specified wavelength.[Citation36] Results were expressed as milligrams of Trolox equivalent per 100 g of honey (mg TE/100 g). The ferric reducing antioxidant capacity (FRAC) was determined spectrophotometrically.[Citation37] Reducing capacity was expressed in milligram of ascorbic acid equivalent in 100 g of honey (mg AAE/100 g).
Sensory Analysis
The honey samples were tested for odor, taste, appearance, and color in comparison to respective references.[Citation38] The panel of assessors was formed by seven people. Procedure was developed to evaluate the samples and developed the following scales and references: (1) scale for the assessment of the current tastes sweet, salty, sour, and bitter; (2) rating scale for odor intensity; (3) scale for the assessment of odor intensity of caramelized/burned notes; and (4) scale for the assessment of graininess and fluidity. Examples of trigeminal sensations were presented, using ferrous Al and K sulfate dodecahydrate aqueous solutions. For the pungency, it was adopted the presentation with strips of paper embedded in acetaldehyde or propionaldehyde ethanolic solutions. Examples of smoothness (Marroc snacks), adhesiveness (current style toffee), easy dissolution (gelatin) and coolness (menthol candy) were given. To quantify the intensity of sensory variables, we used a seven-point scale, where zero indicated no property.
Statistical Analysis
For the evaluation of the data we performed a linear discriminant analysis (LDA) and canonical correlation analysis (CCA) using SAS® v. 9.3 (SAS Institute, Cary, USA, 2011). LDA was applied to identify the set of “best discriminating” variables between defined groups. CCA was computed to identify the relationships between two sets of variables: polinic versus physicochemical and polinic versus sensory variables.[Citation39]
RESULTS
Palynological Characteristics
The pollens from Eucalyptus spp. appeared in 83.95% of the samples, and the pollen from Trifolium sp. appeared in 51.85%; they were classified as very frequent. Lotus sp. (49.4%), Melilotus sp. (48.15%), Carduus sp. (42.0%), Helianthus annuus (30.9%), Medicago sativa (27.2%), and Brassicaceae (24.7%) were found as frequent pollen; while Echium plantagineum (14.8%), Cycorea (13.6%), Poaceae (13.6%), and Convolvulus arvensis (12.3%) were found as uncommon. The 31 remaining pollen types had a very low frequency of occurrence and were classified as rare.
Physicochemical Characteristics
This set of honeys met the international quality specifications.[Citation25] shows the mean and standard deviation values for all physicochemical tests for both clover and eucalyptus honeys.
TABLE 1 Physicochemical characteristics and antioxidant activities of clover and eucalyptus honeys
Phenolic Compounds, Flavonoid, and Radical Scavenging Activity
shows the mean and standard deviation values for all phenolic and flavonoid compounds as well as the antioxidant capacity for both unifloral honeys. The values were consistent with those reported by other authors.[Citation40–Citation42]
Sensory Characteristics
The mean values for sensory variables describing physical as well as odor and taste status are depicted in . No honey sample featured salty, bitter, or sour taste. The UNI 59.00.642[Citation43] states that eucalyptus honeys have scents and aromas of medium intensity, with mildly sweet taste without bitterness. It was found slight acidic and salty notes for eucalyptus Spanish honeys with secondary pollen presence of broom and heather.[Citation44] Clover honeys (Trifolium alexandrinum L. and Trifolium incarnatum L.) have been described as weak odor with no particular characteristics, at best a vegetal note. A note of caramel milk was perceived for honeys of white clover (Trifolium repens L.).[Citation45] No trigeminal sensations such as coolness and pungency were identified. They were soft texture, not sticky and easy solution. Both types of honey unifloral spontaneously crystallized in a short time after harvest.
TABLE 2 Sensory characteristics of clover and eucalyptus honeys
Correlations Between Sensory Parameters
The graininess and size of crystals were highly correlated (r2 = 0.87) indicating that the visual appreciation of the size of crystals matches the oral perception of texture. Sweetness intensity and persistence are also associated (r2 = 0.54); the sweetness may be a component of the overall sensations in the mouth. The intensity of color and odor were also significantly correlated (r2 = 0.56); this could indicate that substances contribute to the color and the odor both. Other authors informed correlations between odor intensity, persistence, bitterness, and color when multifloral honeys were analyzed.[Citation46]
Discriminant Analysis of the Three Sets of Variables
Palinological characteristics
Applying LDA to all variables that identified the pollen composition, it was found that there was significant difference between the average vectors of both eucalyptus and clover honeys (Λ = 0.751, p-associated = 0.0001). Besides, the LDA correctly assigned both sets of honeys to each botanical origin. The standardized coefficients of the linear discriminant function are shown in . The melisopalynological variables which scored the greatest differences between eucalyptus and clover honeys were Lotus and Eucalyptus, followed by Melilotus and Medicago sativa. The centroid for eucalyptus honeys was equal to 3.83 and equal to –2.02 for clover honeys. It correctly assigned 100% of the total samples to each group of honeys. According to the LDA, eucalyptus honeys were characterized by a high percentage of pollen from Eucalyptus sp. and low content of pollen from Lotus sp., Melilotus, and Medicago sativa. While clover honeys were characterized by high percentages of pollen from Lotus sp., Melilotus, and Medicago sativa and low percentage of pollen from Eucalyptus sp.
TABLE 3 Standardized coefficients of the Linear Discriminant Analysis Function of palynological, physicochemical and antioxidant variables and sensory variables of clover and eucalyptus honey
Physicochemical characteristics and antioxidant parameters
Before the application of LDA, it was performed a step-wise selection of physicochemical and antioxidant capacity variables. At each step a one-way ANalysis Of VAriance (ANOVA) was performed, and the variable with the highest F-to-enter value was selected. Thus, the LDA technique was performed from the physicochemical and antioxidant variables selected by stepwise discriminant analysis. It was obtained a linear discriminant function which coefficients are showed in . This classification function can be used to predict the probability for the inclusion of a new sample whose group membership is unknown, in one of the two botanical origins studied. The centroid for eucalyptus honeys was equal to 1.23 and for clover honeys equal to –0.66. The correct classification for eucalyptus honey samples was up to 96.4% and for clover honeys group was up to 100%. The estimation of cases correctly classified was 87.6%.
According to the linear discriminant function, eucalyptus honeys have more glucose, and electrical conductivity than clover honeys. The European Union directives for honey[Citation3] include eucalyptus honeys among those which, by exception, may have values higher than the limits for the electrical conductivity of floral honeys (<0.800 mS/cm). In Italy, the U59.00.642 UNI [Citation43] states that the electrical conductivity of eucalyptus honeys should be between 0.400 and 0.700 mS/cm. This agrees with Argentinean,[47] Algerian,[Citation48] and Spanish eucalyptus honey.[Citation49]
Sensory Characteristics
There were significant differences between the average vectors for both floral origins. The standardized coefficients of the linear discriminant function are shown in . The centroid was equal to 1.33 for eucalyptus honey samples and was equal to –0.59 for clover honey samples. The error estimate of misclassification was 28% for eucalyptus honeys and 16% for clover honeys. Crystal size and fluency were larger for the clover honey, in accordance with Persano Oddo and Piro.[Citation45] However, the characteristics of crystallization and fluidity of honey depend on the time for harvesting, moisture, storage temperature, extraction conditions, and packaging, among others. These variables should be controlled prior to the consideration of crystal size or fluency as parameters of characterization for these unifloral honeys.[Citation4] Eucalyptus honeys were characterized by more intense odor and color than clover honeys. Some authors consider that odor intensity is not a descriptor of the types of honey. They reported that the variables that best discriminated floral and honeydew honeys were the sweetness, the bitterness, color, and granularity, while the acidity, the adhesiveness and viscosity showed similar values for all honeys tested.[Citation46]
Canonical Correlations Analysis Between Polinic and Physicochemical Variables
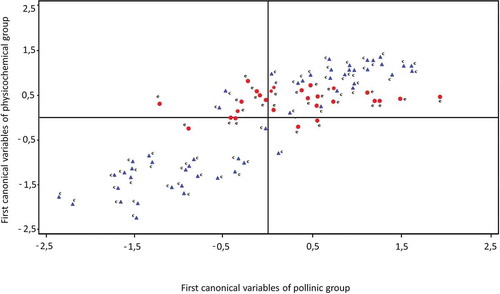
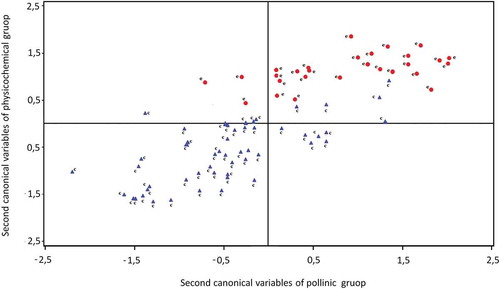
The canonical correlations technique was performed to identify the relationship between polinic and physicochemical variables. The group included pollen variables: Eucalyptus, Trifolium, Melilotus, Lotus, Carduus, Brassicaceae, Medicago sativa, and Helianthus annuus. The physicochemical variables analyzed were moisture, color, ash, acidity, diastase index, reducing sugars, apparent sucrose, fructose, glucose, maltose, sucrose, HMF, specific rotation, electrical conductivity, phenols compounds, flavonoids, FRAC, DPPH, TEAC, and OH. The first canonical correlation value was 0.79 and the second value was 0.65 both statistically significant (p < 0.0001). Standardized coefficients of the two first pair of canonical variables were shown in . The outputs for the no significant canonical correlation were not reported.
TABLE 4 Canonical correlation analysis between physicochemical and palynological variables of clover and eucalyptus honeys
The first pair of canonical variables pointed out that honeys with less percentage of pollen from Trifolium and Medicago sativa had a higher content of maltose and higher electrical conductivity. shows the sample configuration and clearly revealed separate subgroups according to the floral origin of honeys. The second pair of canonical variables suggested that honeys with a minor percentage of pollen from Lotus had a higher content of fructose and higher color intensity. The general shape of the distribution of all sample scores on a scatter diagram whose axes are the second pair of canonical variables is shown in . Several authors found that color, electrical conductivity, specific rotation, diastase, acidity, fructose, and glucose were the parameters that best differentiate honeys from different floral origins,[Citation50–Citation52] pH, and moisture.[Citation9] However, other authors concluded that the chemometric evaluation of the physicochemical properties of honey, did not allow distinguish unifloral from multifloral honeys.[Citation53]
CCA Between Polinic and Sensory Variables
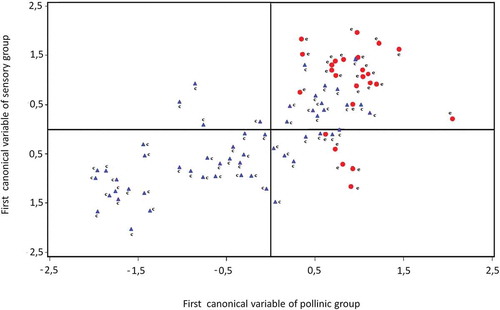
A CCA was applied to determine if there was a relationship between polinic and sensory variables. The polinic variables were Eucalyptus, Trifolium, Melilotus, Lotus, Carduus, Brassicaceae, Medicago sativa, and Helianthus annuus. The sensory variables analyzed were crystal size, sweetness, persistence, graininess, and odor and color intensities. The value of the first canonical correlation was 0.71. It was statistically significant at an alpha level of 0.05 (p < 0.0001), the remaining canonical correlations were not statistically significant. Significative standardized coefficients of the first pair of canonical variables were shown in . Only the results for the statistically significant canonical correlations were reported.
TABLE 5 Canonical correlation analysis between sensory and palynological variables of clover and eucalyptus honeys
According to the first pair of canonical variables, the honeys with a lower percentage of pollen from Lotus had more intense color. In this sense, Italian honeys from Lotus (Lotus corniculatus L.) were described as very clear, with delicate odor and flavor without particular notes,[Citation45] while Spanish Lotus honeys were described as very clear, with faint floral odor and farinaceous notes.[Citation44] shows the sample configuration and clearly revealed separate subgroups according to the botanical origin of honeys.
FIGURE 3 Valorization of clover (c) and eucalyptus (e) honeys on the plane formed by the first set of canonical variables of pollinic and sensory group.
In brief and considering the multivariate analysis from palynological, sensory, and physicochemical parameters analyzed for this set of clover and eucalyptus honeys, the most physicochemical discriminate variable was the electrical conductivity. It was associated with a higher content of pollen from Eucalyptus and a lower content of pollen from Trifolium and Medicago sativa. Among sugars, maltose was associated with a lower presence of pollen from Trifolium and Medicago sativa; fructose was associated to low content of pollen from Lotus, and glucose was associated to the presence of pollen from Eucalyptus. Color and odor intensities were related to the presence of pollen from Eucalyptus, and the absence of pollen from Lotus. Antioxidant capacity indicators could not be associated with particular pollen content, although honeys with highest percentage of pollen from Eucalyptus showed the highest TEAC and FRAC capacity. The experimental data for the variables selected by discriminant analysis have been contrasted with those available in the literature for unifloral honey. In general our data for electrical conductivity and sugars agree well with previous results[Citation22] and Karabourniti et al.[Citation54] It agrees with Marini et al.[Citation55] for eucalyptus honey.
CONCLUSIONS
In an effort to find the best combination of quality parameters to characterize Argentine clover and eucalyptus honey, some physicochemical and sensory variables were found, associated to the presence of pollens from different botanical origins. Classification functions were obtained. The estimation of cases correctly classified by them was high; although, predictability of the procedure should be tasted by cross-validation methods. Sensory, physicochemical and palynological analyses are complementary tools for a successful authentication of honey, in response to consumer demands.
FUNDING
The authors thank Universidad Tecnológica Nacional (UTN 25\M045) for providing financial support for this study.
Additional information
Funding
REFERENCES
- D’oliveira Sant’ana, L.; Baird Buarque Ferreira, A.; Affonso Lorenzon, M.C.; Louro Berbara, R.L.; Castro, R.N. Correlation of Total Phenolic and Flavonoid Contents of Brazilian Honeys with Colour and Antioxidant Capacity. International Journal of Food Properties 2014, 17(1), 65–76.
- Rodríguez Flores, M.S.; Escuredo Pérez, O.; Seijo Coello, M.C. Characterization of Eucalyptus Globulus Honeys produced in the Eurosiberian area of the Iberian Peninsula. International Journal of Food Properties 2014, 17(10), 2177–2191.
- EU-European Commission. Council Directive 2001/110/EC of 20 December 2001 relating to honey. Official Journal of the European Communities OJ L10, 2002; 47–52 pp.
- Piana, M.L.; Persano Oddo, L.; Bentabol, L.; Bruneau, E.; Bogdanov, S.; Guyot Declerk, C. Sensory Analysis Applied to Honey: State of the Art. Apidologie 2004, 35, S26–S37.
- Anupama, D.; Bhat, K.; Sapna, V. Sensory and Physico-Chemical Properties of Commercial Samples of Honey. Food Research International 2003, 36, 183–190.
- Ferreira, E.L.; Lencioni, C.; Benassi, M.T.; Barth, M.O.; Bastos, D.H.M. Descriptive Sensory Analysis and Acceptance of Stingless Bee Honey. Food Science and Technology International 2009, 15(3), 251–258.
- Iordachescu, G. From Bee to Plate. A Sensory Foray in Honey World. Bulletin of the University of Agricultural Sciences and Veterinary Medicine 2009, 62, 264–269.
- La Serna Ramos, I.; Gómez Ferreras, C. Pollen and Sensorial Characterization of Different Honeys from El Hierro (Canary Islands). Grana 2009, 45, 146–159.
- Naab, O.A.; Tamame, M.A.; Caccavari, M.A. Palynological and Physicochemical Characteristics of Three Unifloral Honey Types from Central Argentina. Spanish Journal of Agricultural Research 2009, 6(4), 566–576.
- Etzold, E.; Lichtenberg-Kraag, B. Determination of the Botanical Origin of Honey by Fourier-Transformed Infrared Spectroscopy: An Approach for Routine Analysis. European Food Research and Technology 2008, 227, 579–586.
- Hennessy, S.; Downey, G.; O’Donnell, C.P. Attempted Confirmation of the Provenance of Corsican PDO Honey Using FT-IR Spectroscopy and Multivariate Data Analysis. Journal of Agricultural and Food Chemistry 2010, 58, 9401–9406.
- Kukurová, K.; Karovičová, J.; Kohajdová, Z.; Bíliková, K. Authentication of Honey by Multivariate Analysis of Its Physico-Chemical Parameters. Journal of Food Nutrition Research 2008, 47, 170–180.
- Ruoff, K.; Luginbühl, W.; Bogdanov, S.; Bosset, J.O.; Estermann, B.; Ziolko, T.; Amadò, R. Authentication of the Botanical Origin of Honey by Near-Infrared Spectroscopy. Journal of Agricultural and Food Chemistry 2006, 54, 6867–6872.
- Sanz, S.; Pérez, C.; Herrera, A.; Sanz, M.; Juan, T. Application of Statistical Approach to the Classification of Honey by Geographical Origin. Journal of the Science of Food and Agriculture 2004, 69(2), 135–140.
- FAOSTAT Statistical report. In: http://faostat.fao.org/site/339/default.aspx. ( accessed on March 2014).
- Fagúndez, G.A.; Caccavari, M.A. Pollen Analysis of Honeys from the Central Zone of the Argentine Province of Entre Rios. Grana 2006, 45, 305–320.
- Tellería, M.C. Asteraceae Visited by Honeybees in Argentina: A Record from Entomopalynological Studies. Boletin de la Sociedad Argentina de Botánica 2009, 44, 1–2.
- Valle, A.; Andrada, A.; Aramayo, E.; Gil, M.; Lamberto, S. A Melissopalynological Map of the South and Southwest of the Buenos Aires Province, Argentina. Spanish Journal of Agricultural Research 2007, 5(2), 172–180.
- Chirife, J.; Zamora, M.C.; Motto, A. The Correlation Between Water Activity and Percentage Moisture in Honey: Fundamental Aspects and Application to Argentine Honeys. Journal of Food Engineering 2006, 72, 287–292.
- Conforti, P.; Lupano, C.; Malacalza, N.; Arias, V.; Castells, C. Crystallization of Honey at –20°C. International Journal of Food Properties 2009, 9(1), 99–107.
- Sánchez, V.; Baeza, R.; Ciappini, M.C.; Zamora, M.C.; Chirife, J. Comparison Between Karl Fischer and Refractometric Method for Determination of Water Content in Honey. Food Control 2010, 21(3), 339–341.
- Corbella, E.; Cozzolino, D. Classification of the Floral Origin of Uruguayan Honeys by Chemical and Physical Characteristics Combined with Chemometrics. LWT–Food Science and Technology 2006, 39, 534–539.
- Malacalza, N.; Caccavari, M.A.; Fagúndez, G.A.; Lupano, C.E. Unifloral Honeys of the Province of Buenos Aires, Argentine. Journal of the Science of Food and Agriculture 2005, 85, 1389–1396.
- Ciappini, M.C.; Gatti, M.B.; Di Vito, M.V.; Gatusso, S.; Gatusso, M. Characterization of Different Floral Origins Honey Samples from Santa Fe (Argentine) by Palynological, Physicochemical and Sensory Data. Apiacta 2008, 43, 25–36.
- Codex Stand. Codex Alimentarius Comission. Revised Codex Standard for Honey. Codex STAN 12 (1981) Rev. 1 (1987). Rev. 2 (2001).
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of Melissopalynology. Bee World 1978, 59, 139–157.
- Markgraf, V.; D’Antoni, H.; Pollen Flora of Argentina. University of Arizona Press: Tucson, AZ, 1978; 208 pp.
- Erdtman, G. Pollen Morphology and Plant Taxonomy. Angiosperms. Hafner Pub.: New York, NY, 1966; 553 pp.
- Feller-Demalsy, M.; Parent, J.; Strachan, A. Microscopic Analysis of Honeys from Alberta. Canada. Journal of Apicultural Research 1987, 26, 123–132.
- Von Der Ohe, W.; Persano Oddo, L.; Piana, L.; Morlot, M.; Martin, P. Harmonized Methods of Melissopalynology. Apidologie 2004, 35, S18–S25.
- Association of Official Analytical Chemists. Official methods of analysis of AOAC International. Association of Official Analytical Chemists. Washington; 1995.
- Woisky, R.; Salatino, A. Analysis of Propolis: Some Parameters and Procedures for Chemical Quality Control. Journal of Apicultural Research 1998, 37, 99–105.
- Halliwell, B.; Gutteridge, J.; Aruoma, O. The Desoxyrribosa Method: A Simple Test Tube Assay for Determination of Rate Constants for Reactions of Hydroxyl Radicals. Analytical Biochemistry 1987, 165, 215–219.
- Singleton, V.L.; Orthofer, R.; Lamuela Raventos, R.M. Analysis of Total Phenol and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteau Reagent. Methods in Enzymology 1999, 299, 152–178.
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of Free Radical Method to Evaluate Antioxidant Activity. LWT–Food Science and Technology 1995, 28, 25–30.
- Arnao, M.B.; Cano, A.; Acosta, M. The Hydrophilic and Lipophilic Contribution to Total Antioxidant Activity. Food Chemistry 2001, 73, 239–244.
- Oyaizu, M. Studies on the Products of Browning Reaction Prepared from Glucosamine. Japanese Journal of Nutrition 1986, 44, 307–315.
- Ciappini, M.C.; Di Vito, M.V.; Gatti, M.B.; Calviño, A.M. Development of a Quantitative Descriptive Sensory Honey Analysis: Application to Eucalyptus and Clover Honeys. Advance Journal of Food Science and Technology 2013, 5(7), 829–838.
- Morrison, D. Multivariate Statistical Methods, 4th Ed; McGraw Hill: New York, NY, 2005; 252 p.
- Baltrusaityt, V.; Venskutonia, P.R.; Ceksteryt, V. Radical Scavenging Activity of Different Floral Origin Honey and Beebread Phenolic Extracts. Food Chemistry 2007, 101, 502–514.
- Blasa, M.; Candiaracci, M.; Accorsi, A.; Piacentini, M.; Piatti, E. Honey Flavonoids As Protection Agents Against Oxidative Damage to Human Red Blood Cells. Food Chemistry 2007, 104, 1635–1640.
- Vit, P.; Gutiérrez, M.G.; Titera, D.; Vendar, M.; Rodríguez Malaver, A.J. Mieles Checas Categorizadas Según su Actividad Antioxidante (Czech Honeys Categorized by their Antioxidant Activity). Acta Bioquímica Clínica Latinoamericana 2008, 42(2), 237–244.
- UNI U59.00.642. Ente Nazionale Italiano di Unificazione. Milano, Italy. 2010.
- Gómez Pajuelo, A. Mieles de España y Portugal, Conocimiento y Cata. Castellón: Madrid, España, 1998; 88 p.
- Persano Oddo, L.; Piro, R. European Unifloral Honeys: Descriptive Sheets. Technical Report from the International Honey Commission. Basilea, 2004.
- González, M.; De Lorenzo, C.; Pérez, R.A. Development of a Structured Sensory Honey Analysis: Application to Artisanal Madrid Honeys. Food Science and Technology International 2004, 16(1), 19–29.
- Tamaño, G.; Locaso, D.; Bacigalupo, R.; Todote, P.; Vales, J.; Gómez, M. In Características Fisicoquímicas de Miel de Eucalipto (Eucalyptus sp.). Proceedings of the III Congreso Internacional de Ciencia y Tecnología de los Alimentos, Córdoba, Argentina, 2009, ept 52. 47(44).
- Makhloufi, C.; Schweitzer, P.; Azouzi, B.; Persano Oddo, L.; Choukri, A.; Hocine, L. Some Properties of Algerian Honey. Apiacta 2007, 42, 73–80.
- Serra, B.J. A Study of Physicochemical Properties of Eucalyptus Honey in Spain. Anales de Bromatología 1989, 41, 41.
- Paramas, A.M.; Gómez Barez, J.A.; Villanova, R.J.; Pala, T.R.; Albajar, R.A.; Sánchez, J.S. Geographical Discrimination of Honey by Using Mineral Composition and Common Chemical Quality Parameters. Journal of the Science of Food and Agriculture 2000, 80(1), 157–165.
- Piro, R.; Guidetti, G.; Persano Oddo, L.; Piazza, M.C. Mathematical Diagnosis of Unifloral Honeys. In Il Ruolo Della Ricerca in Apicoltura; Sabatini, A.G.; Bolchi Serini, G.; Frilli, R.; Porrini, C.; Eds.; Litosey, Bologne, Italy, 2002; 235–239.
- Devillers, J.; Morlot, M.; Pham-Delegue, M.; Dores, J. Classification of Monofloral Honeys Based on Their Quality Control Data. Food Chemistry 2004, 86(2), 305–312.
- Bogdanov, S.; Gallman, P. Authenticity of Honey and Other Bee Products: State of the Art. ALP Science 2008, 520, 2–12.
- Karabourniti, S.; Thrasyvoulou, A.; Eleftheriou, E.P. A Model of Predicting Geographic Origin of Honey from the Same Floral Source. Journal of Apicultural Research 2006, 45(3), 117–124.
- Marini, F.; Magri, A.L.; Balestrieri, F.; Fabretti, F; Marini, D. Supervised Pattern Recognition Applied to the Discrimination of the Floral Origin of Six Types of Italian Honey Samples. Analytica Chimica Acta 2004, 515(1), 117–125.