Abstract
This study analyzed the aromatic composition and established the soluble carbohydrate profiles of the Chriha, Razeki, Assli, and Meski varieties of Tunisian raisin (Vitis vinifera L.). A total of 80 compounds obtained by headspace solid phase micro-extraction were detected by gas chromatography-mass spectrometry. Non-terpene hydrocarbon derivatives were the major compounds of the Chriha and Assli varieties (35.8 and 26.3%, respectively). The Razeki variety accumulated 25.5%. apocarotenoids. Oxygenated non-terpene derivatives, including esters, alcohols, aldehydes, acids, ketones, and others were the most abundant (57.5%) in the Assli variety. The presence and amounts of volatiles were different among varieties and might be responsible, in part, for the aroma and taste of raisins. The concentration of total sugars was highest in the Razeki variety and lowest in the Chriha variety. The content of individual sugars differed quantitatively among the varieties in this study. The Razeki variety had the best fruit quality with higher contents of individual and total sugars compared to the other three varieties.
INTRODUCTION
Raisins (dried grapes; Vitis vinifera L.) are produced in most regions of the world where grapes are grown, and consumption occurs in all cultures and demographic sectors. Grapes were probably dried for storage and travel in the Neolithic period, leading to the early production of raisins, and there is evidence of the early use of raisins as food and decorations. Raisins are a favored dried fruit throughout the world because of their high nutritional value and pleasant flavor. They are rich in several micronutrients, including carotenoids, polyphenols, tocopherols, vitamins, and oligo elements, whose beneficial effects on human health are well known.[Citation1,Citation2] Drying methods have a significant impact on the sensory characteristics and consumer preference for raisins. The main difference between grapes and raisins is the water content and two main methods of drying grapes are used to produce raisins. One method is sun-drying for 2–3 weeks and the other is a short (15–20 s) exposure to hot water (87–93ºC) followed by dehydration at 71°C for 20–24 h.[Citation2,Citation3] Many new methods of dehydration have been developed, including microwave vacuum-drying,[Citation2–Citation4] dipping pretreatments to expedite the drying process or chamber-drying at a controlled temperature.[Citation2–Citation5] In Tunisia, sun-drying is the most ancient and most frequently used method, which consists of exposure to hot water (87–93°C) followed by drying for 2–3 weeks under direct sunlight.[Citation6] Chriha, Razeki, Asli, and Meski are the main varieties of raisin (Zbib) produced in Tunisia.
Analysis of the volatile compounds in plant extracts has attracted increasing interest due to the use of these components in both the food and pharmaceutical industries. Moreover, phenolic and aromatic compounds as well as carbohydrates are concentrated during grape dehydration.[Citation7–Citation10] These are very important parameters for judging the market quality of fruit.[Citation11] To evaluate the volatile compounds, various methods have been developed for their extraction, including hydrodistillation, supercritical fluid extraction and headspace solid phase micro-extraction (HS-SPME). HS-SPME is a simple, sensitive, and solvent-free sampling and concentration technique that can be used in combination with gas chromatography–mass spectrometry (GC–MS) for analysis of the volatile components of natural products and foods.[Citation12]
Only the physicochemical compositions, including moisture, ash, protein, fat, and some minerals, have been reported for the Chriha, Razeki, Assli, and Meski varieties.[Citation6] The objective of this study was to qualify and quantify the aromatic volatile composition and the carbohydrate profiles of different varieties of raisin (Vitis vinifera L.) produced in Tunisia.
EXPERIMENTAL
Plant Material
Four varieties of dried raisins (Vitisvinifera L.; Chriha, Raseki, Assli, and Meski) were harvested in August–mid September 2013 season from different regions of Tunisia: Chriha and Meski from Rafraf (located in the region of bizerte), Raseki from Tunis, and Assli from Sfax. Specimens have been deposited in the Herbarium of the Laboratory of Biochemistry, Faculty of Medicine of Monastir (Tunisia). Samples were immediately used for analysis of aromatic composition and carbohydrates profiles.
HS-SPME and GC–MS Analysis
SPME analyses were performed using a Supelco SPME device coated with polydimethylsiloxane (PDMS, 100 µm). The fiber was pre-conditioned according to the manufacturer instructions. After the equilibration time, the fiber was exposed to the headspace for 20 min at room temperature. When sampling finished, the fiber was withdrawn into the needle and transferred to the injection port of the GC or GC–MS system. GC analyses were accomplished with an HP-5890 series II instrument equipped with a DB-5 and DB-WAX capillary columns (30 m × 0.25 mm, 0.25 µm film thickness), using the following temperature program: 60°C for 10 min, ramp of 5°C/m into 220°C; injector and detector temperatures, 250°C; carrier gas, helium (2 mL/min); detector, dual flame ionization detection (FID); split ratio, 1:30. The identification of the components was performed, for both columns, by comparison of their retention times with those of pure authentic samples and by means of their linear retention indices (LRI) relative to the series of n-hydrocarbons. Gas chromatography–electron impact mass spectrometry (GC–EIMS) analyses were performed with a Varian CP 3800 gas chromatograph (Varian, Inc. Palo Alto, CA) equipped with a DB-5 capillary column (Agilent Technologies Hewlett-Packard, Waldbronn, Germany; 30 m × 0.25 mm, coating thickness 0.25 µm) and a Varian Saturn2000 ion trap mass detector. Analytical conditions were as follows: injector and transfer line temperature at 250 and 240°C, respectively; oven temperature was programmed from 60 to 240°C at 3°C/min; carrier gas, helium at 1 mL/min; splitless. Identification of the constituents was based on comparison of the retention times with those of the authentic samples, comparing their LRI relative to the series of n-hydrocarbons, and on computer matching against commercial NIST 98 (U.S. National Institute of Standards and Technology) and ADAMS and homemade library mass spectra built from pure substances and components of known samples and MS literature data.[Citation13–Citation18] Moreover, the molecular weights of all the identified substances were confirmed by gas chromatography–chemical ionization mass spectrometry (GC–CIMS), using methanol as chemical ionization gas. All the analyses were performed at least in triplicate.
Carbohydrates Analysis
Carbohydrates were extracted according to the method described by Bartolozzi et al.[Citation19] Extracts were dried and converted into trimethylsilyl ethers with a silylation mixture made up of pyridine, hexamethyldisilazane, and trimethylchlorosilane. An aliquot of 1 µL of each silylated total extract of the raisins samples was analyzed using a Hewlett-Packard 5890 series II gas chromatograph equipped with a FID system and a HP-5MS capillary column (30 m × 0.25 mm) as described by Bartolozzi et al.[Citation19] Using this program, 23.6 min were required to elute the trimethylsilyl derivatives. Identification of individual carbohydrates was achieved by the use of the relative retention times, i.e., in comparison with the standard used. These were compared to those identified earlier by GC–MS.
Statistical Analysis
All parameters analyzed were carried out in triplicate. The results were reported as mean values of three repetitions and standard deviation. The significance of differences between mean values was determined by one-way analysis of variance. A post-hoc analysis using Tukey’s test was carried out to compare the means, using the SPSS program, release 11.0 for Windows (SPSS, Chicago, IL, USA). Differences were considered to be significant when p < 0.05. Principal component analysis (PCA) was carried out using XLSTAT (2014) for Windows (Addinsoft, New York, USA).
RESULTS AND DISCUSSION
Volatile Compounds
The results of GC–MS analysis of the volatile compounds () present in different varieties of raisin obtained by HS-SPME are given in . A total of 80 volatile compounds belonging to seven chemical classes were identified; namely, five monoterpene hydrocarbons, 15 oxygenated monoterpenes (OM), one sesquiterpene hydrocarbon, six AP, two phenylpropanoids (PH), 44 oxygenated non-terpene derivatives (ONTD), and seven non-terpene hydrocarbon (NTH) derivatives. Using HS-SPME, a total of 17, 16, 15, and 26 compounds, accounting for 91.3, 90.6, 95.5, and 95.4% of the total aromatics were identified for the Chriha, Razeki, Assli, and Meski varieties, respectively. Only four of the 80 compounds identified were found in all four varieties; namely (E)-geranylacetone, n-tetradecane, n-pentadecane, and n-hexadecane.
TABLE 1 Composition of volatiles (%) extracted by head space micro extraction of Tunisian dried raisins
FIGURE 1 Volatile compounds chromatograms obtained from headspace micro extraction of four varieties of dried raisins (A [Chriha], B [Razeki], C [Assli] and D [Meski]).
![FIGURE 1 Volatile compounds chromatograms obtained from headspace micro extraction of four varieties of dried raisins (A [Chriha], B [Razeki], C [Assli] and D [Meski]).](/cms/asset/100f8501-97d1-4bf7-a3a2-edc0eb8ef5c5/ljfp_a_1027920_f0001_oc.jpg)
Our analysis showed the number of aromatic compounds differed among the raisin varieties. Each volatile compound is characterized by an odor threshold and even if different fruits have similar qualitative compositions, the aroma can differ considerably when the relative proportions differ.[Citation20,Citation21] Quantitatively, non-terpene derivative hydrocarbons were found to be the most abundant group of volatiles in all the varieties of raisin in this study, except in the case of the Meski variety (). The amounts of non-terpene derivative hydrocarbons were present in the four varieties of raisin in the order Chriha (35.8%) > Assli (26.3%) > Razeki (25.5%), and Meski (2.9%). Among this group of volatiles, which impart the characteristic intense fruity aromas to raisins, the most abundant were n-pentadecane (Chriha, 11.9%), n-hexadecane (Razeki, 8.2%) and n-tetradecane (Assli, 7.8%). ONTD were accumulated in Meski (57.7%) > Razeki (24.7%) > Chriha (24.3%) > Assli (22.7%). This group of compounds includes alcohols (ALC), esters (ES), aldehydes (ALD), ketones, and acids, which are responsible for citrus, floral, and fruity aromas.[Citation20] ALC were the most abundant group of volatiles identified in the order Meski (28.9%) > Chriha (11.9%) > Razeki (8%; ). ALD were present in higher proportions (). The Assli variety was quantitatively the richest (13.1%) because of a considerable amount of benzaldehyde, followed by the Razeki (10.9%) and Meski (6.9%) varieties (). The other volatile compounds of this group were distributed in different proportions among the four varieties. ALD are known to be derived from thermal oxidative degradation of amino acids and fatty acids; also, SPME extracted hexanal, which is present naturally in plants and fruits.[Citation22]
FIGURE 2 Oxygenated non-terpene derivatives composition (%) according to compound group of the volatiles obtained from headspace micro extraction of four varieties of dried raisins.
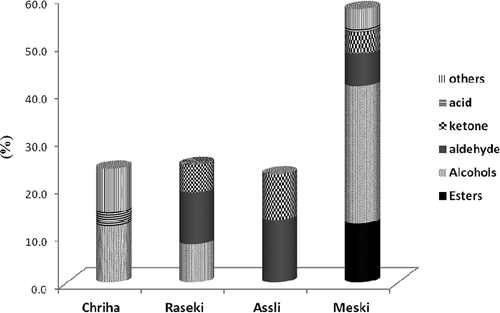
Using HS-SPME, ES were detected only in the Meski variety (12.4%). This group was represented mainly by fatty acid ethyl and methyl ES related to lipid metabolism, including ethylacetate present only in the Meski extract (11.2%). Finally, ketones were present in the order Assli (9.6%) > Razeki (5.8%) > Meski (4.6%) but were absent from the Chriha variety ().
Terpenes constitute another important group of volatiles that cover a wide range of herbaceous, fruity, citrus, floral, and fungal odors.[Citation20] In this study, the terpenoids could be placed into three classes: monoterpene hydrocarbons, OM, and sesquiterpene hydrocarbons. The Assli variety had qualitatively and quantitatively the richest content of monoterpene hydrocarbons. It was the only variety containing appreciable proportions of two of the five monoterpene hydrocarbons identified, including γ-terpinene (12.5%) and δ-3carene (3%). By contrast, only γ-terpinene was detected in the Chriha and Meski varieties (2.1 and 2%, respectively).
OM were present in greater proportions in the Meski and Assli extracts with total contents of 26.4 and 16.7%, respectively. Meski was the only variety containing appreciable amounts of seven of the 15 monoterpene hydrocarbons identified, including mostly pyranoid (8.7%) and furanoid (7.3%). Linalool and carvone were the major compounds of this group in the Assli variety (7.8 and 6%, respectively). The sesquiterpene class was represented by a single component, β-caryophyllene, which was present only in the Assli and Meski varieties (3.6 and 0.7%, respectively).
AP, known also as carotenoid cleavage products (CCPs), have become exciting molecules with excellent prospects for novel functions.[Citation23,Citation24] In this study, the apocarotenoid components were qualitatively and quantitatively different among the four varieties of raisin in the order Razeki (25.5%) > Chriha (23.4%) > Assli (7.2%) > Meski (6.1%). (E)-Geranylacetone, detected in all varieties, was the most abundant compound of this chemical class in the Assli (7.2%) and Meski (6.1%) varieties, whereas allyl ionone 2 was the most abundant compound in the Razeki (16.6%) and Chriha (14%) varieties. This group of volatile compounds contributes to the flavor and/or aroma of flowers and a variety of fruits and vegetables.[Citation25–Citation27]
PH, a diverse group of compounds derived from the carbon skeleton of phenylalanine, are involved in the defense, structural support, and survival of plants.[Citation28] This group, which was present mostly in the Razeki (15%), Assli (3.6%), and Chriha (3.2%) varieties, was represented mainly by (E)-anethole (9.5%) and methyl chavicol (5.5%) in the Razeki variety. The PH pathway, an important source of metabolites in plants, is required for the biosynthesis of lignin and serves as a starting point for the production of many other important compounds, including flavonoids, coumarins, and lignans.[Citation28] Furthermore, diverse physiological and pharmacological properties of these natural compounds, including antioxidant,[Citation7–Citation32] anti-inflammatory and antinociceptive,[Citation33] hypoglycemic,[Citation7–Citation34] antidepressant effects,[Citation35] and antitumor activity[Citation36] have been reported.
The four varieties of raisin, which were collected on the same date and dried under the same conditions, could be distinguished on the basis of the qualitative and quantitative difference in the contents of volatile components. The accumulation of metabolites depends on the genetically determined enzyme composition of the fruit.[Citation7–Citation37] By contrast, the method of analysis chosen can affect the aromatic composition. HS-SPME was used in this study to evaluate aroma compounds because it is a non-destructive technique,[Citation38] which has become the method of choice in aroma analysis. HS-SPME offers rapid solvent-free sampling with facility of operation and low cost; moreover, it is sensitive, selective, and compatible with low detection limits.[Citation39] Because of these advantages, HS-SPME is used frequently to extract volatile and semi-volatile compounds from biological, environmental, food, and drink samples.[Citation22,Citation40,Citation41]
Carbohydrate Profile
The concentrations of monosaccharides (mannose, glucose, fructose, galactose, arabinose, rhamnose, xylose), sugar ALC (mannitol, sorbitol, inositol, and adonitol) and polysaccharides (sucrose, trehalose, and raffinose) detected in the Chriha, Razeki, Assli, and Meski varieties of raisin expressed as mg/kg dry weight (DW) are given in . The total sugars content in the raisins ranged from 3501.4 to 9017.6 mg kg–1 DW. The highest and lowest values were found for the Razeki and Chriha varieties, respectively, and there was no significant difference between the Assli and Meski varieties ().
TABLE 2 Carbohydrates profiles of Tunisian dried raisins (mg/kg)
The total monosaccharides content followed the same trend as the total sugars content and all differences between the varieties were statistically significant (p < 0.05). Of the free sugars, glucose was present at a significantly higher concentration compared to the other free sugars, followed by galactose and fructose. The concentration of glucose ranged from 1407.6 to 4692.6 mg kg–1 DW, which corresponded to 40–52% of the total sugar content. In addition, the four varieties of raisin exhibited some homogeneity and some differences. However, all of the varieties tested contained glucose, galactose, and fructose as the most abundant free sugars in accord with an earlier report.[Citation42] Dehydration alters the texture, color, taste, and nutritional value of grapes due to the high temperatures and long drying times required in the process. Carranza-Concha et al. reported that glucose losses were less marked when grapes were pretreated with NaOH, whereas the fructose content was decreased.[Citation42] This might be due to the keto–enolic equilibrium of fructose in a basic medium; i.e., the conversion of fructose into its enediol and of the latter into glucose. In general, sugars were concentrated or produced during the dehydration of grapes.[Citation8,Citation43]
Our results showed that total sugar ALC were concentrated significantly in the Razeki variety (671.3 mg kg–1 DW; p < 0.05) compared to the Assli, Meski, and Chriha varieties, which had similar contents. Sorbitol, which was the principal sugar alcohol in all varieties with an appreciable amount in the Razeki variety (587.3 mg kg–1 DW), is known to have a significant role in the translocation of photosynthates.[Citation44] For human nutrition, there is increasing interest in fruits that are rich in sorbitol because this sugar alcohol is beneficial with regard to diet control, dental health and the avoidance of gastrointestinal problems. Moreover, sorbitol can be used by diabetics as a substitute for glucose and as a natural sweetener alternative to sucrose.[Citation45,Citation46] The other sugar ALC differed quantitatively among the varieties; significant differences of individual sugars in fruits likely reflect the metabolic behavior of each variety in relation to different environmental conditions. Finally, the Razeki variety had the highest values and the Meski variety had the lowest total polysaccharides content (p < 0.05).
Sucrose was the major free sugar in the Razeki, Chriha, and Assli varieties, whereas trehalose was the principal sugar in the Meski variety (). The differences observed were statistically significant (p < 0.05), except those between the Chriha and Assli varieties. Raffinose was present in very low concentrations in all varieties. Our results are in disagreement with those reported elsewhere,[Citation42] showing the sucrose content was the most strongly affected by dehydration (not detected in raisins) likely due to its susceptibility to hydrolysis induced by high temperatures. According to Tarachiwin et al.,[Citation11] sucrose is the predominant biochemical component that contributes to the sweetness of fruit.
PCA
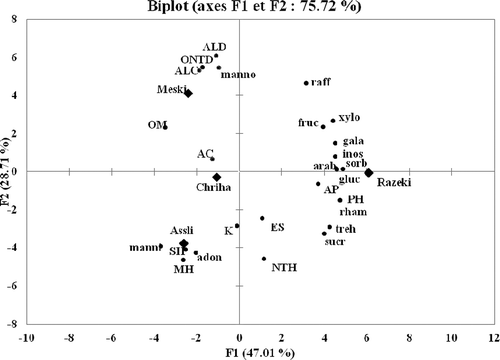
PCA was used to analyze the volatile compounds () and the carbohydrate profiles () of four varieties of Tunisian raisin. shows the correlation between the different groups of volatile compounds and free sugars. PCA accounted for 47.01% of the total variance (75.72%) associated with the first component and the second component accounted for 28.71% of the total variance. The position of each variable in this plot shows its relationship with the other variables. Variables close together were highly correlated; those on the same side of the origin (0.0) were positively correlated and those on the opposite side of origin were negatively correlated. The analysis of correlation showed the varieties of raisins could be discriminated on the PCA plane. The first main component (PC1) was positively related to NTH derivatives, ES, AP, PH, raffinose, fructose, xylose, galactose, inositol, sorbitol, arabinose, glucose, rhamnose, trehalose, and sucrose. The second main component (PC2) was related more closely to ALD, ALC, ONTD, OM, and mannose. The Razeki variety was located on the positive side of PC1, whereas the Meski, Chriha, and Assli varieties were located on the negative side. Each variety was located quite apart from the other varieties, which suggests its composition in terms of some of the volatile compounds and free sugars differs significantly compared to the other varieties. Thus, PCA provided clear discrimination between the four varieties of raisin in this study.
FIGURE 3 Principal component analysis (scores and loading plots, Biplot) applied to the dataset of volatile compounds and carbohydrates profiles of 4 varieties of dried. MH: monoterpene hydrocarbons; OM: oxygenated monoterpenes; SH: sesquiterpenes hydrocarbons; AP: apocarotenoids; PH: phenylpropanoids; ONTD: oxygenated non-terpene derivatives; ALC: alcohols; ALD: aldehydes; ES: esters; K: ketones; AC: acids; and NTH: non-terpene derivatives hydrocarbons. Arab: arabinose; rham: rhamnose; adon: adonitol; xylo: xylose; manno: mannose; fruc: fructose; gala: galactose; gluc: glucose; manni: mannitol; sorb: sorbitol; inos: inositol; sucr: sucrose; treh: trehalose; raff: raffinose.
CONCLUSION
This work increased our knowledge of the volatile compounds and carbohydrate constituents of the Chriha, Razeki, Assli, and Meski varieties of Tunisian raisin. A total of 80 compounds in the Chriha, Razeki, Assli, and Meski varieties were identified by GC–MS following HS-SPME, which allowed rapid extraction of the highly volatile compounds without apparent hydrolysis or formation of artifacts. The number of volatile compounds detected differed among the varieties. The same behavior was observed for different classes of volatile compounds, which varied qualitatively and quantitatively among these varieties of raisin. The types and amounts of sugars were observed to have a direct influence on the organoleptic quality of fruit.[Citation11] According to our results, the Razeki variety had, in general, greater sweetness based on individual and total sugars compared to the Assli, Meski, and Chriha varieties. Finally, as the harvesting period and extraction conditions were similar for the four varieties, our results indicated that besides genetic factors, environmental conditions might be implicated in the aromatic and carbohydrate compositions. The study of a large number of samples from various years of production will hopefully confirm the results obtained by this first screening.
FUNDING
This study was supported by the Ministry of Higher Education and Scientific Research in Tunisia. We express our sincere thanks to the members of LR-NAFS/LR12ES05 « Nutrition-Functional Food et Vascular Health » at Faculty of Medicine—University of Monastir (Tunisia). Part of this study was carried out at the Dipartimento di Farmacia, Università di Pisa, Italy.
Additional information
Funding
REFERENCES
- Karakaya, S.; El, S.; Tas, A. Antioxidant Activity of Some Foods Containing Phenolic Compounds. International Journal of Food Sciences and Nutrition 2001, 52, 501–508.
- Shahidi, F.; Tan, Z. Raisins: Processing, Phytochemicals, and Health Benefits. In Dried Fruits: Phytochemicals and Health Effects; Alasalvar, C.; Shahidi, F.; Eds.; Wiley-Blackwell: Hoboken, NJ, 2013; 372 p.
- Moreno, J.J.; Cerpa-Calderón, F.; Cohen, S.D.; Fang, Y.; Qian, M.; Kennedy, J.A. Effect of Postharvest Dehydration on the Composition of Pinot Noir Grapes (Vitisvinifera L.) and Wine. Food Chemistry 2008, 109, 755–762.
- Vega-Mercado, H.; Gongora-Nieto, M.; Barbosa-Canovas, G.V. Advances in Dehydration of Foods. Journal of Food Engineering 2001, 49, 274–279.
- Serratosa, M.P.; Lopez-Toledano, A.; Medina, M.; Merida, J. Drying of Pedro Ximenez Grapes in Chamber at Controlled Temperature and with Dipping Pretreatments. Changes in the Colour Fraction. Journal of Agriculture and Food Chemistry 2008, 56, 10739–10746.
- Ghrairia, F.; Lahouar, L.; El Arem, A.; Brahmi, F.; Ferchichi, A.; Achour, L.; Said, S. Physicochemical Composition of Different Varieties of Raisins (Vitis Vinifera L.) from Tunisia. Industrial Crops and Product 2012, 43, 73–77.
- Bhat, N.R.; Desai, B.B.; Suleiman, M.K. Flavor in Grapes: Its Characterization and Commercial Applications. In Handbook of Fruit and Vegetable Flavors; Hui, Y.H.; Ed.; Wiley-Blackwell: Hoboken, NJ, 2010; 279 p.
- Costantini, V.; Bellincontro, A.; De Santis, D.; Botondi, R.; Mencarelli, F. Metabolic Changes of Malvasia Grapes for Wine Production During Post Harvest Drying. Journal of Agriculture and Food Chemistry 2006, 54(9), 3334–3340.
- De Torres, C.; Díaz-Maroto, M.C.; Hermosín-Gutiérrez, I.; Pérez-Coello, M.S. Effect of Freeze-Drying and Oven-Drying on Volatiles and Phenolics Composition of Grape Skin. Analytica Chimica Acta 2010, 660, 177–182.
- Ruiz, M.J.; Zea, L.; Moyano, L.; Medina, M. Aroma Active Compounds During the Drying of Grapes cv. Pedro Ximenez Destined to the Production of Sweet Sherry Wine. European Food Research and Technology 2010, 230(3), 429–435.
- Tarachiwin, L.; Masako, O.; Fukusaki, E. Quality Evaluation and Prediction of Citrulluslanatus by1H NMR-Based Metabolomics and Multivariate Analysis. Journal of Agriculture and Food Chemistry 2008, 56, 5827–5835.
- Delgado, F.J.; González-Crespo, J.; Cava, R.; García-Parra, J.; Ramírez, R. Characterisation by SPME-GC–MS of the Volatile Profile of a Spanish Soft Cheese P.D.O. Torta del Casar During Ripening. Food Chemistry 2010, 118, 182–189.
- Stenhager, E.; Abrahamsson, S.M.C.; Lafferty, F.W. Registry of Mass Spectral Data; Wiley: New York, NY, 1974.
- Massada, Y. Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry; Wiley: New York, NY, 1976.
- Jennings, W.; Shibamota, T. Quantitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Chromatography; Academic Press: New York, NY, 1980.
- Swigar, A.A.; Silvestein, R.M. Monoterpenes; Aldrich Chemical Company, Milwaukee, WI, 1981.
- Davies, N.W. Gas Chromatographic Retention Indexes of Monoterpenes and Sesquiterpenes on Methyl Silicone and Carbo-Wax 20M Phases. Journal Chromatography 1990, 503, 1–24.
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography Mass Spectroscopy; Allured: Carol Stream, IL, 1995.
- Bartolozzi, F.; Bertazza, G.; Bassi, D.; Cristoferi, G. Simultaneous Determination of Soluble Sugars and Organic Acids As Their Trimethylsilyl Derivatives in Apricot Fruits by Gaz Liquid Chromatography. Journal of Chromatography A 1997, 758, 99–107.
- Richard, H. Connaissance de la Nature des Arômes. In Les Arômes Alimentaires; Richard, H.; Multon, J.L.; Eds.; Tec & Doc Lavoisier: Paris, 1992; 21–37 pp.
- Visai, C.; Vanoli, M. Volatile Compound Production During Growth and Ripening of Peaches and Nectarines. Scientia Horticulturae 1997, 70(1), 15–24.
- Barra, A.; Baldovini, N.; Loiseau, A.M.; Albino, L.; Lesecq, C.; LizzaniCuvelier, L. Chemical Analysis of French Beans (Phaseolusvulgaris L.) by Head Space Solid Phase Microextraction (HS-SPME) and Simultaneous Distillation/Extraction (SDE). Food Chemistry 2007, 101, 1279–1284.
- Akiyama, K.; Matsuzaki, K.I.; Hayashi, H. Plant Sesquiterpenes Induce Hyphal Branching in Arbuscularmycorrhizal Fungi. Nature 2005, 435, 824–827.
- Bouvier, F.; Isner, J.C.; Dogbo, O.; Camara, B. Oxidative tailoring of carotenoids: a prospect towards novel functions in plants. Trends in Plant Science 2005, 10, 187–194.
- Simkin, A.J.; Schwartz, S.; Auldridge, M.; Taylor, M.G.; Klee, H.J. The Tomato Carotenoid Cleavage Dioxygenase1 Genes Contribute to the Formation of the Flavor Volatiles b-Ionone, Pseudoionone, and Geranylacetone. Plant Journal 2004, 40, 882–892.
- Auldridge, M.E.; Block, A.; Vogel, J.T.; Dabney-Smith, C.; Mila, I.; Bouzayen, M.; Magallanes-Lundback, M.; DellaPenna, D.; McCarty, D.R.; Klee, H.J. Characterization of Three Members of the Arabidopsis Carotenoid Cleavage Dioxygenase Family Demonstrates the Divergent Roles of This Multifunctional Enzyme Family. Plant Journal 2006, 45, 982–993.
- Walter, M.H.; Flos, D.S.; Hans, J.; Fester, T.; Strack, D. Apocarotenoid Biosynthesis in Arbuscularmycorrhizal Roots: Contributions from Methylerythritol Phosphate Pathway Isogenes and Tools for Its Manipulation. Phytochemistry 2007, 68, 130–138.
- Vogt, T. Phenylpropanoid Biosynthesis. Molecular Plant 2010, 3, 2–20.
- Ralph, A.; Provan, G.J. Phytoprotectants. In Human Nutrition and Dietetics; Garrow, J.S.; James, W.P.T.; Ralph, A.; Eds.; Elsevier Limited: London, United Kingdom, 2005; 417–424 pp.
- Katalinic, V.; Mozina, S.S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic Profile, Antioxidant Capacity, and Antimicrobial Activity of Leaf Extracts from Six Vitis Vinifera L. Varieties. International Journal of Food Properties 2013, 16, 45–60.
- Ghatak, A.A.; Chaturvedi, P.A.; Desaia, N.S. Indian Grape Wines: A Potential Source of Phenols, Polyphenols, and Antioxidants. International Journal of Food Properties 2014, 17, 818–828.
- Radovanovic, B.; Radovanovic, A.; Tomic, V. Relations Between the Phenolic Composition and Free Radical Scavenging, and Antibacterial Activities of Red Wines from Different Cultivars of Vitis vinifera L. International Journal of Food Properties 2012, 15, 725–735.
- Choi, J.; Shin, K.M.; Park, H.J.; Jung, H.J.; Kim, H.J.; Lee, Y.S.; Rew, J.H.; Lee, K.T. Anti-Inflammatory and Antinociceptive Effects of Sinapyl Alcohol and Its Glucosidesyringin. Planta Medicinal 2004, 70, 1027–1032.
- Niu, H.S.; Liu, I.M.; Cheng, J.T.; Lin, C.L.; Hsu, F.L. Hypoglycemic Effect of Syringing from Eleutherococcussenticosus in Streptozotocin-Induced Diabetic Rats. Planta Medicinal 2008, 74, 109–113.
- Kurkin, V.A.; Dubishchev, A.V.; Ezhkov, V.N.; Titova, I.N.; Avdeeva, E.V. Anti-Depressant Activity of Some Phytopharmaceuticals and Phenylpropanoids. Pharmaceutical Chemistry Journal 2006, 40, 614–619.
- Wei, Z.; Zhang, W.D.; Chuan, Z.; Liu, R.H.; Li, T.Z.; Peng, F.; Lei, S. Antitumor Activities of Extracts and Compounds from the Roots of Daphne Tangutica Maxim. Phytotherapy Research 2007, 21, 1113–1115.
- Maia, J.G.S.; Andrade, E.H.A; Zoghbi, M.G. Aroma Volatiles from Two Fruit Varieties of Jackfruit (ArtocarpusheterophyllusLam.). Food Chemistry 2004, 85, 195–197.
- Hamm, S.; Lesellier, E.; Bleton, J.; Tchapla, A. Optimization of Headspace Solid Phase Micro-Extraction for Gas Chromatography/Mass Spectrometry Analysis of Widely Different Volatility and Polarity Terpenoids in Olibanum. Journal of Chromatography A 2003, 1018, 73–83.
- Pawliszyn, J. Solid-Phase Microextraction. In Theory and Practice; Wiley-VCH: New York, NY, 1997.
- Berlardi, R.; Pawliszyn, J. The Application of Chemically Modified Fused Silica Fibres in the Extraction of Organics from Water Matrix Samples and Their Rapid Transfer to Capillary Columns. Water Pollution Research Journal of Canada 1989, 24, 179–191.
- Kataoka, H.; Lord, H.L.; Pawliszyn, J. Applications of Solid Phase Microextraction in Food Analysis. Journal of Chromatography A 2000, 880(1–2), 35–62.
- Carranza-Concha, J.; Benlloch, M.; Camacho, M.M.; Martínez-Navarrete, N. Effects of Drying and Pre-Treatment on the Nutritional and Functional Quality of Raisins. Food and Bioproducts Process 2012, 90, 243–248.
- Bellincontro, A.; De Santis, D.; Botondi, R.; Villa, I.; Mencarelli, F. Different Postharvest Dehydration Rates Affect Quality Characteristics and Volatile Compounds of Malvasia, Trebbiano and Sangiovese Grapes for Wine Production. Journal of the Science of Food and Agriculture 2004, 84(13), 1791–1800.
- Moriguchi, T.; Sanada, T.; Yamaki, S. Seasonal Fluctuations of Some Enzymes Relating to Sucrose and Sorbitol Metabolism in Peach Fruit. Journal of the American Society for Horticultural Science 1990, 115, 278–281.
- Forni, E.; Erba, M.L.; Maestrelli, A.; Polesello, A. Sorbitol and Free Sugar Contents in Plums. Food Chemistry 1992, 44, 269–275.
- Muir, J.G.; Rose, R.; Rosella, O.; Liels, K.; Barrett, J.S.; Shepherd, S.J. Measurement of Short-Chain Carbohydrates in Common Australian Vegetables and Fruits by High-Performance Liquid Chromatography (HPLC). Journal of Agriculture and Food Chemistry 2009, 57, 554–565.
