Abstract
The effect of drying methods (freeze-drying and tray-drying) on the rheological, thermal and functional properties of truffle flour dispersion was studied. Sieve analysis showed the number of finest particles in the freeze-drying samples were significantly higher than the tray-drying samples whereas no significant difference was observed in the particle size distribution through laser diffraction measurement. The freeze-drying sample had significantly higher water holding capacity, sediment volume fractions, and superior instrumental color values than the tray-drying sample. Upon thermal scanning, two distinct starch-lipid complex melting transitions for the freeze-drying and one for the tray-drying sample were detected. Contrary to differential scanning calorimetric measurement, the oscillatory shear measurement successfully detected a distinct gelatinization temperature for the freeze-drying sample between 64 and 67.6°C. The mechanical rigidity of the dispersion was significantly influenced by the drying method and the freeze-drying sample exhibited the higher mechanical strength over the tray-drying sample throughout the frequency range (0.1 to 10 Hz). Viscoelasticity of the dispersion was estimated by a power-type relationship correlating the elastic modulus and frequency. Various shapes of truffle particles including rod, round, oval, and irregular were observed through the scanning electron microscope. This study provided in-depth knowledge on structural/rheological properties of truffle powder produced by two drying processes.
INTRODUCTION
Truffles are hypogeous ascomycetous fungi belonging to the genus Tuber (Ascomycota, Pezizales) exceptionally appreciated, mostly because of their excellent flavor and sensory properties.[Citation1,Citation2] Some species of truffles are among the most expensive foods and owe their high value due to the complex emanating aroma, even if today, unlike in the past, truffles are usually used as a flavoring rather than as food.[Citation3] Among desert truffles, the genera Terfezia and Balsamia are most important species because of their highly appreciated edible and commercial value, particularly in Mediterranean and Arabic peninsula countries.[Citation4] However, the desert truffles are not so intensely flavored like the European counterpart.[Citation5] In addition to unique aroma, there are sufficient scientific evidences that truffles have potential usages for therapeutic purposes.[Citation6]
Truffles are the mostly appreciated in fresh form due to its intense and distinctive aroma. Various volatile compounds are responsible for their aroma comprise a blend of alcohols, aldehydes, esters, ketones, and aromatic and sulfur compounds.[Citation7] Desert truffles are available for a limited period of time (February to April) in the Middle Eastern countries with a moderate price ($20–$70 per kg in Kuwait).[Citation8] Truffles are highly perishable mostly due to bacterial and mold growth, therefore, it is apparent to process those immediately to ensure the availability of this expensive commodity throughout the year.
Various preservation techniques have been reported in the literature for preservation of truffles including blanching, modified atmosphere packaging, canning, freezing, drying, pureeing, and even irradiation.[Citation7–Citation12] However, drying is the best way to preserve moist produce like truffles for longer periods and dehydrated truffles could be used as a valuable food ingredient in a variety of products (e.g., soup, sauce). Quality of dried powder depends upon type of drying, temperature used, duration, air velocity, and tray loading, etc. Furthermore, a higher drying temperature affects food components (e.g., starch and some proteins) negatively, and the extent of modification/degradation depends on source, moisture content, drying temperature, the presence of other compounds. The chemical and biological functions of truffles from different sources/locations are still unknown and, therefore, a thorough knowledge on the drying effect on functionality and physicochemical changes of truffle flour or powder is required.
Upon heating, food macromolecules undergo thermal transitions like starch gelatinization and protein denaturation and affects the functionality. Those thermal transitions and structural behavior of truffle dispersions can be measured through various fundamental rheological techniques like oscillatory rheological measurement which is very sensitive to changes in physical structure of the sample. The rheological properties of dried food powders are sensitive to mode of preparation and may be significantly altered by the drying processes. However, the rheological properties of reconstituted dispersions from truffles powder have not been sufficiently explored. To the best of our knowledge, there is no similar published report investigating the effect of different drying techniques on the rheological properties and functional properties of truffles. Therefore, these data could be useful for food formulation and process industries, and also process equipment design professionals. The main objectives of this work were to study the functional, rheological, and thermal properties of truffle powders in dispersions as affected by two drying processes namely tray-drying (TD) and freeze-drying (FD). Furthermore, microstructures of truffle powders were also examined.
MATERIALS AND METHODS
Materials
A single batch of white Zabide truffles (Tirmania nivea) was purchased from the local market in the state of Kuwait. The truffles were collected from the Kuwaiti desert during the winter season (January to March) of 2013–2014. The average weight of each truffle was 83 ± 3.5 g. Samples were washed thoroughly with running tap water to remove sands from the surface, peeled, and cut into small pieces with a sharp knife. Samples were divided into two batches for drying. The first batch was placed inside a tray dryer (Harvest Saver, Model R5A, Commercial Dehydrator Systems, Inc., OR, USA) for drying at a constant temperature of 60°C, 30% of relative humidity, and at a constant air velocity of 2.9 m s–1 until the sample achieved constant moisture content. The selection of temperature was based on some preliminary runs at selected temperatures (50, 55, 60, and 65°C) and it was observed that the color and texture of the product was superior at 60°C than other temperatures. The drying period was about 11 h until the moisture content became constant. The second batch was dried through FD. The samples were frozen in a freezer, and later transferred to the freeze-drier (GAMMA 2-16 LSC; Martin Christ GmbH, Osterode am Harz, Germany) for 38 h at a temperature between −47 and −50°C, and a pressure of 0.7 Pa.
Sieve Analysis
Truffle samples were ground in a laboratory grinder (Robot Coupe R5, France), and passed through a series of U.S. Standard sieve numbers 20-mesh (841-μm), 30-mesh (595-μm), 50-mesh (297-μm), 100-mesh (149-μm), 140-mesh (105-μm), 200-mesh (74-μm), and 230-mesh (63-μm; Endecotts, London, UK), following the method described by Ahmed et al.[Citation13] The fractions retained in each sieve after the sieving were designated as 595 (–30; +50), 297 (–50; +100), 149 (–100; +140), 105 (–140; +200), and 74 (–200; +230) μm. The –ve sign represents truffle particles passed through the sieve and the retained particles are expressed through +ve sign. Fraction yields were calculated. The fractionation process was performed in triplicate. Fractionated samples were packed in amber glass bottles and stored at 5°C till further use.
Particle Size Distribution (PSD) Using Light Scattering
The particle-size distribution of the truffle flours was measured by laser light scattering using a Malvern Mastersizer 3000 instrument (Malvern Instruments Ltd., Worcestershire, UK) attached to Hydro EV Flexible volume wet dispersion. The lens used had a focal length of 300 mm which allowed particle size measurement from 0.01 to 3500 μm. The PSDs, i.e., particle size at 10% (Dv10), 50% ([Dv50], median diameter), 90% (Dv90) of the volume distribution were all calculated automatically using the Mastersizer 3000 software based on Fraunhofer theory. The measurement was carried out in triplicate.
Physico-Chemical Properties
The proximate compositions of the fresh and the dried truffle flours were analyzed according to AOAC methods[Citation14] for the determination of moisture, ash, and crude fat contents. Protein was calculated as nitrogen content (N) × 6.25. Protein for each particle fraction was estimated by elemental analysis procedure using a FLASH 2000 CHNS/O Analyzers (Thermo Scientific, Germany) based on combustion method.[Citation14] The loose bulk density was determined by weighing the mass of the dried powder sample which freely was poured in a 100 mL graduated cylinder and expressed as weight per unit volume (kg/m3; ASTM D7481−09). The volume of bulk aggregate material includes the volume of the individual particles and the volume of the voids between the particles. Water activity of the dried truffle flours was measured using water activity meter (Aqualab 3TE series, Decagon Devises, Pullman, WA). Duplicate samples were measured at 25 ± 1°C. Total starch content was estimated by Megazyme Total Starch Assay Kits (Megazyme International, Wicklow, Ireland) following the method described in the instructions.
Water and Oil Holding Capacity
The water holding capacity (WHC) of the truffle samples was determined according to the method described by McConnell et al.[Citation15] at 25 and 70°C. Briefly, 100 mg truffle samples were transferred to 10 mL of deionized water in a 50 mL centrifuge tube and stirred for 6 h at 25°C. The mixture was centrifuged at 14,000 × g for 15 min and the supernatant was carefully decanted. WHC was expressed as g of water adsorbed/g sample. The oil holding capacity of truffle samples was determined according to the method described by Caprez et al.[Citation16] Briefly, 10 mL of canola oil was transferred to 100 mg truffle flour in a 50 mL centrifuge tube, and stirred well. The resultant mixture was centrifuged at 1500 × g for 30 min. Oil holding capacity (OHC) was expressed as g oil absorbed/g sample at 25°C.
Determination of Sediment Volume Fraction
The volume fraction of the truffles flour was measured using a simple centrifugation method as described by Hemar et al.[Citation17] with some modification. The measurement was carried out at 25 and 70°C. Simply, 1 g of flour was dispersed in 20 mL deionized water in a graduated centrifuge tube, mixed well in a vortex and kept for 6 h for hydration followed by centrifugation (Beckman GS-6R, USA) at constant centrifugation force (3000 × g) for 60 min. After centrifugation, the total height HT of the sample and the height of the sediment HS were measured and the effective volume fraction ϕ occupied by the truffle particles was expressed as
Equation (1) is valid only for closely packed particles without interstitial space and without damage during the packing. The volume fraction determination was performed at least in duplicate.
Tristimulus Color Measurement
Visual color was measured using a Hunter colorimeter model ColorFlex (Hunter Associates Laboratory, Reston, VA) in terms of L (lightness), a (redness and greenness), and b (yellowness and blueness) as described earlier by Ahmed et al.[Citation18] The instrument (45°/0° geometry, 10° observer) was calibrated with a standard black and white tile followed by measurement of samples. A glass cell containing the truffle powder sample was placed above the light source and L, a, and b values were recorded. Color measurements were taken in triplicates, and average values were taken for calculation.
Differential Scanning Calorimetric (DSC) Measurement
A differential scanning calorimeter (TA Q 2000, TA Instruments, New Castle, DE, USA) was employed to measure the thermal analysis of truffle dispersion. The DSC was calibrated with indium and sapphire for temperature and heat capacity calibration. The samples (10–12 mg) were heated from –20 to 150°C at a heating rate of 2.5, 5, 10, and 20°C/min in a nitrogen atmosphere (flow rate 50 mL/min). The thermal properties of the dispersions were detected during the scanning. An empty pan was used as a reference. The DSC measurements were done in triplicate. Instrument software provides the onset temperature, end point temperature, and the change of heat flow of the glass transition region. The enthalpy (∆H) of the transition (associated with starch-lipid complex) was calculated from the area of the peak endotherm using the Universal Analysis Software (version 4.5A, TA Instruments, New Castle, DE, USA).
SEM Observation for Average Particle Size and Tissue Structure
The microstructure and particle dimension of truffle particles were examined through a scanning electron microscope (SEM; JEOL, JSM-5410LV, Tokyo, Japan). Each sample was coated with gold in a sputter coater (Structure Probe, West Chester, PA) before being scanned and photographed at various magnifications (30, 50, 200, 500, and 1000×). Particle size was measured by the software attached to the instrument which allows for detailed (average particle diameter and maximum length) measurements. About 100 particles were chosen randomly for the particle size measurement.
Rheological Measurement
Oscillatory rheological measurements of truffle dispersions were carried out using a Discovery Hybrid Rheometer HR-3 (TA Instruments, New Castle, DE, USA) as per method described earlier by Ahmed and Thomas.[Citation19] The powder (flour) to water (F/W) ratio in the dispersion was 1 to 3 for the rheological measurement. Samples were placed in a 1500-μm gap between two stainless steel parallel plates (plate diameter 40 mm) and the perimeter of the sample was covered with a thin layer of high-temperature-resistant silicone oil to prevent sample dehydration. The sample temperature was controlled by a Peltier system and monitored by platinum resistance thermometer sensors (accuracy of ± 0.1°C) which are positioned at the upper and lower plates. The studied temperature range for oscillatory measurement was 25 to 85°C.
Small-amplitude oscillatory strain sweep experiments (0.001 to 10%) were performed, and the elastic (G′) and viscous (G″) shear moduli, at a constant frequency of 0.1 Hz were monitored to determine the limit of the linear visco-elastic region (LVR). The LVR was carried out for the entire studied temperature range (data are not shown), and the measurement was carried out accordingly. Frequency sweep tests (0.1–10 Hz) were carried out in the linear regime, at constant strain (0.1%) at selected temperatures. Following an initial equilibration of samples for 5 min at 25°C, ramp heating was carried out at selected heating rate 2.5, 5, and 10, 20°C/min to an endpoint of 95°C (non-isothermal heating) at a frequency of 1 Hz. All rheological measurements were carried out in triplicate and rheological parameters were obtained directly from the manufacturer supplied computer software (TRIOS, TA Instruments, New Castle, DE, USA).
Statistical Analysis
The regression analysis, in particular the analysis of variance (ANOVA) of data was carried out using the Minitab Statistical Software (Version 16; Minitab Corp., USA). Lack-of-fit tests were performed on the fitted models. For statistical tests, significance was defined at p ≤ 0.05.
RESULTS AND DISCUSSION
Proximate Composition
The average moisture content of fresh truffles was 76.3% (db) which is in close agreement with the reported value by Al-Laith[Citation20] for samples collected from the neighboring countries such as Saudi Arabia and Bahrain. Drying of truffles slices significantly reduced the moisture content. The moisture contents for the TD and FD samples were 4.10 and 2.15% (dry basis), respectively. The ash contents and crude fibers of those powders were not significantly affected by the drying process (). The ash contents (4.15–4.35%) are close to those previously reported values (4.9–5.9%) for different desert truffles.[Citation21] The drying methods did not cause a significant change in the fat (5.88–5.91%) and protein contents (24–24.5%). The values of protein content obtained in this study are within reported value of 26%.[Citation22] However, the value was significantly higher than the value (16%) as reported by Bokhary and Parvez.[Citation23] The variation in protein content within the truffle strain is mostly attributed by the fungi cultivars, sources, tissue types, and geographical locations, and also in accuracy of the analytical methods used.
TABLE 1 Physico-chemical properties of tray and freeze-dried truffle flours
Sieve Analysis
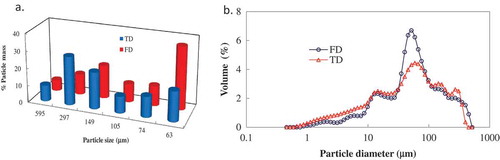
Dried truffles showed a broader distribution of particle sizes. The particle mass (%) differed significantly between samples obtained by two different drying processes as illustrated in . For the TD samples, it was observed that the maximum weight percent of particles were about 297 μm (≈28%) followed by 149 μm (≈21%), 63 μm (≈17%), 74 μm (≈12%), 105 μm (≈10%), and 595 μm (≈9%). The maximum weight percent of the FD particles were about 63 μm (≈36%) followed by 149 μm (≈19%), 297 μm (≈12%), 74 μm (≈12%), 105 μm (≈11%), and 595 μm (≈6%), respectively. Overall, the FD samples contained finer particle fractions than the TD sample. It has been reported that the size reduction could depend on the type of grinding forces involved as well as the internal structure of the sample.[Citation24]
Both TD and FD truffle flours showed similar bimodal PSDs in dispersion as demonstrated by laser diffraction analysis (). Interestingly, no particles larger than 516–μm was found in the size distribution and it supported the sieve analysis adequately. Particle sizes at 10% (Dv10), 50% (Dv50), and 90% (Dv90) volume distribution of the TD sample were 5.49-, 52.1-, and 232-μm and the corresponding values for the FD samples were 11.3-, 57.3-, and 243-μm. The median PSD values indicated that no significant differences observed between the two samples. Those observed particle sizes are within the range of reported values (63-380-μm) for mushrooms.[Citation25]
Physico-Chemical Properties
Physico-chemical properties of truffle powders are presented in . The loose bulk density of truffle samples is significantly influenced by the drying methods. The bulk density of the FD sample was significantly lower (347 kg/m3) than that of the TD sample (753 kg/m3). It indicates the lighter mass of the FD sample with porous structure than the powder sample produced by the TD process. The water activity values of the dried samples (0.17–0.20) confirmed their shelf stability during storage. The lower total starch contents (7.2%) of the TD sample over the FD sample (8.9%) supports possible starch damage during the hot-air drying.
The drying methods had significant effect on the WHC of the powders. The WHC of the FD samples was significantly higher (4.75 and 4.13 g/g, dry basis) than the TD sample (3.84 and 3.77 g/g, dry basis) at 25 and 70°C (). The pore size distributions in the internal structure have been contributed toward the higher WHC of FD truffles. In the case of TD sample, it was difficult for water to intrude within the truffle matrix because the surface of truffle formed a crust during hot-air drying at 60°C. Similar range of WHC values had been reported earlier for mushroom powder.[Citation26] Broadly, the WHC is a function of temperature, and therefore, it is expected that the WHC values should increase with temperature; however, a reverse trend had been observed at 70°C for both samples. The drop in the WHC at higher temperature could be associated with presence of lower amount of starch in the truffle sample, and furthermore, the swelling during heating was not sufficient compared to other starch-rich sample. The OHC of the TD sample was lower (0.78 g/g) than the FD sample (1.16 g/g). An increase in OHC for the FD sample is possibly attributed by more porous structure of the sample that developed during the FD process.
The volume fraction (ϕ) of suspended particles in aqueous dispersions of truffle flours at 25 and 70°C is shown in . The volume fraction measures the particles occupancy after the centrifugation and it generally estimates the effective volume fraction of the particles. At 25°C, the ϕ value for the FD sample was higher than the TD sample, whereas the values reversed at higher temperature. The ϕ value increased significantly at 70°C for the TD sample over the FD sample. Mostly, the magnitude of ϕ for powdery materials is a direct function of the particle sizes. At 70°C, the coarser particles enriched tray dried powdery sample exhibited significant effect on the ϕ value. In our earlier study on pumpkin flour,[Citation27] it is observed that more water was absorbed by porous particles compared to finer particles and the observation was also supported by the microscopic study.
Color of Truffle Flours
As can be seen from the , the type of drying process significantly affected the tristimulus color values. The application of TD significantly reduced the lightness, L, value (80.2) compared to the FD sample (90.0) and the product became darker. Other color parameters namely the redness (+a value) and yellowness (+b value) values of the TD sample (15.4 and 46.7) were abruptly increased as compared to the FD sample (1.25 and 25.6). The loss of lightness in TD process could be attributed to browning due to Maillard reactions,[Citation28] and also the dark color of truffle sample during TD was facilitated by higher temperature and oxygen content in drying operations.[Citation29] An increase in yellow color and red color after TD is most probably attributed to enzymatic browning of truffles.
Thermal Properties
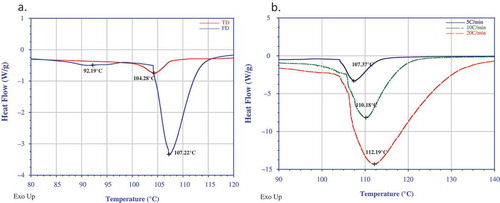
Drying has significant effect on thermal properties of truffle flours. Thermal transitions of both TD and FD truffle flours in dispersions were studied as function of water content (F/W of 1:2; 1:3 and 1:4) and heating rate (2.5, 5, 10, and 20°C/min). Truffle flour undergoes structural and morphological changes during heating in excess of water. However, no gelatinization peak was detected during the DSC measurement. Typical thermograms for both truffle dispersions (F/W = 1:3) at 5°C/min are illustrated in . For the FD dispersion, two distinct transitions were detected, whereas only single peak was found for the TD sample during thermal scanning. The enthalpy values of the FD samples were significantly higher than the TD samples. As expected, the peak transition temperature (Td) shifted to lower values as F/W ratio increased from 1:2 to 1:4 due to plasticizing effect of water. For example, the Td values of the FD sample were 112 and 124°C at F/W ratio of 1:2 and those values dropped drastically to 91 and 109°C when the F/W ratio changed to 1:3. The TD samples followed a similar drop in the Td value. In the earlier publication, two similar peaks were detected in truffle puree sample and the peak detected in the higher temperature range could be attributed to protein-lipid complex.[Citation8] The absence of the second peak for the TD sample in the higher temperature range could be associated with hot-air drying, and associated thermal effect on the sample. The Td values also shifted to higher values as the heating rate increased from 5 to 20°C/min ().
Rheology of Truffle Flour Dispersions
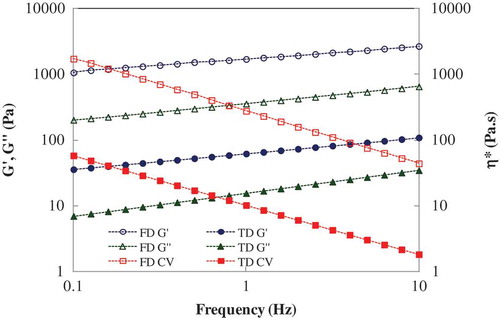
Typical mechanical spectra of both TD and FD truffle flour dispersion (F/W = 1:3) at 40°C are shown in . The elastic modulus (G′) showed predominance over the viscous modulus (G′′) throughout the frequency range for both samples, and therefore, the truffle dispersion typically behaved like a biopolymer gel. As it can be seen from the figure, all dynamic modulii G′, G′′ and η* of the FD sample were significantly higher than the TD sample. Both G′ and G′′ increased systematically within the studied frequency range (0.1 to 10 Hz) and confirmed the frequency-dependency of the truffle dispersion. The complex viscosity, η* decreased with increasing frequency from 0.1 to 10 Hz whereas the phase angle, δ varied from 11 to 14° at the similar condition. The δ values confirmed the viscoelasticity of the dispersion.
Effect of Non-Isothermal Heating
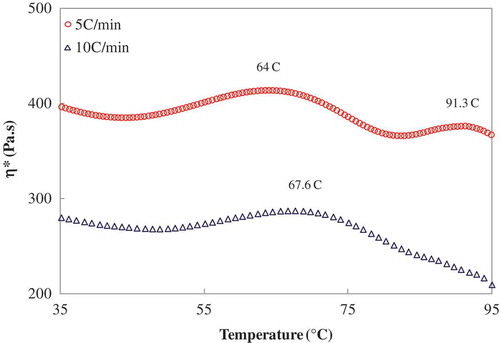
The truffle flour dispersions were heated non-isothermally from 25 to 95°C at selected heating rate (2.5, 5, 10, and 20°C/min) at a constant frequency of 1 Hz. The TD sample did not exhibit any thermal transition during heat treatment irrespective of heating rate (data are not shown). This can be attributed to thermal degradation of the polysaccharides/proteins present in truffles. The high drying temperature could cause partial hydrolysis of the sample and possibly generates smaller molecules of the original polymer. However, the FD sample showed distinct thermal transitions at similar condition. Typical non-isothermal heating of the FD truffle dispersions are illustrated in . Contrary to DSC scan, a distinct thermal transition was detected in η*-T curve which is attributed by swelling of starch granules and gelatinization where amylopectin crystallites melted during the thermal exposure, and amylose leached during the process. It can be argued that rheometric measurement is more precise and authentic to detect thermal transition even the starch content is too low (sample contains only about 8% starch). The peak starch gelatinization temperature (Tp) of the truffle dispersion shifted to higher temperature as expected when the heating rate was increased from 2.5 to 20°C. For example, the Tp value shifted from 64 to 67.6°C when the heating rate increased from 5 to 10°C/min as shown in . A second thermal peak was detected between 88 and 91°C when the heating rate was maintained between 2.5 and 5°C/min. It indicates that the second peak was heating rate dependent, and at higher heating rate the peak was not traceable. The appearance of the peak has been supported by the DSC measurement and it is believed this peak is attributed by protein denaturation of the truffle sample. However, the DSC Tp value was marginally higher (91°C) than the rheometric Tp value (88°C) at similar F/W ratio (1:3) and heating rate (5°C/min). The difference could be explained from the sensitivity of the instrument, sample size and other factors.
Effect of Isothermal Heating
Isothermal heating of the FD and the TD truffle dispersions (F/W = 1:3) at selected temperatures are presented in and , respectively. There was significant difference in mechanical rigidity (G′) between FD and hot-air-dried truffle dispersions. Generally, the mechanical strength in dispersion is influenced by the particle size and its distribution, molecular weight, and ratio of soluble to insoluble matters.[Citation28,Citation30] Although, there was significant variation in particle size between two samples; however, the particle size and its distribution has the least effect, whereas other factors (e.g., composition, particle surface properties) dominate the mechanical strength of the truffle dispersions.
FIGURE 5 Mechanical spectra of truffle flour dispersions (flour to water ratio of 1:3) at selected temperatures (a) Freeze-dried and (b) tray-dried sample.
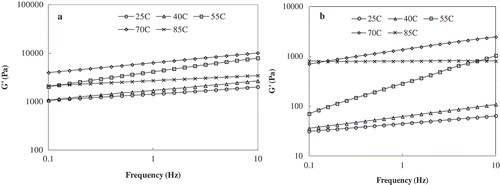
At a constant temperature, the TD dispersion exhibited significantly lower G′ value than the FD sample within the frequency range studied. The G′ value did not change significantly with frequency when the measurement temperature increased from 25 to 40°C; however, the G′ value increased drastically at and above 55°C which is at close vicinity of starch gelatinization temperature and also attributed by the swelling of starch granules. The G′ value of both TD and FD samples dropped significantly during isothermal heating at 85°C, which is believed to be associated with the melting of starch crystallites and leaching of amylose in hot paste above the gelatinization temperature. The significant difference in mechanical behavior could be due to the substantial impact of drying process on the chemical composition of the truffle sample. A comparison between the two drying methods on green peppers indicated that the texture was more sensitive to hot-air convective drying, and particularly at the higher temperature, than the freeze drying process.[Citation31] Furthermore, Giri and Prasad[Citation32] reported that the FD mushroom showed greater rehydration capacity than hot-air drying because the internal structure of the product remains quite undistorted.
The viscoelasticity of truffle dispersions can be evaluated by logarithmic plot of elastic modulus (G′) versus frequency (ω) following the power-type relationship:
where, A is a constant and n is the frequency exponent (slope). The intercept corresponds to the magnitude of G′ and the slope will provide information about the frequency dependency of the sample. Three-dimensional polymer networks should have zero slope, whereas low values would indicate an intermediate network structure containing highly cross-linked material mixed with some uncross-linked ones.[Citation33]
provides the magnitudes of the slopes, intercepts, and the goodness of fitting (R2) for truffle dispersions. Experimental data were fitted well with R2 values being always higher than 0.99. An increase of the temperature resulted in a steady increase of the intercept of G′, with the exception at 85°C where a decrease in intercept was observed and the trends with temperature follow the aforementioned behavior for G′ and η*. It is evident from the table that temperature had a significant effect on the solid-like properties as assessed by slope, n. The slope of the equation for both FD and TD samples gradually increased from 25 to 55°C, and decreased, thereafter. Those values indicate that the truffle dispersions exhibited less solid-like property below 70°C, whereas the sample heated at 85°C exhibited the peak solid-like property.
TABLE 2 Slope of equation (2) of tray and freeze-dried truffle dispersions (flour to water ratio of 1:3) as function of temperature
SEM Analysis
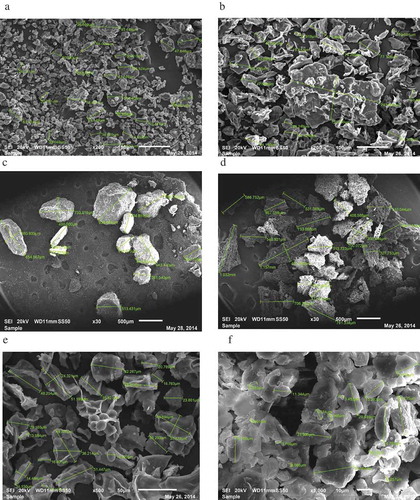
The scanning electron micrographs of truffle flour produced by the two drying methods are shown in . The particle images were obtained from SEM at selected magnification (30, 200, 500, and 1000×). These images are typical for all samples investigated showing a strong heterogeneity in shape and size, and similar observation was found for truffle puree.[Citation8] The truffle particles exhibited various shapes including rod, round, oval, and irregular. The microstructure of the whole FD sample was more distinct than the TD sample ( and ) and as shown in this figure ( and ), the FD sample possessed a large number of pores and honeycomb-like microstructure. Contrary to the TD sample, the FD truffles had a uniform and small porous structure and there is little or no damage of cell walls on the surface. The largest and the smallest particle diameters of TD truffle samples were 939 and 2 μm with an average value of 146 μm; the corresponding values for the FD sample are 1157 and 3 μm, respectively, with an average value of 176 μm. The FD starch granules are very distinct at higher magnification than the TD ( and ). It has been reported that starch granules lost their integrity in a high degree if the drying operation is carried out at high temperature and the gelatinization will be faster in starch granules.[Citation34]
FIGURE 6 Scanning electron microscopy of truffle flours at different magnifications (a) whole TD sample 200×; (b) whole FD sample 200×; (c) 297 mm particle enriched TD sample 30×; (d) 297 mm particle enriched FD sample 30×; (e) 63 mm particle enriched FD sample 500× and (f) 63 mm particle enriched TD sample 1000×.
CONCLUSIONS
Drying has a potential to preserve excess truffle production during glut season and could be used as a food ingredient in the food formulation for its characteristics aroma to truffle lovers. In this work, two drying methods (TD and FD) have been compared for their functionality and rheological properties in dispersions. It has been observed that the FD process resulted in superior powder quality (e.g., color, WHC, thermal property) compared to the TD product. Thermal transitions associated to starch gelatinization and protein denaturation of truffle dispersion was detected by oscillatory rheological measurement, whereas the DSC scanning could not able to trace the starch gelatinization at similar condition. Overall, the truffle dispersions showed predominantly solid-like behavior and the FD dispersions exhibited significantly higher mechanical strength than the TD sample. The significant difference in mechanical properties of truffle dispersions indicated that the hot-air drying significantly affects the chemical composition and microstructure of the resulting powders. Those studies provided valuable information for understanding the applicability of drying techniques for newer product.
FUNDING
The authors express their gratitude to Kuwait Institute for Scientific Research for providing the grant for the research work (Grant number FB098K).
ACKNOWLEDGMENTS
Dr. Abdulsalam and Shaji Samuel are acknowledged for providing the SEM facility and other technical assistance.
Additional information
Funding
REFERENCES
- Cullere, L.; Cacho, J.; Ferreira, V. Comparative Study of the Aromatic Profile of Different Kinds of Wine Cork Stoppers. Food Chemistry 2009, 112, 381–387.
- Villares, A.; García-Lafuente, A.; Guillamón, E.; Ramos, A. Identification and Quantification of Ergosterol and Phenolic Compounds Occurring in Tuber spp. Truffles. Journal of Food Composition and Analysis 2012, 26, 177–182.
- Pacionia, G.; Cerretani, L.; Procidac, G.; Cichelli, A. Composition of Commercial Truffle Flavored Oils with GC–MS Analysis and Discrimination with An Electronic Nose. Food Chemistry 2014, 146, 30–35.
- Pérez-Gilabert, M.; Morte, A.; Honrubia, M.; García-Carmona, F. Partial Purification, Characterization, and Histochemical Localization of Fully Latent Desert Truffle (Terfezia claVeryi Chatin) Polyphenol Oxidase. Journal of Agricultural Food Chemistry 2001, 49, 1922–1927.
- Al-Sheik, A.M.; Trappe, J.M. Desert Truffles: The Genus Tirmania. Transactions of the British Mycological Society 1983, 81, 83–90.
- Hannan, M.A.; Al-Dakan, A.A.; Aboul-Enein, H.Y.; Al-Othaimeen, A.A. Mutagenic and Antimutagenic Factor(S) Extracted from Desert Mushroom Using Different Solvents. Mutagenesis 1989, 4, 111–114.
- Palacios, I.; Guillamon, E.; Garcia-Lafuente, A.; Villares, A. Effects of Freeze-Drying Treatment on the Aromatic Profile of Tuber spp. Truffles. Journal of Food Processing and Preservation 2012, 174, 4549, 1–6.
- Ahmed, J. Effect of pH and Temperature on Rheological and Calorimetric Behaviour of Desert Truffles (Terfezia claveryi). Food Research International 2013, 54, 1813–1820.
- Al-Ruqaie, I.M. Effect of Different Treatment Processes and Preservation Methods on the Quality of Truffles: I. Conventional Methods (Drying/Freezing). Journal of Food Processing & Preservation 2006, 30, 335–351.
- Murcia, M.A.; Martinez-Tome, M.; Jimenez, A.M.; Vera, A.M.; Honrubia, M.; Parras, P. Antioxidant Activity of Edible Fungi (Truffles And Mushrooms): Losses During Industrial Processing. Journal of Food Protection 2002, 65, 1614–1622.
- Rivera, C.S.; Blanco, D.; Salvador, M.L.; Venturini, M.E. Shelf-Life Extension of Fresh Tuber Aestivum and Tuber Melanosporum Truffles by Modified Atmosphere Packaging with Microperforated Films. Journal of Food Science 2010, 75, E225–E233.
- Culleré, L.; Ferreira, V.; Venturini, M.E.; Marco, P.; Blanco, D. Chemical and Sensory Effects of the Freezing Process on the Aroma Profile of Black Truffles (Tuber Melanosporum). Food Chemistry 2013, 136, 518–525.
- Ahmed, J.; Al-Jassar, S.; Thomas, L. A Comparison in Rheological, Thermal, and Structural Properties Between Indian Basmati and Egyptian Giza Rice Flour Dispersions As Influenced by Particle Size. Food Hydrocolloids 2015, 48, 72–83.
- AOAC INTERNATIONAL. Official Methods of Analysis of AOAC INTERNATIONAL, 17th Ed; AOAC INTERNATIONAL: Washington, DC, 2000.
- McConnell, A.A.; Eastwood, M.A.; Mitchell, W.D. Physical Characteristics of Vegetable Foodstuffs That Could Influence Bowel Function. Journal of the Science of Food and Agriculture 1974, 25, 1457–1464.
- Caprez, A.; Arigoni, E.; Amado, R.; Neukom, H. Influence of Different Types of Thermal Treatment on the Chemical Composition and Physical Properties of Wheat Bran. Journal of Cereal Science 1986, 4, 231–239.
- Hemar, Y.; Lebreton, S.; Xu, M.; Day, L. Small-Deformation Rheology Investigation of Rehydrated Cell Wall Particles—Xanthan Mixtures. Food Hydrocolloids 2011, 25, 668–676.
- Ahmed, J.; Shivhare, U.S.; Kaur, M. Thermal Colour Degradation Kinetics of Mango Puree. International Journal of Food Properties 2002, 5, 359–366.
- Ahmed, J.; Thomas, L. Effect of β-Glucan Concentrate on the Water Uptake, Rheological, and Textural Properties of Wheat Flour Dough. International Journal of Food Properties 2015, 18, 1801–1816.
- Al-Laith, A.A.A. Antioxidant Components and Antioxidant/Antiradical Activities of Desert Truffle (Tirmania Nivea) from Various Middle Eastern Origins. Journal of Food Composition and Analysis 2010, 23, 15–22.
- Hussain, G.; Al-Ruqaie, I.M., Occurrence, Chemical Composition, and Nutritional Value of Truffle: An Overview. Pakistan Journal of Biological Sciences 1999, 2, 510–514.
- Hamza, A.; Zouari, N.; Zouari, S.; Jdir, H.; Zaidi, S.; Gtari, M.; Neffati, M. Nutraceutical Potential, Antioxidant, and Antibacterial Activities of Terfezia Boudieri Chatin: A Wild Edible Desert Truffle from Tunisia Arid Zone. Arabian Journal of Chemistry 2013, in press.
- Bokhary, H.A.; Parvez, S. Chemical Composition of Desert Truffles Terfezia claveryi. Journal of Food Composition and Analysis 1993, 6, 285–293.
- Vishwanathan, K.H.; Subramanian, R. Particle Size Characteristics of Ground Soy and Red Gram. International Journal of Food Properties 2014, 17, 1469–1481.
- Kim, J.; Lim, J.; Bae, I.Y.; Park, H.; Lee, H.G.; Lee, S. Particle-Size Effect of Lentinus Edodes Mushroom (Cahamsong-I) Powder on the Physicochemical, Rheological, and Oil-Resisting Properties of Frying Batters. Journal of Texture Studies, 2010, 41, 381–395.
- Zhang, Z.; Song, H.; Peng, Z.; Luo, Q.; Ming, J.; Zhao, G. Characterization of Stipe and Cap Powders of Mushroom (Lentinus Edodes) Prepared by Different Grinding Methods. Journal of Food Engineering 2012, 109, 406–413.
- Ahmed, J.; Al-Foudari, M.; Al-Salman, F.; Almusallam, A.S. Effect of Particle Size and Temperature on Rheological, Thermal, and Structural Properties of Pumpkin Flour Dispersion. Journal of Food Engineering 2014, 124, 43–53.
- Pei, F.; Yang, W.J.; Shi, Y.; Sun, Y.; Mariga, A.M.; Zhao, L.Y.; Fang, Y.; Ma, N.; An, X.X.; Hu, Q.H. Comparison of Freeze-Drying with Three Different Combinations of Drying Methods and Their Influence on Colour, Texture, Microstructure, and Nutrient Retention of Button Mushroom (Agaricus Bisporus) Slices. Food Bioprocess Technology 2014, 7, 702–710.
- Cui, Z.-W.; Li, C.-Y.; Song, C.-F.; Song, Y. Combined Microwave–Vacuum and Freeze Drying of Carrot and Apple Chips. Drying Technology 2008, 26, 1517–1523.
- Wang, S.; Marcone, M.F. The Biochemistry and Biological Properties of the World’s Most Expensive Underground Edible Mushroom: Truffles. Food Research International 2011, 44, 2567–2581.
- Guinéa, R.P.F.; Barroca, M.J. Effect of Drying Treatments on Texture and Color of Vegetables (Pumpkin and Green Pepper). Food and Bioproducts Processing 2012, 90, 58–63.
- Giri, S.K.; Prasad, S. Quality and Moisture Sorption Characteristics of Microwave Vacuum, Air and Freeze-Dried Button Mushroom (Agaricus Bisporus). Journal of Food Processing and Preservation 2009, 33, 237–251.
- Kokini, J.L.; Cocero, A.M.; Madeka, H.; de Graaf, E. The development of State Diagrams for Cereal Proteins. Trends in Food Science and Technology 1994, 5, 281–288.
- Correia, P.; Leitão, A.; Beirão-da-Costa, M.L. The Effect of Drying Temperatures on Morphological and Chemical Properties of Dried Chestnuts Flours. Journal of Food Engineering 2009, 90, 325–332.