Abstract
Estimating oxidative stability with successive heating temperatures provides a qualitative assessment of oil in the food industry. As one of the prime factors contributing to oil characteristics, the flow behavior of highly refined sesame oil and mustard oil were determined experimentally between the temperatures 25 and 85°C. The variation of viscosity with temperature was fitted in two variable empirical equations and the mathematical models were compared. Various physicochemical parameters of the oils like conductivity, density, viscosity, ultrasonic velocity, saponification value, iodine value, and free fatty acid content were observed to estimate their characteristics and stability on heating to cooking temperature. The physical and chemical parameters were compared and correlated between the oils to analyze the oxidative stability at different times of heating. A novel relation was demarcated between ultrasonic velocity and the chemical parameters. The study helped in the identification of the best oil suited for repeated cycles of heating.
INTRODUCTION
Oil has been a vital part of people’s regular dietary consumption all over the world and its usage has been found to increase several folds over the decades. The importance of using the appropriate oil for cooking goes a long way in affecting the consumer’s health. Improper methods of oil-aided cooking can lead to cardiovascular diseases and increased cholesterol in the blood. They have also been found to be cancer-inducing. Hence, it is highly essential to use the right oil for cooking. Oils exhibit various physicochemical properties depending upon the amount of heating they are exposed to while cooking.[Citation1] These properties in turn determine the quality of the oil.
Any type of oil exhibits a change in its characteristic features when it is subjected to repeated heating with respect to different ranges of temperatures achieved or in terms of time of heating.[Citation2] Vegetable oils are mainly composed of monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA). Upon heating, these unsaturated compounds begin to degrade and various oxidation products are formed. Extreme and higher durations of heating leads to increased amount of free fatty acids (FFAs) in the oil, which tends to bring down the smoke point.[Citation3] This restricts repeated usage of the oil. Upon repeated heating, oil reaches a condition called rancidity where all the unsaturated fatty acids undergo double-bond cleavage and release volatile compounds like aldehydes and ketones.[Citation4] This phenomenon results in development of undesirable flavors and odors in the oil, making it non-ideal for consumption.[Citation5] Subjecting oil to high temperatures will affect its chemical parameters, such as acid value, iodine value (IV), amount of MUFA and PUFA present, etc.[Citation6] Ultrasonic velocity (UV) is a non-invasive parameter for indexing the quality of oil by its propagation through liquids.[Citation7]
Analysis of oils like olive oil, palm oil, peanut oil, soybean oil, sunflower oil, and rice bran oil has been done in earlier research.[Citation5,Citation6] The drawback of the analytical study is time consumption and cost. In western countries, quantity of FFAs, and in central and eastern countries, polar compounds are used as index of degradation. Hence, simple physicochemical properties can be used as a quality index of the oil that undergoes cycles of heating.[Citation8] The study of chemical and physical changes in oil on successive heating is very important as in small scale food and snack industries, mess and marriage halls, the public is unaware of the antagonistic effect caused by repeated oil usage.[Citation9]
Mustard oil is derived from the seeds of the plants of the family Brassicaceae. It has a distinct, characteristic pungent taste associated to the plant and is frequently used for cooking, frying, and eating in northern and eastern India. Its distinctive pungent odor is due to the presence of allyl isothiocyanate. It has about 21% of PUFA and 12% saturated fats. However, in earlier studies, mustard oil was heated to its smoke point in cooking and degradation of omega-3-fatty acids was reported which causes a toxic effect in the human system.[Citation10]
Sesame oil is used as a flavor enhancer in various countries like Korea, China, and Japan. It is used as major cooking oil in South India. It has MUFA (35%), PUFA (37%), and saturated fats (18%). The amount of antioxidants and fatty acids present in the oil enhances its capacity to control blood pressure when used in appropriate cooking conditions. [Citation11] In this study, physical properties like density, viscosity, UV, acoustic impedance, conductivity, refractive index, and adiabatic compressibility along with chemical properties, like saponification value (SV), acid number, and IV, were estimated separately and then correlated to examine the quality of sesame and mustard oils individually. Depending upon the level of unsaturation reached in each of the oils with varied durations of heating, a comparative analysis is done to show the better oil between the two for regular usage, with minimal harmful effects. The changes observed in the properties of these oils are used as indicators to determine the re-usability of the oil, safety, and its quality.
MATERIALS AND METHODS
To compare the oxidative stability of mustard and sesame oils, the oils were purchased in a local supermarket in Trichy, Tamil Nadu. The basic composition of the oil was noted for reference. The various reagents used for titration in determination of chemical parameters were purchased from standard laboratory reagent (SLR) grade. Sesame (Sesamum indicum) and mustard (Brassica juncea) oils were heated from 25 to 85°C using microcontroller based temperature regulator. The oil (150 mL) of was heated by keeping the sample in a copper beaker placed in a water bath aided by an electric device. A uniform temperature was maintained by constant stirring with glass rod manually. For the heated oil sample, 250 mL of oil was exposed to frying temperatures for 0.5, 1.0, 1.5, and 2 h using castor oil instead of water.
Physical Parameters
Viscosity measurement
Each of the oils was exposed to four cycles of heating (0.5, 1.0, 1.5, and 2 h) to 180°C temperature and the oxidative stability was observed by measuring viscosity with respect to time. Oils were periodically removed from heating and cooled to room temperature (25°C) after each desired time of heating and the kinematic viscosity (ν) of oils were measured using redwood viscometer (manufactured by Associated Instrument Manufacturers, India Private Limited, New Delhi, India).
Ultrasonic measurement
The ultrasonic interferometer (F-80, Mittal Enterprises, Delhi, India) working at frequency 2 MHz with an overall correctness of ±0.01 ms–1 was used in measuring the ultrasonic velocities of oil samples.
Density measurement
The density was measured using Pycnometer with an accuracy of ±0.2 kg m–3 in accordance with the American Society for Testing and Materials (ASTM) standard method D891-09.
Conductance
Conductance is a measure of the ability of oil to allow electrical charges to pass through. Conductivity of oils was measured by the method ASTM D2624.
Refractive index
Refractive index is the resistance offered to light to pass through a liquid medium. The refractive indices of oils exposed to cycles of heating were measured by using an Abbe’s refractometer (Sinotech), falling within the specifications mentioned as per ASTM D1218. The refractometer was been calibrated by measuring the refractive indices of triply distilled water and toluene at room temperatures. The reproducibility of refractive index measurements was within ±0.0001.
Chemical Parameters
Acid value
A simple titrimetric method was used to measure acid value. The sample oil to be titrated was weighed to 3 g and dissolved in a combination of ethanol and diethyl ether used as solvent. Phenolphthalein was used as an indicator. Potassium hydroxide of 0.1 N in ethanol was taken as the burette solution and titrated against the sample oil (ASTM D664). Pink color persisting for 10–15 s denotes the end point. Blank titration was done with just the sample to eliminate the effects of the reagents.
SV
Four grams of oil sample was dissolved in appropriate solvent system (ethanol and diethyl ether). Twenty-five milliliters of 0.5 N alcoholic potassium hydroxide (KOH) was added to this solution (ASTM D5558). The contents of the conical flask were titrated against 0.5 N HCl, once with sample containing reagents and once with blank solution. Phenolphthalein was used as an indicator. The end point was denoted by appearance of pale permanent pink color. The amount of KOH consumed by the fat represents the SV of the oil sample.
IV
The amount of unsaturated fatty acids in oil is estimated using IVs. The double bonds react with iodine compounds. The higher the iodine number, the more double bonds are present in the fat. Sample of oil was taken in an Erlenmayer Flask. Two grams of oil sample was dissolved in 15 mL of benzene and added with 25 mL of Wijs (Iodine monochloride) solution (ASTM D5768).
Statistical Analysis
All data on viscosity, density, conductivity, refractive index, and UV were recorded as mean ± SD and analyzed by SPSS (version 12). One-way analysis of variance was performed by ANOVA procedures. Significant differences between the parameters were determined by Duncan’s multiple range tests. Taking time of heating as the independent parameter and the other physical and chemical parameters as dependent, the variances were computed. The correlation analyses were carried out using graph pad software.
TABLE 1 Viscosities (10−6 m2/s) of mustard and sesame oil at different temperature
TABLE 2 Values of constants obtained using different models and equations of mustard oil
TABLE 3 Values of constants obtained using different models and equations of sesame oil
TABLE 4 Physical properties of sesame and mustard oil
TABLE 5 Chemical properties of sesame and mustard oil
RESULTS AND DISCUSSION
Physicochemical Characteristics
Each physical parameter was correlated to a chemical parameter and the observed relationship was used to estimate the level of unsaturation in each of the oils with increased heating durations.
Prediction of Viscosity Using Mathematical Modeling
Seven different models were used in the study of variation of flow properties of sample with temperature. The viscosity varies nonlinearly with temperature as given in . Two parameter equations—Arrhenius, Andrade, Walther, Wright ASTM, and Erying have been used for this purpose. The following Arrhenius equation can be used to determine the activation energy of the viscous flow of oils.
Ea is the activation energy (kJ/kg), R is universal gas constant (8.314 kJ/kg mol K) and A is a constant (m2/s).[Citation3] A plot of natural logarithm of viscosity and reciprocal of temperature showing a linear graph was drawn that exhibits the Newtonian behavior of the oil, from which the value of Ea was evaluated.[Citation10–Citation12] and list out the values of constants A and B, along with the correlation coefficient and standard error estimate (SEE) with least square approximation. The correlation coefficient varies from 0.952 to 0.990 and the activation energy for viscous flow ranges from 2.344 to 3.217 kJ/mol. Experimental variation of viscosity with temperature follows a simple Andrade equation represented by Eqs. (3) and (4):
where, ln ƞ is the natural logarithm of kinematic viscosity, A and B are the constants determined by non-linear estimation procedure and T is the temperature in °C. The results of the regression analysis for the four oil samples are presented in and . For Eq. (3) the correlation coefficient varies from 0.959 to 0.993 and the deviation of calculated viscosity from the measured viscosity varies by 2 to 6%. The analysis was used to find the exponential and logarithmic variation of kinematic viscosity with temperature.[Citation13] The calculated correlation coefficient for Eq. (4) ranges from 0.962 to 0.995 and the deviation between calculated and experimental value ranges from 1.5 to 8%. The variation of viscosity with temperature can also be studied using Walther equation which is depicted as follows:
In 1932, ASTM collected the values and correlations for viscosity–temperature connections in order to develop a standard chart.[Citation14] The chart states that the linear variation between the viscosity and temperature is due to the molecular structure of the long chain molecules at high temperatures. The calculated correlation coefficient for Eq. (5) ranges from 0.990 to 0.999. The Walter formula is one of the ASTM chart equations (using a constant of 0.8) and it correlates the data sets within 2–4% accuracy.
Wright equation (ASTM 341-93):
In 1969 Wright modified Walter equation significantly by linearizing the variation of viscosity with temperature for a liquid containing hydrocarbon for –73 to 371°C.[Citation15,Citation16] The correlation ranges from 0.943 to 0.980 with the data accuracy of 2–4%.
Erying equation:
It is an alternative approach of two parameter equations, used to correlate kinematic viscosity with the variation of temperature. Here, B could be a fraction of boiling point temperature and A denotes the American Petroleum Institute (API) gravity for liquid that contains hydrocarbons.[Citation17] The equation is also called as Andrade’s equation. B takes the value ranging from 58 to 77 (fraction of boiling point temperature) and A varies between 1.6 and 2.6 constant. The R2 ranges from 0.946 to 0.998.
Physical Parameters
Flow behavior
The viscosity of the oil is measured from the laminar flow of the liquid. exemplifies the variation of viscosity of sesame and mustard oil with time of heating. It is observed that the viscosity increases with the time of heating. Sesame and mustard oil contain large amounts of unsaturated fatty acids and fewer amounts of saturated fatty acids. Hence, the viscosity is low after first time of heating. The increase in viscosity with time of heating indicates that the unsaturated fatty acids are getting saturated by the conversion of double bonds to single bonds in the fatty acids. The viscosity increases by 62% for sesame oil after the fourth time of heating and in mustard oil it increases by 75.8%. The percentage of increase shows the formation of oxidized compounds and the increase in formation of monomers, dimers, and trimers during polymerization reaction that takes place in the oil.[Citation10,Citation18,Citation19] The lower increase of viscosity percentage in sesame oil may be due to the oxidative stability of antioxidants present in the oil.
Molecular clustering
Oil contains large amounts of fatty acids and glycerol. On hydrolysis there may be the addition of one, two, or three fatty acids to the glycerol, forming molecular clusters. Density is the molecular fraction per unit volume.[Citation11,Citation20] Viscosity and density are linearly correlated. Hence, from it can be pragmatic that density also increases with time of heating. The density of sesame after fourth time of heating is observed to increase by 7.15% and for mustard oil it increases by 12.3%. From the increase in density it is observed molecular clustering is empirically high in mustard oil.
Measurement of electricity flow
Conductance of electricity depends on number of double bonds present in the chemical species of the oil. The presence of MUFA and PUFA in cis form varies the conductivity in the oil.[Citation21] shows the decrease in conductance with increase in time of heating. This elucidates the decrease in the concentration of unsaturated fatty acids with time of heating. As the time of heating increases the fatty acids with a lesser double bond count decreases. The decrease in conductance of the oil is due to the reduction of free electrons in double bonds that move in the direction of electric flow.
Measurement of optical property
The color of the oil becomes dark, even black, with increased time of heating due to molecular clustering, polymerization, and upsurge in saturated compounds. This changes the refractive index and it increases with the time of heating, indicating the degree of degradation in the oil.[Citation18,Citation22] The percentage of increase in refractive index for sesame and mustard (0.5%) oil is established using and is found to be almost the same.
Measurement of sonic property
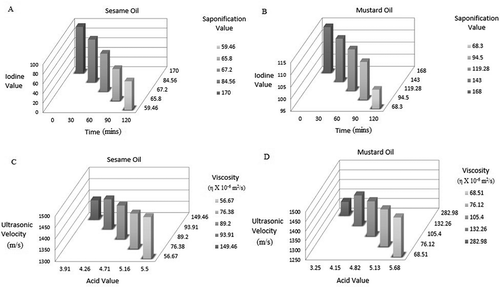
Measurement of UV is one of the few non-invasive methods to find the quality of the sample.[Citation23] UV increases with increase in the number of double bonds in the fatty acids. The ultrasonic parameters are highly used in real time analysis and it has been observed that the UV increases with escalation in hydrogen bonding and intermolecular forces.[Citation24] The variation of UV also depends on the chain length of fatty acids in oil. It is observed that all these parameters depend on the chemical reactions and complexes present in the oil. Since molecular clustering is high in heated oils, they offer an enhanced resistance to the flow of sound. Thus, the percentage of reduction observed in sesame oil is 6.7 and 9.6% for mustard oil.
Chemical Parameters
Measurement of acidity
Acid value is an indication of the amount of carboxylic acids present in the fatty acid chains. When oil is heated for a specific time, the carboxylic acid groups react to form esters and peroxides which degrade to aldehydes, ketones, and other secondary compounds. Hence, the number of available free hydrogen ions reduces.[Citation25] Thus, as the time of heating increases acid value keeps reducing. In it is observed that acid value decreases by 29% for sesame oil and 44% for mustard oil.
Measurement of average chain length of acids
SV for oil indicates the average chain length of all the fatty acids present in the oil.[Citation26] When oil is subjected to multiple heating cycles, the unsaturated fats degrade faster and the average chain length decreases due to various reactions occurring within oil. In it is noted that SV for sesame oil drops by 65% and for mustard oil it drops by 59%.
Measurement of double-bond stability
IV is a measure of the number of double bonds present in the oil. When oil is heated, most of the double bonds are converted into single bonds. Thus, this is a direct measure of the amount of unsaturated fatty acids present in the oil.[Citation27,Citation28] IV also decreases with increase in time of heating. The fall in IV is noted to be 38% for sesame oil and 0.1% for mustard oil as shown in .
Regression Analyses Between Physical and Chemical Parameters
The SVs and the IVs were investigated following Association of Official Analytical Chemists’ (AOCS) technique. Regression analyses were used to develop the equations between the physical parameter viscosity and UV of oils with their chemical properties IV and SV, using quadratic effect.
Viscosity of the oil is affected by multiple cycles of heating due to degradation of fatty acids and molecular clustering. Thus, viscosity (ƞ) can be written as a function of SV and IV. and illustrate the variation of SV and IV with time of heating in minutes. Viscosity is highly correlated with SV and IV where the computed coefficient R2 = 0. 931 and significance p < 0.01.
For sesame oil:
For mustard oil:
Ultrasonic property describes the type of molecules present in oil. It also takes into account the resistance offered by oil against the flow of sound which is an indirect measure of molecular clustering due to oxidation and further reduction of fatty acids to various other compounds. Thus, UV can also be depicted as a function of SV and IV. UV is highly correlated with SV and IV where the computed coefficient R2 = 0. 995 and significance p < 0.05.
For sesame oil:
For mustard oil:
These equations illustrate the correlation analysis between the physical and chemical properties of the oils.
Viscosity and UV are negatively correlated. The changes in viscosity and UV over the heating period arise due to changes in hydrogen bonding. Molecular clustering, being the majorly affecting parameter, increases the viscosity of the liquid. But it offers more resistance to the flow of sound, thereby decreasing UV.
Conductance is an inherent property of the solution to allow the flow of electrical charges. Movement of charge carriers in the oil rely on double bond and unsaturated compounds in oil. Decrease in conductance is due to the lesser number of double bonds present in heated oil which takes away the extra electrons previously present. Thus, conductivity is negatively correlated with viscosity.
Variation of viscosity, UV, and acid value of sesame and mustard oil with the cycles of heating is illustrated in and . It was observed that acid value decreases with increase in the time of heating on degradation. The change in color of oil due to molecular aggregation leads to a variation in the refractive index of the oil.[Citation29] This change, although very small, is noted to increase with increased time of heating. The ability of oil to bend the incident light increases with the angle of incidence. This is due to molecular clustering. Thus, viscosity and refractive index are positively correlated and their correlation significance is shown to be good as observed.
CONCLUSION
The study illustrates that viscosity decreases with increase in temperature due to the molecular interaction and the modeling exhibits the accuracy of data that could be used in pipelining design for oil transfer. From the study of Arrhenius, Andrade, Walther, Wright, and Erying models, Walter was found to be the best model. The physicochemical properties between sesame and mustard oil have been studied with increased times of heating. This aids in the selection of oil during repeated cycles of heating with less adverse effects in the food industry. The correlation between the parameters could be used in indexing the quality of oil and also in real-time measurement.
ACKNOWLEDGMENTs
The authors gratefully acknowledge the Vice Chancellor, SASTRA University, for the support to carry out the research work in the University lab and for their constant encouragement.
REFERENCES
- Makni, M.; Haddar, A.; Fraj, A.B.; Zeghal, N. Physico-Chemical Properties, Composition, and Oxidative Stability of Olive and Soybean Oils under Different Conditions. International Journal of Food Properties 2011, 18(1), 194–204.
- Bansal, G.; Zhou, W.; Philip, J. B.; Ho, H.-L.; Neo, F.-L. Performance of Palm Olein in Repeated Deep Frying and Controlled Heating Process. Food Chemistry 2010, 121, 338–347.
- Fasina, O.O.; Hallman, H.; Schmidt, C.M.; Clements, C.; Predicting Temperature Dependence Viscosity of Vegetable Oils from Fatty Acid Composition. Journal of American Oil Chemical Society 2006, 83, 899–903.
- Williams, D. G. The Chemistry of Essential Oils; Micelle Press: Dorset, England, 2008; pp. 248–316.
- Frankel, E.N. Antioxidants in Lipid Foods and Their Impact on Food Quality. Food Chemistry 1996, 57, 51–55.
- Chang S.S.; Peterson, R.J.; Ho, C.T. Chemical Reactions Involved in the Deep Fat Frying of Foods. Journal of the American Oil Chemists’ Society 1978, 55, 718–727.
- Benedito, J.; Mulet, A.; Velasco, J.; Dobarganes, C.M. Ultrasonic Assessment of Oil Quality During Frying. Journal of Agricultural and Food Chemistry 2002, 50, 531–4536.
- Rubalya, V.S.; Chandiramouli, R.; Neelamegam, P. Detection of Adulteration in Olive Oil Using Rheological and Ultrasonic Parameters. International Food Research Journal 2013, 20, 3197–3202.
- Rubalya, V.S.; Neelamegam, P. Study of Rheological Behavior and Thermal Degradation in Vegetable Oils on Heating. Asian Journal of Chemistry 2012, 24, 1975–1978.
- Ghosh, P.K.; Bhattacharjee, P.; Alternative Methods of Frying and Antioxidant Stability in Sesame and Mustard Oils. Acta Alimentaria 2013, 42(1), 109–123.
- Mohamed, H.M.A.; Awatif, I.I. The Use of Sesame Oil Unsaponifiable Matter As a Natural Antioxidant. Food Chemistry 1998, 62, 269–276.
- Hosahalli, S.R.; Chen, C.R.; Rattan, N.S. Comparison of Viscoelastic Properties of Set and Stirred Yogurts Made from High Pressure and Thermally Treated Milks. International Journal of Food Properties 2015, 18(7), 1513–1523.
- Abramovic, H.; Klofutar, C.; The Temperature Dependence of Dynamic Viscosity for Some Vegetable Oil. Acta Chimica Slovenica 1998, 45, 69–77.
- Seeton, C.J. Viscosity–Temperature Correlation for Liquids. Tribology Letters 2006, 22(1), 67–78.
- Mehrotra, A.K. Development of Mixing Rules for Predicting the Viscosity of Bitumen and Its Fractions Blended with Toluene. The Canadian Journal of Chemical Engineering 1990, 68, 839–848.
- Ali, A.; Nain, A.K.; Chanda, D.; Ahmad, R.; Viscosities and Refractive Indices of Binary Mixtures of Dimethyl Sulphoxide with Some Aromatic Hydrocarbons at Different Temperatures: An Experimental and Theoretical Study. Journal of the Chinese Chemical Society 2006, 53, 531–543.
- Abu-Eishah, S.I.; A New Correlation for Prediction of the Kinematic Viscosity of Crude Oil Fractions as a Function of Temperature, API Gravity, and 50% Boiling-Point Temperature. International Journal of Thermophysics 1999, 20(5), 1425–1434.
- Fountain, C.W.; Jennings, J.; Mckie, C.; Oakman, P.; Fetterolf, M.L. Viscosity of Common Seed and Vegetable Oils. Journal of Chemical Education 1997, 74, 224–227.
- Kim, J.; Kim, D.N.; Lee, S.H.; Yoo, S.-H.; Lee, S. Correlation of Fatty Acid Composition of Vegetable Oils with Rheological Behavior and Oil Uptake. Food Chemistry 2010, 118, 398–402.
- Hassanien, M.F.R.; Sharoba, A.M. Rheological Characteristics of Vegetable Oils As Affected by Deep Frying of French Fries. Food Measurement and Characterization 2014, 8, 171–179.
- Prevc, T.; Cigic, B.; Vidrih, R.; Ulrih, N.P.; Segatin, N. Correlation of Basic Oil Quality Indices and Electrical Properties of Model Vegetable Oil Systems. Journal of Agricultural Food Chemistry 2013, 61, 11355−11362.
- Hui, Y.H. Bailey’s Industrial Oil and Fat Products Edible Oil and Fat Products. New York, NY: Wiley-Interscience Publication 1999, 2, 603–675.
- Izbain, D.; Faiz, B.; Moudden, A.; Taifi, N.; Aboudaoud, I.; Evaluation of the Performance of Frying Oils Using An Ultrasonic Technique. Grasas Y Aceites 2010, 61, 151–156.
- Benedito, J.; Jose, V.; Garcia-Perez; Dobarganes, C.M.; Mulet, A. Rapid Evaluation of Frying Oil Degradation Using Ultrasonic Technology. Food Research International 2007, 40, 406–414.
- Chen, W.-A.; Chiu, C.P.; Cheng, W.-C.; Hsu, C.-K.; Kuo, M.-I. Total Polar Compounds and Acid Values of Repeatedly Used Frying Oils Measured by Standard and Rapid Methods. Journal of Food and Drug Analysis 2013, 21(1), 58–65.
- Shahidi, F. Bailey’s Industrial Oil and Fat Products, 6th Ed; Wiley-Inter Science Publication: New York, NY, 2005; (Vol. 2, Chap. 12).
- Gopinath, A.; Puzhan, S.; Nagarajan, G. Theoretical Modeling of Iodine Value and Saponification Value of Biodiesel Fuels from Their Fatty Acid Composition. Renewable Energy 2009, 34, 1806–1811.
- Adolfo Valdes, F.; Garcia, B. A Study of the Evolution of the Physicochemical and Structural Characteristics of Olive and Sunflower Oils after Heating at Frying Temperatures. Food Chemistry 2006, 98, 214–219.
- Wai, T.N.K. Local Repeatedly Used Deep Frying Oils Are Generally Safe. International Journal of Science, Medicine, and Education 2007, 1(2), 55–60.