Abstract
In this research, the effect of different bicarbonate salts (sodium and ammonium) and their doses (0, 1, 2, and 3 g/100 g raw material) in the coating batter formula use and the sequential use of frying oil (1st, 2nd, 3rd, and 4th) on 5-hydroxymethylfurfural and acrylamide contents in coated fried chicken meat. The addition of sodium bicarbonate was efficient for reducing acrylamide content, but it increased browning and 5-hydroxymethylfurfural content compared to the control. When increasing the doses of sodium and ammonium bicarbonate from 1 to 3 g/100 g of raw material, the acrylamide content of samples did not change significantly, although adding sodium bicarbonate significantly reduced the acrylamide content as a control. These research results showed that using about 1 g/100 g raw material sodium bicarbonate rather than ammonium bicarbonate and as little frying oil as possible use during the production of coated and fried meat results in lower contents of 5-hydroxymethylfurfural and acrylamide.
INTRODUCTION
The coated meat production process is basically formed of battering, breading, and then frying of muscle strips. Different cuts of poultry, fish, pork, and beef meat could be used in production. The desired properties of the end product are dependent on product shape, coating formula, cooking methods (par-fry and full-fry in oil or hot air), frying oil and time and temperature of cooking.[Citation1–Citation4] Deep frying is a quick cooking method in which food material is immersed in edible hot oil, such as sunflower, soybean, corn, canola oil, and so on. Hot oil increases the temperature of food material in a short time as it acts as an excellent heat-transfer medium. Following the quick temperature rise in food, the temperature of the internal region remains below the boiling point of water, while the temperature of the surface continues to increase.[Citation2,Citation5,Citation6]
Both fried food and hot oil have an effect on each other and together promote the occurrence of complex chemical reactions, such as hydrolysis, oxidation, polymerization,[Citation7] caramelization, and Maillard reaction. The brown color of the breaded product arises from the Maillard reaction, rather than caramelization.[Citation8] The Maillard reaction is a non-browning reaction, which occurs mainly from a series of reactions between carbonyl compounds such as reducing sugar and amino compounds during the heat treatment.[Citation9]
These reaction series are caused by the formation of some toxic compounds that are not naturally present in foods, but that may develop during cooking, along with process contaminants like 5-hydroxymethylfurfural (HMF) and acrylamide, which is reported to be potentially carcinogenic to humans.[Citation10–Citation12] Besides, some Maillard reaction products like acrolein are precursors of acrylamide. An oxidation product of acrolein produced a high level of acrylamide in lipid-rich foods.[Citation9,Citation10,Citation13] HMF, as a furanic compound, occurs as an intermediate in the Maillard Reaction and caramelization occurs during the exposure of food materials to the thermal process; this is defined as an important contributor to the characteristics of fried foods.[Citation14,Citation15]
Generally, various ingredients are used in the batter and breading system to provide the desired stable color in coated meat products.[Citation1] According to European and North American Regulations, bicarbonate salts, such as sodium and ammonium bicarbonate, which are easily accessible, and safe food additives can be used at “quantum satis” levels in food products.[Citation16] Polyvalent cations such as Ca2+, Mg2+, and Fe3+ added to food formulation prevent the formation of acrylamide more than monovalent cations such as Na+ and K+. The added cations inhibit the formation of Schiff bases, which is the intermediate of Maillard reaction. Despite this positive feature of cations, it is known that they cause the formation of HMF as a known carcinogen during the cooking of food.[Citation17–Citation19] Sodium and ammonium bicarbonates might inhibit the formation of Schiff bases providing monovalent cations; their effects on toxic compounds such as acrylamide and HMF are explained in this article. The aim of the present research was to investigate the effect of different bicarbonate salts and doses (sodium and ammonium bicarbonates) in used coating batter formula and the multiple use of frying oil on acrylamide and HMF formations in coated fried chicken breast because these toxic compounds become severely in starch-based products like coated materials.
MATERIALS AND METHODS
Materials
Chicken breast pieces, wheat flour, breadstick flour, salt, and sunflower oil were purchased from well-known local markets in Antalya, Turkey. Sodium bicarbonate and ammonium bicarbonate were obtained from a well-known company (Zag, Istanbul, Turkey). Potassium bromide, hydrobromic acid, triethylamine, bromine, sodium thiosulphate, potassium hexacyanoferrate, and zinc sulphate were purchased from Merck (Darmstadt, Germany). Ethyl acetate, hexane, and isotopic purity (13C3) acrylamide were from Sigma Aldrich (St. Louis, USA). Ultrapure water used in the chromatographic analysis was produced by the Millipore/Milli-Q device (Bedford, MA, USA). Chemicals were obtained in analytical and chromatographic analysis which were of analytical and chromatographic grade, respectively.
Sample Preparation
Boneless and skinless chicken breast samples were purchased with a uniform range of 40 g in weight and dimensions of 5 × 5 × 2 cm on average. For double-layer coating, two different coating materials were prepared. The first coating material as a batter was prepared with different doses of bicarbonate salts, wheat flour, salt (1 g/100 g raw material), and water (200 g/100 g raw material) based flour. Flour was replaced with 3, 2, 1, and 0 g/100 g raw material bicarbonate salts for different doses. The coating formulation for the control group (0 g/100 g raw material) did not include any bicarbonate salts. The second coating material as dry dust was prepared with wheat flour (49 g/100 g raw material), breadstick flour (49 g/100 g raw material), and salt (2 g/100 g raw material). For coating, samples were immersed in the first coating material and allowed to stand for 1 min to drip. Then, immersed samples were coated with the second coating material.
The coated samples were fried with the traditional method of a deep-fat fryer. The frying process was performed for a period of 4 min at 180°C in a commercial bench-top deep fryer (Felix FL-269, Istanbul, Turkey) containing sunflower oil. Frying was performed with two pieces of sample in 400 mL frying oil. After 4 min, the internal temperature of samples monitored with a probe thermometer was about 70°C. Four coated samples with the same coating formulation were fried consecutively in the same oil to determine the effect of multiple use of frying oil. The sunflower oil was changed after the four samples were fried.
Physio-Chemical Analysis
The pH value of the first coating batter was measured with a digital pH meter (Hanna HI 2210, Woonsocket, RI, USA) after 10 g batter samples were homogenized in 20 mL pure water.[Citation20] The dry matter of coated and fried chicken meat samples was determined by drying (Memmert UNB 500, Schwabach, Germany) the samples at 105°C to a constant weight.[Citation21] Cooking loss was calculated according to the method of Barmanti and Pasquini.[Citation22] Samples were weighed before and after frying, and cooking loss was calculated by dividing the difference of the first weight as a fraction of the percent. The color of fried samples was measured at 4 points on the sample surface and averaged. Color parameters (L*, a*, b*) were measured by the CIELAB system using a CR-400 Chromameter (Konica Minolta, Japan). Parameter L* represents the light–dark spectrum with a range of 0 (black) to 100 (white). Parameter a* represents the red–green color with positive a* values indicating redness and negative a* values indicating greenness; parameter b* represents yellow–blue color with positive b* values indicating yellowness and negative b* values indicating blueness. The chromameter was calibrated using a white ceramic calibration tile (L* = 98.35, a* = –0.25, b* = 1.83).[Citation23]
HMF Analysis
The HMF content of the samples was determined according to Winkler’s spectrophotometric method.[Citation24] The dried and milled samples were weighed (2.5 g) in a tube (PPCO, Nalgene, USA), and homogenized in 10 mL hexane for 1 min at 1500 rpm (WiseStir HS-30D, Daihan Scientific Co, Seoul, Korea). They were then incubated at 40°C for 30 min, centrifuged in a 3K30 centrifuge (Sigma, Germany) at 4500 × g for 5 min, and the supernatant was removed to separate the lipids. Defatted samples were dried in air and homogenized (Ultraturrax T-25, IKA Labortechnik, Germany) in 10 mL water for 1 min, before being transferred to a 25 mL volumetric flask. The content of the flask was mixed thoroughly with 0.5 mL Carrez I and rested for 2 min, and then 0.5 mL Carrez II was mixed using the same procedure in order to remove co-extractives. The flask was made up to the complete volume with distilled water and then the solution was filtered using Whatman 42 paper (Whatman, Maidstone, UK). Two mL of the filtrate and 5 mL of p-toluidine in two different test tubes were prepared. One milliliter of distilled water was added into one of them for use as a reference solution and 1 mL of barbituric acid solution (0.5%) was added into the other test tube as a sample solution. The maximum absorbance of both tubes was measured at 550 nm using a spectrophotometer.
Acrylamide Analysis
The dried and milled samples were weighed (10 g) in a tube (PPCO, Nalgene, USA), and homogenized in 20 mL hexane for 1 min at 1500 rpm (WiseStir HS-30D, Daihan Scientific Co, Seul, Korea). The homogenate was taken in an ultrasonic bath for 5 min, and then centrifuged in a 3K30 centrifuge (Sigma, Germany) at 4500 × g for 5 min. The supernatant was removed to separate the lipids, and the defatted sample was dried on air.
For acrylamide extraction, 1 g sample, 300 μL Carrez I solution, and 300 μL Carrez II solution were homogenized (Ultraturrax T-25, IKA Labortechnik, Germany) at 20,000 rpm for 1 min with 10 mL pure water in a tube for removing co-extractives. The homogenate was kept in a bath at 60°C for 20 min, and then centrifuged (Centrifuge, 3-18K, Sigma, Germany) at 8000 rpm for 30 min. The supernatant was filtered through a 0.45 mm micro-filter (Sigma, Stenheim, Germany). The filtrate was treated with 300 μL brominating solution (15.2 g KBr, 0.8 mL HBr, 5 mL 1.6% bromine water and 60 mL water) in a tube and kept in an ice box for 1 h in the dark. After 1 h, to terminate the reaction, 1 M sodium thiosulphate solution was added to the sample until the yellow color of the samples disappeared. Then, 1 mL of ethyl acetate was added to the sample solution, mixed with a vortex and after resting, the upper phase containing acrylamide was collected. Then, the same procedure was repeated twice with ethyl acetate. After 1 mL of collected sample and 100 μL triethylamine was mixed in a vial for derivatization, it was injected for gas chromatography/mass spectrometry (GC/MS) analysis.
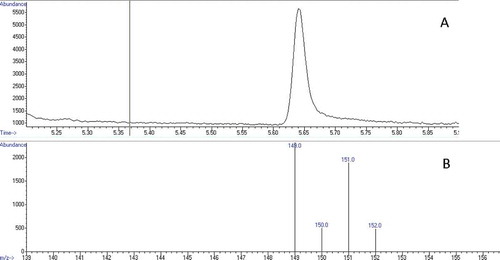
The samples were injected into a gas chromatography system (Agilent 7890A GC, Agilent G4513A automatic sampler, Agilent 5975C MSD, Wilmington, DE, USA) equipped with a column (Agilent HP-1, 100% Dimethylpolysiloxane, 30 m × 0.32 mm × 1.05 µm, Wilmington, DE, USA). The oven temperature was held at 50°C for 1 min, increased to 180°C at a rate of 20°C/min, and then increased to 230°C at a rate of 10°C/min and held for 20 min, before finally being increased to 260°C at a rate of 10°C/min and held for 10 min. The temperatures of injector block and flame ionization detector (FID) were 240 and 250°C, respectively. The carrier gas (helium) was at a constant flow of 1 mL/min.[Citation7,Citation25,Citation26] For the identification of acrylamide, analyses were conducted in electron ionization (EI) mode by selected ion monitoring of the major ion at m/z 149, 150, 151, and 152 for brominated acrylamide. The recovery value was determined to be 89% by quantification of acrylamide in samples before and after the addition of (13C3) acrylamide.
Statistical Analysis
Experiments were designed as factorial. The factors were the type of bicarbonate salts (control and sodium and ammonium bicarbonates), the dose of bicarbonate salts (0, 1, 2, and 3 g/100 g raw material, kg flour) and the number of frying oil use (1st, 2nd, 3rd, and 4th). Production was performed in two replicates and analyses were duplicated. The data were analysed by analysis of variance (ANOVA) using the SAS statistical software package (v.7.00, SAS Institute Inc., Cary, NC, USA) to compare the means with respect to factors. Duncan’s multiple range test was used to determine significant differences at the 5% level. Results are given as the mean ± standard error.
RESULTS AND DISCUSSION
The pH of first-coating materials, total dry matter content and cooking loss of coated and fried chicken samples are given in . It was determined that adding different bicarbonate salts and their increasing doses significantly (p < 0.01) increased the pH values of first-coating material as the control-coating material contained no bicarbonate salts. Gokmen et al.[Citation27] reported that adding leavening agent such as ammonium and sodium bicarbonate provided the alkaline pH of cookie dough. Adding ammonium or sodium bicarbonate changed the pH of food systems to alkaline.[Citation28] Bicarbonate salt type, dose, and the number of frying oil use significantly (p > 0.05) affected total dry matter content and cooking loss of coated and fried samples. Dry matter and cooking loss of samples were determined to be 42.3 and 20.8%, respectively. The cooking loss was seen in the fried samples because water was removed from the coating material and raw breast meat rather than oil being absorbed by weight during frying; for this reason, the dry matter of sample increased. It is reported that the water removal is simultaneous with oil uptake during the frying process.[Citation29]
TABLE 1 pH, total dry matter content, and cooking loss findings on coated and fried chicken meats
Color values of coated meat samples are given in . Except for the L* value, statistically significant differences (p < 0.01) were found in a* and b* parameters from both bicarbonate salt types and doses as controls. It was determined that a* and b* values of samples containing ammonium bicarbonate were higher than in those containing sodium bicarbonate. This indicates that adding ammonium bicarbonate increases non-enzymatic browning reactions like the Maillard reaction because it provides additional nitrogen. It has been reported that a high correlation exists between HMF content and browning development; HMF formation follows the same pathways, leading to brown compounds.[Citation30,Citation31]
TABLE 2 Color L*, a*, and b* values of coated and fried chicken meats
Adding bicarbonate salts improved browning as a control, but increasing their doses did not affect the color values of samples. Also, the number of uses of frying oil significantly (p < 0.05) improved browning as the first use of frying oil but sequential use of frying oil did not have an effect (p > 0.05). It was also seen during the literature research that different parameters have no effect on b* values of coated meat products.[Citation5,Citation32] The deep-frying oils play a significant role in the a* value of the fried product due to the frying fats being used once or more. This could be related to complex chemical reactions that take place in both coated and fried materials and frying oil.[Citation7]
The HMF content of coated and fried chicken samples is given in . It was determined that bicarbonate salts, their doses and the number of frying oil uses significantly (p < 0.01) increased the HMF content of coated and fried chicken breast meat. The HMF contents of control and samples containing sodium bicarbonate and ammonium bicarbonate were determined as 11.11, 17.29, and 23.66 mg/kg, respectively. The higher HMF content in samples containing ammonium bicarbonate compared to others might be from the ammonium enhanced Maillard reaction. The increasing HMF contents depend on increasing pH values of first-coating material because of adding bicarbonate salts and their increasing doses. During the four times use of frying oil, the HMF contents of samples containing sodium bicarbonate and ammonium bicarbonate were raised from 7.57 to 32.67 and 11.84 to 39.64 mg/kg, respectively.
TABLE 3 HMF findings on coated and fried chicken meats
The amount of HMF in foods is related to factors such as temperature, pH, and water activity during the processing of carbohydrate-rich products.[Citation10] It is reported that when of bicarbonate salts are added to batter, they have an impact on the final pH value and are effective in the formation of chemical reactions during the cooking process.[Citation33] Gokmen et al.[Citation27] reported that the HMF content (3493.1 mg/kg) in cookies containing ammonium bicarbonate was higher than in cookies (239.5 mg/kg) containing sodium bicarbonate. In the other researches, the HMF value was found to be 1.78 mg/kg in breaded fish products deep-fried in sunflower,[Citation15] 6.9–240.5 mg/kg in breakfast cereals,[Citation34] 0–57.18 mg/kg in baby food,[Citation35,Citation36] 2.20–87.70 mg/kg in bread,[Citation37] and 0.5–74.6 mg/kg in biscuits.[Citation38]
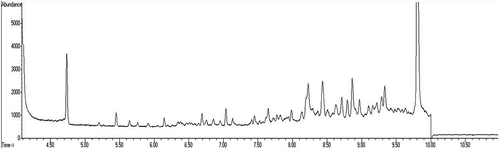
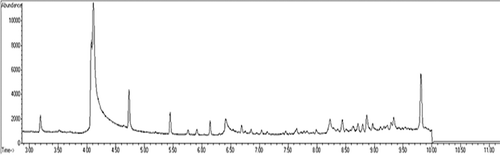
GC–MS chromatogram and mass spectra of acrylamide (13C3) are given in . The sample chromatograms of samples containing sodium bicarbonate and ammonium bicarbonate are given in and , respectively. The acrylamide content of samples is given in . While adding increased doses of sodium bicarbonate to batter significantly reduced (p < 0.01) the content of acrylamide in samples as a control, adding ammonium carbonate did not affect (p > 0.05) the acrylamide content of samples. While the acrylamide content of samples with added sodium bicarbonate reduced from 63.83 to 33.05 µg/kg, the other acrylamide content was maintained at approximately 61.58 µg/kg.
TABLE 4 Acrylamide content findings on coated and fried chicken meats
The acrylamide content in samples with different bicarbonate salts added significantly increased (p < 0.01) depending on the increasing frequency of frying oil use. The acrylamide content in samples with added sodium and ammonium bicarbonate for frying four times increased to 29 and 44%, respectively. This result indicates that ammonium bicarbonate enhances acrylamide formation. In some similar research, it has been determined that ammonium salts increase acrylamide content in different ways, like acting as an additional source of nitrogen and by indirectly catalyzing the decomposition of sugars to produce new reactive carbonyls.[Citation26,Citation39,Citation40] In the literature, with regard to cereal products, it was determined that ammonium bicarbonate used in the formulation enhanced the formation of acrylamide, but the replacement of ammonium salt with sodium or potassium salt reduced the formation of acrylamide.[Citation33,Citation41,Citation42] It was reported that acrylamide formation reduced by about 70% when sodium bicarbonate (NaHCO3) instead of ammonium bicarbonate was used in bakery products like cookies, biscuits, and crackers.[Citation37,Citation43]
Similar to the results in this research, findings in the literature show that acrylamide content in some meat products was between 87.90–110.95 µg/kg in battered and breaded fried chicken that was produced using conventional deep frying method,[Citation5] 34 µg/kg in chicken schnitzel,[Citation44] 159 µg/kg in chicken nuggets trademark-A,[Citation45] < 50 µg/kg in chicken nuggets trademark-B,[Citation45] and 68 µg/kg in meat patty.[Citation44]
CONCLUSION
HMF and acrylamide are known toxic compounds which occur in starchy food during frying and baking. Hence, any reduction of their content in food, like coated meat products, is very important for health. HMF contents increased by 65% with the addition of sodium bicarbonate and 113% with the addition of ammonium bicarbonate to batter materials; in contrast, acrylamide content reduced by approximately 38% with the addition of sodium bicarbonate but it did not change with the addition of ammonium bicarbonate. Furthermore, increasing doses of bicarbonate salts affected acrylamide content in the same way. When the number of frying oil uses in the production of coated fried meat was increased from 1st to 4th this significantly enhanced the HMF and acrylamide content of samples. Moreover, the type of bicarbonate salts in the first coating material had an influence on the color of coated and fried meat samples. Due to the provision of additional nitrogen, the highest browning development as a* and b* color values was determined in samples containing ammonium bicarbonate, with HMF and acrylamide contents that were higher than in other samples. As a result, using about 1 g/100 g raw material sodium bicarbonate rather than ammonium bicarbonate and as low as reasonably achievable (ALARA principle) the number of frying oil use during the production of coated and fried meat results in lower contents of HMF and acrylamide.
FUNDING
This research was supported by Akdeniz University Research Fund.
Additional information
Funding
REFERENCES
- Barbut, S. Frying—Effect of Coating on Crust Microstructure, Color, and Texture of Lean Meat Portions. Meat Science 2013, 93(2), 269–274.
- Chen, S.D.; Chen, H.H.; Chao, Y.C.; Lin, R.S. Effect of Batter Formula on Qualities of Deep-Fat and Microwave Fried Fish Nuggets. Journal of Food Engineering 2009, 95(2), 359–364.
- Adedeji, A.A.; Ngadi, M.O. Microstructural Properties of Deep-Fat Fried Chicken Nuggets Coated with Different Batter Formulation. International Journal of Food Properties 2011, 14(1), 68–83.
- Maskat, M.Y.; Kerr,W.L. Effect of Surfactant and Batter Mix Ratio on the Properties of Coated Poultry Product. International Journal of Food Properties 2004, 7(2), 341–352.
- Barutcu, I.; Sahin, S.; Sumnu, G. Acrylamide Formation in Different Batter Formulations During Microwave Frying. LWT–Food Science and Technology 2009, 42(1), 17–22.
- Albert, Á.; Salvador, A.; Hough, G.; Fiszman, S. Influence of Outer Layer Formulation on the Sensory Properties of Microwaved Breaded Nuggets. International Journal of Food Properties 2014, 17(4), 829–841.
- Zhang, Q.; Saleh, A.S.M.; Chen, J.; Shen, Q. Chemical Alterations Taken Place During Deep-Fat Frying Based on Certain Reaction Products: A Review. Chemistry and Physics of Lipids 2012, 165(6), 662–681.
- Erbas, M.; Sekerci, H.; Arslan, S.; Durak, A.N. Effect of Sodium Metabisulfite Addition and Baking Temperature on Maillard Reaction in Bread. Journal of Food Quality 2012, 35(2), 144–151.
- Yasuhara, A.; Tanaka, Y.; Hengel, M.; Shibamoto, T. Gas Chromatographic Investigation of Acrylamide Formation in Browning Model Systems. Journal of Agricultural and Food Chemistry 2003, 51(14), 3999–4003.
- Capuano, E.; Fogliano, V. Acrylamide and 5-Hydroxymethylfurfural (HMF): A Review on Metabolism, Toxicity, Occurrence in Food and Mitigation Strategies. LWT–Food Science and Technology 2011, 44(4), 793–810.
- Gerrard, J.A. The Maillard Reaction in Food: Progress Made, Challenges Ahead-Conference Report from the Eighth International Symposium on the Maillard Reaction. Trends in Food Science & Technology 2006, 17(6), 324–330.
- IARC. Monographs on the Evaluation of Carcinogen Risk to Humans: Some Industrial Chemicals. International Agency for Research on Cancer 1994, 60, 389–433.
- Jin, C.; Wu, X.Q.; Zhang, Y. Relationship Between Antioxidants and Acrylamide Formation: A Review. Food Research International 2013, 51(2), 611–620.
- Anese, M.; Suman, M. Mitigation Strategies of Furan and 5-Hydroxymethylfurfural In Food. Food Research International 2013, 51(1), 257–264.
- Perez-Palacios, T.; Petisca, C.; Henriques, R.; Ferreira, I. Impact of Cooking and Handling Conditions on Furanic Compounds in Breaded Fish Products. Food and Chemical Toxicology 2013, 55, 222–228.
- Samapundo, S.; Devlieghere, F.; De Meulenaer, B.; Lamboni,Y.; Osei-Nimoh, D.; Debevere, J.M. Interaction of Water Activity and Bicarbonate Salts in the Inhibition of Growth and Mycotoxin Production by Fusarium and Aspergillus Species of Importance to Corn. International Journal of Food Microbiology 2007, 116(2), 266–274.
- Gokmen, V.; Senyuva, H.Z. Acrylamide Formation Is Prevented by Divalent Cations During the Maillard Reaction. Food Chemistry 2007, 103(1), 196–203.
- Keramat, J.; LeBail, A.; Prost, C.; Jafari, M. Acrylamide in Baking Products: A Review Article. Food and Bioprocess Technology 2011, 4(4), 530–543.
- Mustafa, A.; Andersson, R.; Kamal-Eldin, A.; Aman, P. Fortification with Free Amino Acids Affects Acrylamide Content in Yeast Leavened Bread. In Flour and Breads and their Fortification in Health and Disease Prevention; Preedy, V.; Watson, R.; Patel, V.; Eds.; Elsevier Inc.: Philadelphia, PA, 2013; 325–333 pp.
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, 1984.
- AOAC. Official Methods of Analysis, 16th Ed; Association of Official Analytical Chemists: Washington, DC, 1995.
- Barbanti, D.; Pasquini, M. Influence of Cooking Conditions on Cooking Loss and Tenderness of Raw and Marinated Chicken Breast Meat. LWT–Food Science and Technology 2005, 38, 895–901.
- Candogan, K.; Kolsarici, N. Storage Stability of Low-Fat Beef Frankfurters Formulated with Carrageenan Or Carrageenan with Pectin. Meat Science 2003, 64(2), 207–214.
- Zappala, A.; Fallico, B.; Arena, E.; Verzera, A. Methods for the Determination of HMF in Honey: A Comparison. Food Control 2005, 16(3), 273–277.
- Cengiz, M.F.; Gunduz, C.P.B. Acrylamide Exposure Among Turkish Toddlers from Selected Cereal-Based Baby Food Samples. Food and Chemical Toxicology 2013, 60, 514–519.
- Bent, G.A.; Maragh, P.; Dasgupta, T. Acrylamide in Caribbean Foods—Residual Levels and Their Relation to Reducing Sugar and Asparagine Content. Food Chemistry 2012, 133(2), 451–457.
- Gokmen, V.; Acar, O.C.; Serpen, A.; Morales, F.J. Effect of Leavening Agents and Sugars on the Formation of Hydroxymethylfurfural in Cookies During Baking. European Food Research and Technology 2008, 226(5), 1031–1037.
- Eskin, N.A.M.; Ho, C.T.; Fereidoon, S. Browning Reaction in Foods. In Biochemistry of Foods, 3rd Ed; Eskin, N.A.M.; Fereidoon, S.; Eds.; Academic Press: San Diego, CA, USA, 2013; 245–228.
- Mellema, M. Mechanism and Reduction of Fat Uptake in Deep-Fat Fried Foods. Trends in Food Science & Technology 2003, 14(9), 364–373.
- Capuano, E.; Ferrigno, A.; Acampa, I.; Ait-Ameur, L.; Fogliano, V. Characterization of the Maillard Reaction in Bread Crisps. European Food Research and Technology 2008, 228(2), 311–319.
- Capuano, E.; Ferrigno, A.; Acampa, I.; Serpen, A.; Acar, O.C.; Gokmen, V.; Fogliano, V. Effect of Flour Type on Maillard Reaction and Acrylamide Formation During Toasting of Bread Crisp Model Systems and Mitigation Strategies. Food Research International 2009, 42(9), 1295–1302.
- Dogan, S.F.; Sahin, S.; Sumnu, G. Effects of Soy and Rice Flour Addition on Batter Rheology and Quality of Deep-Fat Fried Chicken Nuggets. Journal of Food Engineering 2005, 71(1), 127–132.
- Graf, M.; Amrein, T.M.; Graf, S.; Szalay, R.; Escher, F.; Amado, R. Reducing the Acrylamide Content of a Semi-Finished Biscuit on Industrial Scale. LWT–Food Science and Technology 2006, 39(7), 724–728.
- Rufian-Henares, J.A.; Delgado-Andrade, C.; Morales, F.J. Application of a Fast High-Performance Liquid Chromatography Method for Simultaneous Determination of Furanic Compounds and Glucosylisomaltol in Breakfast Cereals. Journal of AOAC International 2006, 89(1), 161–165.
- Gokmen, V.; Senyuva, H.Z. A Simplified Approach for the Kinetic Characterization of Acrylamide Formation in Fructose-Asparagine Model System. Food Additives and Contaminants 2006, 23(4), 348–354.
- Gokmen, V.; Senyuva, H.Z. Improved Method for the Determination of Hydroxymethylfurfural in Baby Foods Using Liquid Chromatography-Mass Spectrometry. Journal of Agricultural and Food Chemistry 2006, 54(8), 2845–2849.
- Özçandır Kıvanç, S. The Effect of Bugs (Eurygaster spp. and Aelia spp.) Damaged Flours on Formation of Acrylamide and Hydroxmethylfurfural (HMF) in Cookies, Cakes and Breads; Hacettepe University Press: Ankara, Turkey, 2013; 94 p.
- Ameur, L.A.; Trystram, G.; Birlouez-Aragon, I. Accumulation of 5-hydroxymethyl-2-Furfural in Cookies During the Backing Process: Validation of An Extraction Method. Food Chemistry 2006, 98(4), 790–796.
- Biedermann-Brem, S.; Noti, A.; Grob, K.; Imhof, D.; Bazzocco, D.; Pfefferle, A. How Much Reducing Sugar May Potatoes Contain to Avoid Excessive Acrylamide Formation During Roasting and Baking? European Food Research and Technology 2003, 217(5), 369–373.
- Grob, K. Reduction of Exposure To Acrylamide: Achievements, Potential of Optimization, and Problems Encountered from the Perspectives of a Swiss Enforcement Laboratory. Journal of AOAC International 2005, 88(1), 253–261.
- Friedman, M.; Levin, C.E. Review of Methods for the Reduction of Dietary Content and Toxicity of Acrylamide. Journal of Agricultural and Food Chemistry 2008, 56(15), 6113–6140.
- Grob, K. Options for Legal Measures to Reduce Acrylamide Contents in the Most Relevant Foods. Food Additives and Contaminants 2007, 24, 71–81.
- Gündüz, C.P.B.; Cengiz, M.F. Acrylamide Contents of Commonly Consumed Bread Types in Turkey. International Journal of Food Properties 2015, 18(4), 833–841.
- Olmez, H.; Tuncay, F.; Ozcan, N.; Demirel, S. A Survey of Acrylamide Levels in Foods from the Turkish Market. Journal of Food Composition and Analysis 2008, 21(7), 564–568.
- Tateo, F.; Bononi, M.; Andreoli, G. Acrylamide Levels in Cooked Rice, Tomato Sauces, and Some Fast Food on the Italian Market. Journal of Food Composition and Analysis 2007, 20(3–4), 232–235.