Abstract
Effect of cabinet-drying, vacuum-drying, and freeze-drying methods on the surface hydrophobicity, secondary structure, emulsifying, and foaming properties of protein concentrates prepared from different cultivars of cowpea and Bambara bean was investigated. The vacuum-drying method reduced hydrophobicity, while freeze-dried concentrates presented high hydrophobicity. The concentrates prepared by freeze drying presented more β-sheet (40–43%) and less β-turn (19–24%) structures. Bambara bean protein concentrates prepared by freeze-drying presented higher emulsifying activity (56–59%) compared to those by vacuum-drying and cabinet-drying, while emulsifying activity varied significantly among cultivars of cowpea (46–61%). Protein concentrates prepared by cabinet-drying showed the highest foaming ability.
INTRODUCTION
Legumes are good sources of low-cost proteins and low glycemic carbohydrates, which are consumed by low-income populations in developing countries. Legumes have many beneficial physiological effects in controlling and preventing various metabolic diseases, such as diabetes mellitus, coronary heart disease, and colon cancer.[Citation1] Tropical legumes, such as cowpea and Bambara bean, are a good source of cheap, accessible, and safe proteins. However, it is essential that protein as an ingredients must exhibit good functional properties in addition to nutritional value.
Functional properties of proteins affect consumer acceptance. Formation and stabilization of emulsions and foams is critical in many applications, such as chopped and comminuted meats, cake batters, coffee whiteners, milk, mayonnaise, salad dressings, bakery products, and frozen desserts.[Citation2] Surface hydrophobicity (HO), ligand binding, molecular flexibility, and structure stability of proteins affect the emulsifying and foaming properties.[Citation3] Physicochemical and conformational properties of proteins are influenced by the extraction procedure as well as the drying method. The most common method of preparation of protein concentrates in the food industry is the isoelectric precipitation technique. It involves alkaline solubilization, centrifugation to remove insoluble matter and isoelectric precipitation.[Citation4,Citation5] This technique has been successfully used to prepare cowpea and Bambara bean protein concentrates.[Citation6,Citation7] The precipitates obtained from isoelectric precipitation process are dried using various techniques to get a dry powder. Drying techniques affect the physico-chemical and functional properties of protein concentrates.
This study evaluated the influence of vacuum-drying (VD), cabinet-drying (CD), and freeze-drying (FD) methods on the emulsifying and foaming properties of protein concentrates of four cultivars of cowpea and Bambara bean. The appropriate drying method will help to utilize the protein concentrate of these legumes in the food industry.
MATERIALS AND METHODS
Materials
Four local cultivars of cowpea [Vigna unguiculata (L.) Walp] and Bambara bean [Vigna subterranea (L.) verde] with different characteristics were purchased at the local market of Maroua (Far North region, Cameroon). The four cultivars of cowpea were respectively: medium size, smooth and creamy seed coat (Cw); medium size, smooth and black seed coat (Cb); medium size, wrinkled and brown seed coat (Cbr); and medium size, smooth and red seed coat (Cr). The four cultivars of Bambara bean were respectively: large size, smooth and cream seed coat (Bw); large size, smooth and black coat seed (Bb); smooth and light brown with black spots coat seed (Bbr); and large size, smooth and brown seed coat (Br). According to the classification of Pasquet and Fotso,[Citation8] based on morphological traits and geographic distribution, cowpea cultivars Cw, Cb, and Cr, and Bambara bean cultivars were included into the “campestris” group, whereas the Cbr cultivar was included into the “melanophtalmus” group. All these cultivars were harvested at the 2012 growing season.
Preparation of Flour
The seeds were hand-picked, washed and rinsed in deonized water at room temperature (25 ± 2°C). They were dried in an air convection oven at 50°C for 72 h, then cracked and dehulled. The dried seeds were ground into flour, and passed through a 200 µm mesh sieve. The resultant flour was stored in polyethylene bag at –20°C.
Defatting of Flour
Bambara bean and cowpea flours were extracted twice with petroleum ether in a 1:3 ratio (w/v), for 4 h at 37°C to remove fats. Separation between solid and liquid was done by filtration using Whatman No. 1 filter paper. The flour was air-dried at ambient temperature and stored at –20°C.
Preparation of Protein Concentrates
Extraction
Bambara bean and cowpea protein concentrates were prepared by the isoelectric precipitation method.[Citation9,Citation10] Defatted flour was mixed with distilled water in 1:10 ratio (w/w), the pH was adjusted to 10 using a 1.0 M NaOH solution, and the mixture was stirred for 120 min at room temperature (~25°C). The resultant slurry was then centrifuged at 8000 × g for 20 min and at 4°C using a Remi centrifuge (Remi Instruments, Mumbai, India). The pH of the resultant supernatant was adjusted to 4.5 using a 1.0 M HCl solution with constant stirring, the precipitated proteins were recovered by centrifugation at 8000 × g for 20 min (4°C). The proteins were re-suspended in distilled water maintaining about 5% (w/v) total solid, and the pH was adjusted to 7.0 with constant stirring. The protein suspensions were then dried.
FD
Protein solutions were poured into aluminium plates of 10 cm of diameter to about 5 mm depth, then frozen for 2 h at –30°C. Frozen samples were then freeze-dried at –50°C for 48 h using a Heto freeze-dryer at a pressure of about 15 kPa (Heto Power Dry, Allerod, Denmark). The freeze-dried samples were ground using a mortar and pestle, passed through a 150 µm mesh sieve and then kept at –20°C (the room temperature was ~25°C).
VD
VD was performed at 50°C and 3.33 kPa for 24 h, using aluminium plates of 10 cm of diameter (Narang Scientific Works, New Delhi, India) for about 5 mm depth protein solution. The vacuum-dried samples were collected, then ground, sieved, and stored at –20°C.
CD
CD was performed at 50°C for 24 h, at constant air flow (1.5 m/s), using aluminium plates (Narang Scientific Works, New Delhi, India) and about 5 mm depth protein solution. The cabinet-dried samples were collected, then ground, sieved, and stored at –20°C.
HO
Surface HO was determined by the bromophenol blue (BPB) fixation method as described by Chelh et al.[Citation11] BPB has been shown to bind to the same hydrophobic sites on proteins as fluorescent probes,[Citation12] and the absorption difference spectroscopy of BPB provides a valuable supplement to ANS for determining in solution surface HO.[Citation11] HO of protein concentrates was determined using BPB sodium salt for electrophoresis (Sigma, St. Louis, MO, USA). To 1 mL of protein concentrate suspension (5 mg/mL, protein solution in 20 mM phosphate buffer pH 7), 200 µL of BPB solution (1 mg/mL BPB in distilled water) was added and mixed well. A control, without protein concentrate, consisted of the addition of 200 µL BPB solution to 1 mL of 20 mM phosphate buffer at pH 7. Test and control samples were kept under agitation, at room temperature, during 10 min and then centrifuged for 15 min at 2000 × g. The absorbance of the supernatant (diluted 1:10) was measured at 595 nm against a blank of phosphate buffer using a Shimadzu UV-2450 (Kyoto, Japan) spectrophotometer. The amount of BPB bound is given by the formula:
With A = absorbance at 595 nm. All tests and analyses were carried out in triplicate.
Secondary Structure Characterization
The secondary structures of protein concentrates were characterized using Fourier Transform Infrared (FTIR) spectra. The dried protein samples were placed between two pieces of aluminium foil and pressed into a pellet. FTIR spectra were obtained in the wavenumber range of 400 to 4000 cm–1 during 32 scans with 4 cm–1 resolution using a FTIR spectrometer (Perkin Elmer, Massachusetts, USA). Data were analyzed by means of the Spectrum software version 3.01 and Peakfit 4.12 (Systat Software, San Jose, USA). Secondary structural features were calculated from the amide I envelope by non-linear regression fitting of Gaussian peaks of the original spectra. Peaks assignments were made using the results of Farrell Jr. et al.[Citation13]
Functional Properties
Emulsifying properties
Emulsifying activity (EA) and stability were determined using the method of Neto et al.[Citation14] Five milliliter portions of protein solution (1%, w/v) were homogenized with 5 mL soybean oil. The emulsions were centrifuged at 1100 × g for 5 min. The height of emulsified layer and that of the total contents in the tube was measured. The EA was calculated as:
Emulsion stability (ES) was determined by heating the emulsion at 80°C for 30 min before centrifuging at 1100 × g for 5 min.
Foaming properties
The foaming properties were determined in triplicate using the method described by Wu et al.[Citation15] Concentrations of 1% protein were prepared in distilled water and adjusted to pH 7.0 with 1.0 N NaOH and 1.0 N HCl if necessary. Volumes of 100 mL (V1) of cowpea and Bambara bean protein concentrate suspensions were blended for 5 min using a magnetic stirrer (Corning PC-420D, NY, USA) at the highest speed, poured into 250 mL graduated cylinders, and the volume of foam (V2) were immediately recorded at 0, 30, and 60 min. Foaming was calculated using the following equation: Foaming = (V2 – V1) × 100/V1. Foaming capacity was determined at 0 min, and foam stability (FS) after 30 and 60 min.
Statistical Analysis
All experiments were carried out in triplicate. Mean values along with standard deviations were reported. The data were analyzed by the Duncan test. Correlation was estimated by the method of Pearson. The significance level was defined as p < 0.05. The computer softwares used in this study were SPSS (SPSS Inc., Chicago, USA) and STATISTICA (Statsoft Inc., Tulsa, USA).
RESULTS AND DISCUSSION
Surface HO
Surface HO is an important property that determined functionality of proteins.[Citation2] Nakai and Li-Chan[Citation16] demonstrated that surface HO showed good correlation with interfacial tension and the surfactant properties of proteins. Furthermore, high surface HO following processing indicated possible exposure of hydrophobic regions that were otherwise buried inside globular proteins due to denaturation.[Citation17]
It was observed that the drying methods had different effects on the surface HO of protein concentrates prepared from cowpea and Bambara bean cultivars (). The HO of Bambara bean protein concentrates prepared by FD was significantly higher than those prepared by CD and VD (p < 0.05). Furthermore, protein concentrates prepared by CD exhibited higher HO than those prepared by VD. On the other hand, CD was found most effective to improve HO of Cw and Cb protein concentrates, while Cr and Cbr protein concentrates prepared by FD showed significantly higher HO than those prepared by CD and VD (p < 0.05). Zhao et al.[Citation17] reported that drying caused denaturation of proteins and unfolding of their structure, then exposure of more hydrophobic groups previously buried inside the protein structure. Significant differences in HO were also observed among cultivars within the same species (p < 0.05). For protein concentrates prepared by CD, HO ranged from 28.17µg BPB (Cr) to 50.48 µg BPB (Cw) for cowpea cultivars, and 43.21 µg BPB (Bb) to 50.82 µg BPB (Bbr) for Bambara bean cultivars. Non-significant difference in HO was found between protein concentrates prepared from Cw and Bbr cultivars (p > 0.05). The highest HO for protein concentrates prepared by VD was observed for Cw (35.30 µg BPB) and Br (43.25 µg BPB) cultivars, and the lowest for Cb (21.58 µg BPB) and Bb (21.49 µg BPB) cultivars. Bambara bean protein concentrates prepared by FD exhibited significantly higher HO than those from cowpea (p < 0.05). In addition, the protein concentrates prepared from Bbr (71.92 µg BPB) and Cb (22.15 µg BPB) cultivars, had respectively the highest and the lowest HO.
TABLE 1 Surface hydrophobicity (µg bound BPB) of cowpea and Bambara bean protein concentrates depending on the drying method
FTIR Spectra Analysis
FTIR spectra adopts the vibrational spectroscopic technique and can be applied to liquid, semi-solid, and solid state samples for the analysis of structural changes, particularly suitable for denatured or aggregated proteins.[Citation18] The study of secondary structure is based on many bands given by a protein in infra-red spectroscopy. Of these bands, the amide I resulting from the C=O stretching vibration of the protein backbone is particularly sensitive to the folding patterns in the secondary structural level.[Citation19] The hydrogen bonding between C=O and N-H groups of the peptide linkage determined the geometry of the polypeptide backbone which again determines the frequency of the infrared (IR) bands. Since a protein usually contains different secondary structural elements such as α-helix, β-sheet, β-turn, and random coil, the amide I band is a composite band constituted of overlapping signals.[Citation20]
FTIR analysis was used in this study to assess the conformation of cowpea and Bambara bean protein concentrates prepared using the different drying methods. The original spectra of protein concentrates showed two major bands, the amide I at 1633 cm–1 and the amide II band at 1550 cm–1 (), indicating the typical structure of protein. The amide I band was further resolved by Fourier self-deconvolution and second derivative (). The analysis clearly resolves bands which have been assigned on the basis of data available in literature. The bands were easily assigned as follows: band at 1626 cm–1 is due to β-sheet, band at 1648 cm–1 is due to loop or helix, and band at 1676 cm–1 is due to β-turn.[Citation13] According to the calculation of the individual component peak area, results on the secondary structure composition of protein concentrates are presented in and , respectively for Bambara bean and cowpea.
FIGURE 1 Original and self-deconvoluted FTIR spectrum and their curve fitting results of amide I and II of freeze-dried cowpea protein concentrate (for the Cb cultivar).
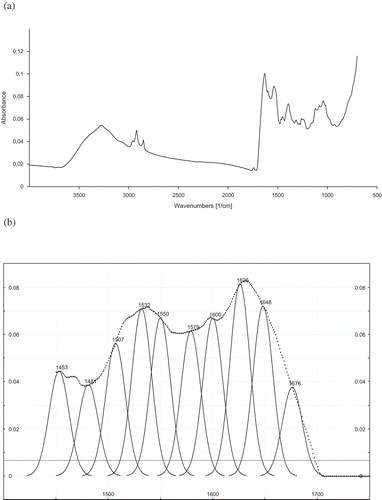
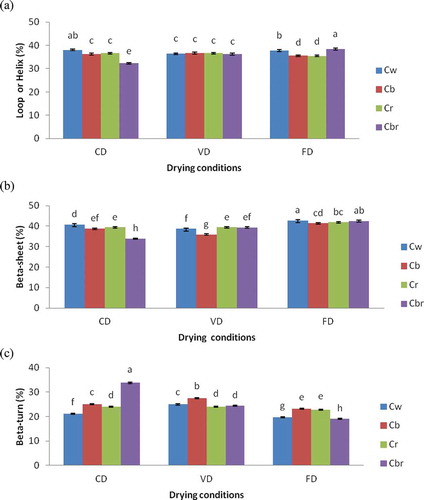
Cowpea and Bambara bean protein concentrates prepared by the different drying methods contained 32–38% and 36–38% loop or helix (unordered structure),[Citation21] 34–42% and 35–43% β-sheet, and 19–38% and 18–25% β-turn, respectively. The β-sheet secondary structure content was significantly higher than unordered and β-turn structure contents (p < 0.05), and no α-helix was found. These results were in agreement with those of Ellepola et al.[Citation18] who reported that many seed globulins from monocotyledonous and dicotyledenous plants were found to possess low levels of α-helix and high levels of β-sheet secondary structure. The drying methods significantly affected the β-sheet content of protein concentrates (p < 0.05). Cowpea and Bambara bean protein concentrates prepared by FD showed significantly (p < 0.05) higher β-sheet structure content compared to those prepared by CD and VD ( and ). In addition, the highest β-sheet structure content (43%) was observed for concentrates prepared from Bb and Cw cultivars and the lowest for Bb (39%) and Cbr (34%) protein concentrates using CD. It was also noticed that Bambara bean protein concentrates prepared by CD and VD showed similar β-sheet structure content, except for the Bb cultivar. On the other hand, non-significant difference was found in unordered structure content of Bambara bean protein concentrates prepared by CD and VD, as well as for cowpea protein concentrates prepared by VD ( and ). In addition, the highest unordered structure content was observed for concentrates prepared by FD particularly from Cbr (38.43%) and Br (39.12%) cultivars, while the lowest was noticed for the Cbr protein concentrate prepared by CD. The drying methods used in this study also significantly affected the β-turn structure content of protein concentrates. It is observed in and that protein concentrates prepared by FD showed significantly lower β-turn structure content than those prepared by CD and VD. And, CD and VD showed similar β-turn structure content for Bambara bean protein concentrates. It was also noticed that the Cbr protein concentrate prepared by CD showed the highest (34%) β-turn structure content.
FIGURE 2 Estimation of the secondary composition of protein concentrates from different cultivars of Bambara bean depending on the drying methods: (a) Loop or Helix, (b) Beta-sheet and, (c) Beta-turn. Values followed by different letters are significantly (p < 0.05) different.
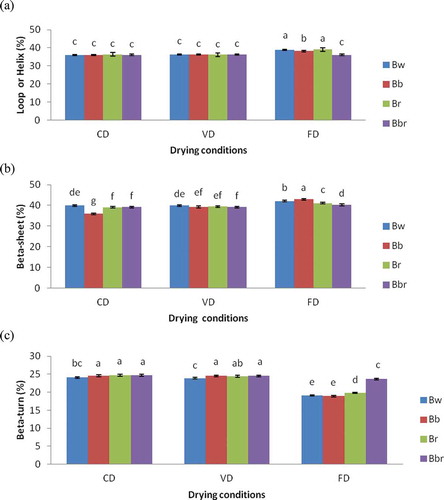
FIGURE 3 Estimation of the secondary composition of protein concentrates from different cultivars of cowpea depending on the drying methods: (a) Loop or Helix, (b) Beta-sheet, and (c) Beta-turn. Values followed by different letters are significantly (p < 0.05) different.
To understand the secondary structure transition following drying, the Pearson correlation method was applied. It was found a negative correlation between β-sheet and β-turn structure content (r = –0.95; p < 0.00), and β-turn and unordered structure content (r = –0.89, p < 0.00). These observations revealed increase in the β-turn structure at the expense of β-sheet structure, and in unordered structure at the expense of β-turn. This remark suggested that the partial unfolding process was dominated in protein structural changes, since the turn structure was considered to be a product of protein unfolding of any higher order structures, whereas anti-parallel β-sheet could be formed in aggregated protein molecules.[Citation18] Moreover, Zhao et al.[Citation17] observed that heat and pressure treatment induced denaturation and unfolding of protein structures and could release more hydrophobic groups. In this connection, the pressure treatment during FD probably promoted protein unfolding and exposure of previously buried hydrophobic groups, as indicated by the higher HO of the freeze-dried protein concentrates. In another hand, the combined heat and pressure treatment during VD probably induced protein unfolding then aggregation by the mean of hydrophobic interaction and disulfide bond formation,[Citation17,Citation22] as noticed by the lower HO and higher β-turn of the VD protein concentrates. It is well known that the conformation stability, i.e., flexibility and their tertiary and/or quaternary conformations affected the protein behavior at the oil-water or air-water interfaces, then determining emulsifying and foaming properties of globular proteins.[Citation23]
Emulsifying Properties
Effect of different drying methods on the emulsifying properties of cowpea and Bambara bean protein concentrates is presented in . It was observed that CD, VD, and FD had significant influence on the EA of concentrates depending on species (p < 0.05). Then, the Bambara bean protein concentrates prepared by FD showed significantly higher EA than those prepared by CD and VD (p < 0.05). In addition, EA of Bambara bean concentrates prepared by VD was significantly lower than those prepared by CD (p < 0.05), excepted for the protein concentrate prepared from the Br cultivar. Similarly, cowpea protein concentrates prepared by VD exhibited the lowest EA, while concentrates prepared by CD showed the highest EA for Cw, Cr, and Cbr cultivars. Then, EA of the Cb protein concentrate prepared by FD was significantly higher than those prepared by CD and VD (p < 0.05). Generally, solubility, surface HO and molecular flexibility influenced the emulsifying behavior of globular proteins. Surface HO was probably determinant for EA of cowpea and Bambara bean protein concentrates, since there was a positive correlation (r = 0.80; p < 0.00) between these two variables. Panyam and Kilara[Citation24] and Mune Mune et al.[Citation25] reported that increase in surface HO facilitated adsorption of proteins at the oil-water interface, then reduced the surface tension and increased emulsifying properties. In addition, the partial unfolding of protein molecules could increase their molecular flexibility, which facilitated their rearrangement at the oil-water interface to form an elastic layer around the fat droplets and then retarded coalescence. On the other hand, EA significantly differed among cultivars within the same species (p < 0.05). For the concentrates prepared by CD, EA ranged between 55.66% (Cw) to 61.46% (Cr), and 38.05% (Br) to 54.51% (Bbr), respectively, for cowpea and Bambara bean. Non-significant difference was found between EA of Bb and Bbr, and Cb and Cw protein concentrates (p > 0.05), while Cbr and Bw showed significantly higher EA than Cb and Br protein concentrates respectively (p < 0.05). The highest EA for the protein concentrates prepared by VD was observed for Cw (53.10%) and Br (46.96%), and the lowest for Cr (46.02%) and Bb (33.31%), respectively, for cowpea and Bambara bean. In addition, non-significant difference in EA was noticed for Cr and Cbr protein concentrates (p > 0.05), while Cb and Bw showed significantly higher EA than Cbr and Bbr respectively (p < 0.05). FD produced less pronounced difference in EA among cultivars of cowpea and Bambara bean. EA of protein concentrates prepared by FD ranged between 55.22% (Cbr) and 58.59% (Bbr), and followed the order: 57.08% (Cb) > 56.05% (Cr) > 55.75% (Cw) > 55.22% (Cbr); and 58.59% (Bbr) > 57.33% (Bb) > 56.75% (Bw) > 56.44% (Br). Inherent genetic differences among cultivars could explain differences in EA of protein concentrates. EA of cowpea and Bambara bean protein concentrates was in the same range than those of soy and sesame protein concentrates.[Citation26]
TABLE 2 Emulsifying activity (EA) and emulsion stability (ES) of cowpea and Bambara bean protein concentrates depending on the drying methods
ES of protein concentrates prepared from different cultivars of cowpea and Bambara bean as affected by different drying methods is presented in . It was observed that ES of concentrates prepared by FD was significantly higher than those prepared by CD and VD (p < 0.05), excepted for the Cb concentrate prepared by VD. Also, CD was generally found more effective to produce cowpea and Bambara bean protein concentrates with high ES than VD. Significant difference in ES was observed among cultivars within the same species (p < 0.05). ES of cowpea protein concentrates prepared by CD was relatively high, and ranged between 61.23% (Cr) and 95.16% (Cb). While, ES of Bambara bean protein concentrates was high for Bb (93.55%) and Bbr (88.19%) cultivars, and low for Bw (9.40%) and Br (13.95%) cultivars. ES of cowpea protein concentrates prepared by VD varied between 7.48% (Cbr) and 101.72% (Cb), and Bambara bean protein concentrates prepared by VD presented low potential to stabilize emulsions. Protein concentrates prepared by FD showed less pronounced difference in ES, which ranged between 98.43–101.52% and 90.23–99.22%, respectively, for cowpea and Bambara bean cultivars. High ES required molecular rearrangement of the adsorbed proteins at the oil-water interface to form a thick layer which prevents coalescence. It could be suggested that the partial unfolding of protein molecules induced by the drying process improved their flexibility which enhanced rearrangement of protein at the oil-water interface, then prevented coalescence. In this regard, a positive correlation (r = 0.85; p < 0.00) was noticed between ES and the β-turn structure content of protein concentrates prepared by VD. Furthermore, there was a negative correlation (r = –0.86; p < 0.00) between ES and the β-sheet structure content.
Foaming Properties
Foaming properties (FC and FS) of protein concentrates prepared from different cultivars of cowpea and Bambara bean as affected by CD, VD, and FD are presented in . The drying methods had significant influence on FA of concentrates. It was observed that the cowpea protein concentrates prepared from Cw and Cb cultivars by CD showed significantly higher FC compared to those by VD and FD (p < 0.05), while the concentrates prepared from Cr and Cbr cultivars using FD showed higher FC. In addition, the cowpea protein concentrates prepared by VD presented significantly lower FC than those prepared by CD and FD (p < 0.05). On the other hand, the Bambara bean protein concentrates prepared by CD exhibited significantly higher FC than those prepared by VD and FD (p < 0.05), except for the Bbr cultivar. Furthermore, lower FC was observed for Bb and Br protein concentrates prepared by VD. Panyam and Kilara[Citation24] reported that the molecular properties of proteins that are relevant for foaming include solubility to enable rapid diffusion at the interface, amphipathicity to enhance interfacial interactions, segmental flexibility to facilitate unfolding at the interface and molecular rearrangement to prevent close approach of bubbles. Drying probably increase foaming ability of protein concentrates by increasing protein solubility, since there was a positive correlation (r = 0.72; p < 0.05)[Citation27] between solubility and FC of protein concentrates prepared by FD. In addition, increasing the unordered structure content decreased FC (r = –0.74; p < 0.05) probably by affecting the segmental flexibility of proteins at the air-water interface.
TABLE 3 Foaming capacity (FC) and foam stability (FS) of cowpea and Bambara bean protein concentrates depending on the drying methods
The drying methods affected FC of protein concentrates depending on cultivars within the same species. It could be pointed out that the Cb protein concentrate prepared by CD could find application in food products where high foaming is required since it presented the highest FC (303%). The maximum FC of Bambara bean protein concentrates prepared by CD was noticed for the Br cultivar (219%), and the minimum for the Bbr cultivar (117%). There was a non-significant difference in FC between Bb and Bw protein concentrates (p > 0.05). Protein concentrates prepared by VD could be divided in two categories depending on FC: those with high FC (Cw and Cb; Bw and Bbr) and those with low FC (Cr and Cbr; Bb and Br). The maximum FC was 216.44% (Cb) and the minimum 106.35% (Br). FC of the cowpea protein concentrates prepared by FD followed the order: 271.15% (Cb) > 264.17% (Cb) > 213.89% (Cw) > 175.56% (Cbr). Non-significant difference in FC was observed between Bw, Br, and Bbr protein concentrates, and this property was significantly higher for the Bb protein concentrate (162.88%). Inherent genetic differences among species and cultivars probably influenced FC of protein concentrates.
FS of protein concentrates after 30 min is presented in . FS of cowpea protein concentrates prepared by the different drying methods followed the same trend as FA, excepted for the concentrate prepared from the Cb cultivar by CD. For food applications where high FS is required, The Cr protein concentrate could be useful (251.19%; FS), while the concentrate prepared from the Cbr cultivar by CD showed the lowest FS (98.53%). In addition, FD was found most effective to prepared cowpea protein concentrates with high FS than CD and VD. On the other hand, FS of Bambara bean protein concentrates prepared by the different drying methods varied significantly among cultivars (p < 0.05). It could be noticed that the Bb (186%) protein concentrate prepared by CD showed the highest FS, while the Bw concentrate prepared by VD (106%) exhibited the lowest FS. After 60 min of stay at the room temperature, relatively low or no change in the FS was recorded. The Cr protein concentrate prepared by FD and the Bb concentrate prepared by CD presented the highest FS, while the Cbr protein concentrate prepared by CD and the Bw concentrate prepared by VD had the lowest FS. Zayas[Citation28] reported that a high FS required a thick, elastic, cohesive, continuous, air-impermeable film around each gas bubble. Results from this study suggested that the partial unfolding of protein following drying was important for their molecular rearrangement at the air-water interface and enhance FS, since there was a positive correlation between FS and the β-turn structure content (r = 0.81: p < 0.00) and a negative correlation between FS and the β-sheet structure content (r = –0.86; p < 0.00) for concentrates prepared by VD.
CONCLUSION
In this study, protein concentrates were prepared from four cultivars of cowpea and Bambara bean using cabinet, vacuum, and freeze dryers. It was found that drying had significant influence on emulsifying and foaming properties of the concentrates, as well as on the surface HO and conformation of proteins depending on species and cultivars. The Bambara bean protein concentrates prepared by FD had higher surface HO than those by CD and FD, while cowpea protein concentrate prepared by CD showed the highest surface HO. In addition, the protein concentrates prepared by FD showed more β-sheet and less β-turn structures than those by CD and VD. The Bambara bean protein concentrates prepared by FD showed higher EA than those by CD and FD, while cowpea protein concentrate prepared by CD showed the highest EA. Protein concentrates prepared by CD showed the highest FA, and FD is useful to prepare cowpea and Bambara bean protein concentrates for food applications where high ES and FS are required. In summary, selecting appropriate drying method and cultivar could determine utilization of cowpea and Bambara bean protein concentrates in applications, such as baking products, mayonnaise, salad, dressing, and frozen dessert, where high emulsifying and foaming properties are required. Further studies are necessary for the determination of amino acids composition of dried protein concentrates and their polypeptide profile, and then define an adequate mathematical model to predict the structure-function relationship for legume proteins.
ACKNOWLEDGMENTS
This work was done under CV Raman International Fellowship for African Researchers to Dr. Martin Alain Mune Mune (March–September of 2013), in GNDU, Department of Food Science and Technology. The authors are grateful to Mr. Pandey Kumar, SLIET, Longowal, for FTIR analysis.
REFERENCES
- Tharanathan, R.N.; Mahadevamma, S. Grain legumes—A Boon to Human Nutrition. Trends in Food Science and Technology 2003, 14(12), 507–518.
- Voutsinas, L.P.; Cheung, E.; Nakai, S. Relationships of Hydrophobicity to Emulsifying Properties of Heat Denatured Proteins. Journal of Food Science 1983, 48, 26–32.
- Withana-Gamage, T.S.; Wanasundara, J.P.D. Molecular Modeling for Investigating Structure-Function Relationships of Soy Glycinin. Trends in Food Science and Technology 2012, 28, 153–167.
- Sanchez-Vioque, R.; Clemente, A.; Viogue, J.; Bautista, J.; Millan, F. Protein Isolates From Chickpea (Cicer Arietinum L.): Chemical Composition, Functional Properties, and Protein Characterization. Food Chemistry 1999, 64, 237–243.
- Chew, P.G.; Casey, A.J.; Johnson, S.K. Protein Quality and Physico-Functionality of Australian Sweet Lupin (Lupinus Angustifolius cv. Gungurru) Protein Concentrates Prepared by Isoelectric Precipitation Or Ultrafiltration. Food Chemistry 2003, 83, 575–583.
- Mune Mune, M.A.; Mbome, I.L.; Minka, S.R. Optimization of Protein Concentrate Preparation from Bambara Bean Using Response Surface Methodology. Journal of Food Process Engineering 2010, 33(3), 398–412.
- Mune Mune, M.A.; Minka, S.R.; Mbome, L.I. Response Surface Methodology for Optimisation of Protein Concentrate Preparation from Cowpea [Vigna Unguiculata (L.) Walp]. Food Chemistry 2008, 110, 735–741.
- Pasquet, R.S.; Fotso, M. Edible legumes in Cameroon, the first results. In Boutrais, J.; Ed.; From the politic to the economy, historical studies in the basin of lake Chad (pp. 317–360). Paris: ORSTOM, 1991.
- Adebowale, Y.A.; Schwarzenbolz, U.; Henle, T. Protein Isolates from Bambara Groundnut (Voandzeia Subterranean L.): Chemical Characterization and Functional Properties. International Journal of Food Properties 2011, 14, 758–775.
- Deng, J.; Sun, T.; Cao, W.; Fan, D.; Cheng, N.; Wang, B.; Gao, H.; Yang, H. Extraction Optimization and Functional Properties of Proteins from Kiwi Fruit (Actinidia Chinensis Planch) Seeds. International Journal of Food Properties 2014, 17, 1612–1625.
- Chelh, I.; Gatellier, P.; Santé-Lhoutellier, V. Technical Note: A Simplified Procedure for Myofibril Hydrophobicity Determination. Meat Science 2006, 74, 681–683.
- Bertsch, M.; Mayburd, A.L.; Kassner, R.J. The Identification of Hydrophobic Sites on the Surface of Proteins Using Absorption Difference Spectroscopy of Bromophenol Blue. Analytical Biochemistry 2003, 15, 187–195.
- Farrell Jr., H.M.; Wickham, E.D.; Unruh, J.J.; Qi, P.X.; Hoagland, P.D. Secondary Structural Studies of Bovine Caseins: Temperature Dependence of Β-Casein Structure As Analyzed by Circular Dichroism and FTIR Spectroscopy and Correlation with Micellization. Food Hydrocolloids 2001, 15, 341–354.
- Neto, V.Q.; Narain, N.; Silva, J.B.; Bora, P.S. Functional Properties of Raw and Heat Processed Cashew Nut (Anarcardium Occidentale, L.) Kernel Protein Isolate. Nahrung/Food 2001, 45, 258–262.
- Wu, H.; Wang, Q.; Tiezheng, M.; Ren, J. Comparative Studies on the Functional Properties of Various Protein Concentrate Preparations of Peanut Protein. Food Research International 2009, 42, 343–348.
- Nakai, S.; Li-Chan, E. Hydrophobic Interactions in Food Systems; CRC Press: Boca Raton, FL, 1988.
- Zhao, Q.; Xiong, H.; Selomulya, C.; Chen, X.D.; Huang, S.; Ruan, X.; Sun, W. Effects of Spray Drying and Freeze Drying on the Properties of Protein Isolate from Rice Dreg Protein. Food Bioprocess Technology 2013, 6(7), 1759–1769.
- Ellepola, S.W.; Choi, S.M.; Ma, C.Y. Conformational Study of Globulin From Rice (Oryza Sativa) Seeds by Fourier-Transform Infrared Spectroscopy. International Journal of Biological Macromolecules 2005, 37(1–2), 12–20.
- Jackson, M.; Mantsch, H.H. Halogenated Alcohols As Solvents for Proteins: FTIR Spectroscopic Studies. Biochemica Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology 1992, 1118(2), 139–143.
- Surewicz, W.K.; Mantsch, H.H.; Chapman, D. Determination of Protein Secondary Structure by Fourier Transform Infrared Spectroscopy: A Critical Assessment. Biochemistry 1993, 32(2), 389–394.
- Liu, G.; Li, J.; Shi, K.; Wang, S.; Chen, J.; Liu, Y.; Huang, Q. Composition, Secondary Structure, and Self-Assembly of Oat Protein Isolate. Journal of Agricultural and Food Chemistry 2009, 57(11), 4552–4558.
- Joshi, M.; Adhikari, B.; Aldred, P.; Panozzo, J.F.; Kasapis, S. Physicochemical and Functional Properties of Lentil Protein Isolates Prepared by Different Drying Methods. Food Chemistry 2011, 129, 1513–1522.
- Tang, C.-H.; Sun, X. A Comparative Study of Physicochemical and Conformational Properties in Three Vicilins from Phaseolus Legumes: Implications for the Structure–Function Relationship. Food Hydrocolloids 2011, 25(3), 315–324.
- Panyam, D.; Kilara, A. Enhancing the Functionality of Food Proteins by Enzymatic Modification. Trends in Food Science and Technology 1996, 7, 120–125.
- Mune Mune, M.A.; Minka, S.; Mbome, I.L. Effects of Increasing Acylation and Enzymatic Hydrolysis on Functional Properties of Bambara Bean (Vigna Subterranea) Protein Concentrate. Acta Alimentaria 2013, 43(4), 1–10.
- Cano-Medina, A.; Jiménez-Islas, H.; Dendooven, L.; Herrera, R.P.; González-Alatorre, G.; Escamilla-Silva, E.M. Emulsifying and Foaming Capacity and Emulsion and Foam Stability of Sesame Protein Concentrates. Food Research International 2011, 44(3), 684–692.
- Mune Mune, M.A.; Sogi, D.S. Some Functional Properties of Protein Concentrates Prepared from Four Cultivars of Cowpea and Bambara Bean As Affected by Different Drying Methods. Unpublished results, 2014.
- Zayas, J.F. Functionality of Proteins in Food; Springer: New York, NY, 1997; p. 373.
