Abstract
The broad-spectrum antibacterial drugs are widely used in food producing animals. Suspected residues of these drugs in meat may have ill effects upon human health. The aim of the present study was to determine the enrofloxacin residues in broiler’s meat and liver samples. Detection of enrofloxacin residues in meat (n = 75) and liver (n = 75) samples was performed by high performance liquid chromatography with UV detector set at 268 nm using C18 column. Overall mean residual concentrations of enrofloxacin in meat and liver samples were 208 ± 55 and 527 ± 84 µg/kg, respectively. This study revealed that 52% (39) meat and 78.7% (59) liver samples were positive for enrofloxacin, out of these 58.3% (21) meat and 71.2% (42) liver samples were having residual concentration above the maximum residual limits. So it can be concluded that the usage of this contaminated meat may cause resistance in consumers and seems to be a public health threat.
Keywords:
INTRODUCTION
The poultry industry is one of the largest and fastest growing agro-industries everywhere in the world, whereas poultry meat is the second most widely used meat. In Pakistan, poultry meat is a chief source of animal proteins. In order to increase the production of broiler meat, various types of antibiotics are used as growth promoters. The ill use of antibiotics raises the risk of multiple resistances to several groups of antibiotics. This cross-contamination is probably the main reason of residues in animals, i.e., contamination of fodder in feed mills.[Citation1] Drugs and their metabolites remain within the body for longer time after their administration. The concentrations of these residues differ significantly from tissue to tissue and are usually detected higher in organs that metabolize and secrete them efficiently such as liver, kidneys, or storage tissues like body fat. Drug resistance is caused by residues of these drugs.[Citation2]
Enrofloxacin (ENRO) is a synthetic chemotherapeutic compound of class fluoroquinolones has its place in the last three generations (II, III, and IV) of quinolones. They were measured to be virtually the best antimicrobial preparations during the 1980s of the last century due to their potency and range of an antimicrobial spectrum of action. ENRO obstructs the role of two enzymes, topoisomerase II and IV. Topoisomerase II (DNA-gyrase) is liable for replication of DNA.[Citation3] For the quantitative detection of fluoroquinolones in biological samples such as animal serum, tissue, or plasma, a number of chromatographic techniques have been developed. The methods for detection include high-performance liquid chromatography (HPLC) along with several detectors, such as photo diode-array, ultraviolet-visible (UV-VIS), mass selective detectors and mass spectrometry/mass spectrometry (MS/MS), etc.[Citation4,Citation5] These methods give an accurate quantitative detection of fluoroquinolones with small quantity of drug detection.[Citation6]
In Pakistan, strict rules and guidelines are usually not followed by most of the poultry farms regarding the administration of veterinary medicines to the food producing animals.[Citation2] The indiscriminate use of ENRO without observing their withdrawal period may create hazards for human health throughout the country. This study aims to determine the residues of ENRO at marketing age by using HPLC with UV detection in broilers and to compare it with the maximum residual limit (MRL) recommended by the Food and Agriculture Organization/World Health Organization (FAO/WHO)[Citation7] and Barbosa[Citation8] as 100 and 200 µg/kg for meat and liver, respectively, to provide a considerable data on the ENRO residues regarding the human safety.
MATERIALS AND METHODS
Punjab is the largest province, and Faisalabad is one of the major industrial cities of Pakistan; it has a big poultry market. This city may carry an exposure of higher risk to human health attributable to excessive use of antibiotics as growth promoters. That is why Faisalabad has been chosen as the study area.
Collection and Storage of Samples
Five birds at marketing age were collected randomly from 15 different farms around Faisalabad, Pakistan. Meat (5–10 gram) from breast (n = 75) and liver (n = 75) samples were collected aseptically from randomly selected birds and transferred to self-sealing polythene bags. These bags were labeled and transferred to pharmacology laboratory, Institute of Pharmacy, Physiology and Pharmacology, University of Agriculture, Faisalabad under chilled conditions and stored at –20°C until analysis.
Chemicals and Reagents
Standard ENRO having a purity of 99.9% was procured from A&K Pharmaceuticals LTD, Faisalabad, Pakistan. Methanol and acetonitrile were purchased from RCI-Labscan Ltd., Thiland whereas n-hexane and sodium dodecyl sulphate were purchased from Deajung Chemicals and Materials, Korea. Sodium dihydrogen phosphate, sodium hydroxide, meta-phosphoric acid, and sodium phosphate monobasic dehydrate of ultra-high purity grade were purchased from Merck, Germany.
Equipment and Instrumentation
The chromatographic system consisted of BDS Hypersil C18 column (250 mm, 4.6 µm, 5 µm), solvent delivery system (Sykam S1122), UV-visible detector (Sykam S3210 UV/VIS), column oven and peak simple version 3.29 integration software for windows. Other instruments include; a sonicator (Transsonic 460/H, Elma®), weighing balance (Shimadzu), homogenizer (IKA Labortechnik, Stefan, Germany), and centrifuge machine (Beckman TJ-6 refrigerated centrifuge, USA) and syringe filters (0.45 µm).
Preparation of Standard Stock Solution and Working Dilution
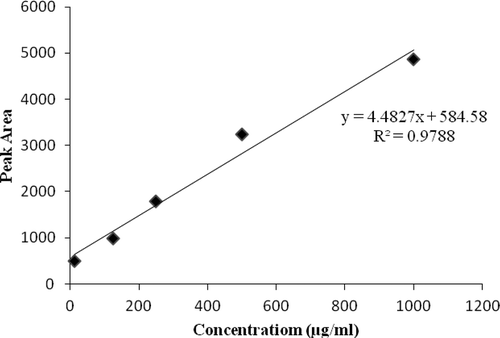
For preparation of standard solution, 1 mg of ENRO was weighed and dissolved in 0.01 NaOH: methanol (2:8, v/v), added 3.5 mM sodium dodecylsulphate and then diluted to the volume with mobile phase. The working dilutions of (10–500 µg/mL) were prepared from the standard stock solution. Each dilution (20 µL) was injected into the HPLC system with a flow rate of 1.0 mL/min and the standard curve was obtained by plotting peak area versus concentration, as shown in .
Extraction of Samples
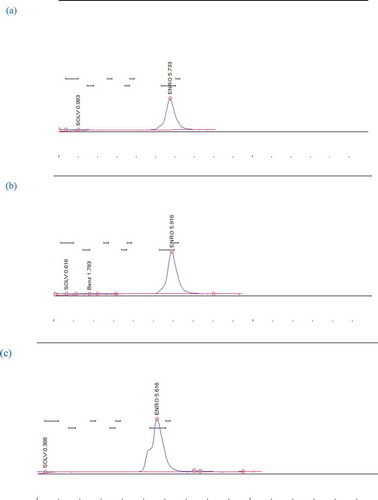
The sample (5 g) was weighed, thawed, and crushed for several minutes by pestle and mortar. The crushed sample was then transferred to 30 mL centrifugation tube with 15 mL of 0.3% meta-phosphoric acid: acetonitrile (1:10, v/v) and homogenized it for 3 min. The mixture was filtered under suction by Buchner funnel and then shook for 5 min with 25 mL of n-hexane. This mixture was saturated with acetonitrile in separatory funnel and the lower layer was separated and filtered through the 0.45 µm filter papers. This procedure was repeated twice while the filtrate was collected and dried at 40°C. The residues were ready for HPLC analysis after dissolving in 1 mL of mobile phase. The resultant aqueous solution was filtered through cellulose nitrate membrane filter having 13 mm diameter with pore size 0.45 µm (Micropore). Mobile phase was acetonitrile: 0.05M NaH2PO4 (pH 2.5; 35: 65, v/v) containing 3.5 mM sodium dodecyl sulphate. The volume of residues (20 µL) was taken and injected into the HPLC system by the manual injector. The flow rate was kept at 1.0 mL/min and wavelength of 286 nm was used for detection.[Citation9] Chromatograms of ENRO for a standard solution, meat, and liver samples of broiler chicken are shown in , , and , respectively.
Identification and Quantitative Analysis
ENRO was identified by finding the retention time, area, and spectra of peaks of unknown substance with standard drug. Quantity of ENRO residues was calculated by the formula:
AUC = area under the curve of sample; AUS = area under the curve of standard.
RESULTS
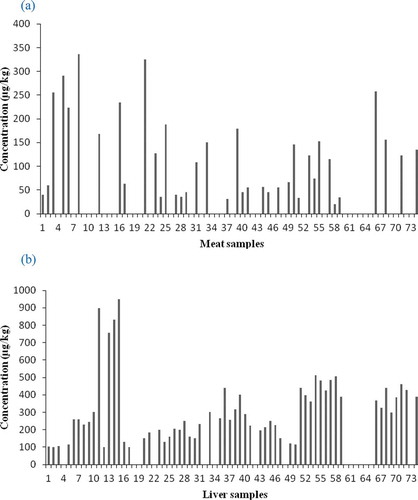
Overall mean concentration of ENRO residues in broiler’s meat samples was found to be 208 µg/kg. The results revealed that 52% (39) meat samples were positive for ERNO residues and out of these 58.3% (21) samples were having residual concentration above the MRL, and 41.7% (15) samples were having residual concentration below the MRL. Whereas, 48% (36) meat samples were negative for ERNO residues as presented in and . Overall mean concentration of ENRO residues in broiler’s liver samples was 527 µg/kg. According to the results, 78.7% (59) liver samples positive for ERNO residues and out of these 71.2% (42) liver samples had having residual concentration above the MRL, and 28.8% (17) liver samples were having residual concentration below the MRL. On the other hand 21.3% (16) liver samples were negative for ERNO residues as presented in and .
TABLE 1 ENRO residual data in broiler chicken samples analyzed by HPLC (n = 75)
DISCUSSION
Poultry meat provides a good alternative source of red meat (beef and mutton).[Citation10] Pakistan is a developing country which largely depends on the use of poultry meat as a dietary animal protein source. The poultry industry has to face various diseases.[Citation11] Antibiotics are used in the treatment and prevention of these diseases as well as a growth promoting agent.[Citation12] The presence of antibiotic residues in poultry meat has received colossal worldwide attention from public health agencies. This is due to the importance and significance of antibiotic residues on public health. Many reports indicated that microbial resistance.[Citation13] Lack of knowledge about the proper withdrawal times of drugs and the overuse or misuse of various drugs lead toward the formation of drug residues into the animal end products.[Citation14] Withdrawal times and MRLs of drugs should be determined to prevent the formation of residues in meat of animals. The drugs that are lipophilic in nature have a high tendency to penetrate into various body tissues, including lungs, liver, kidney, muscles, and skin. Due to the long half-lives of these drugs, they persist in tissues for prolonged periods of times resulting in formation of residues of these drugs. These residues ultimately pose serious health threats to human beings when meat from such animals is consumed.[Citation15] Furthermore, these residues may also be formed if proper withdrawal time of drugs have not been established. All these situations produce somber health effects in humans on consumption of contaminated meat.[Citation16]
The ENRO has a wide spectrum of activity and being widely used as veterinary drug to improve health conditions. It has good absorption and high bioavailability with good penetration into body tissues and found at high concentrations in the excretory organs, especially the liver with high withdrawal time.[Citation17] In present study, 52% (39) of meat and 78.7% (59) of liver samples were having residues of ENRO. Out of this positive samples 58.3% (21) meat and 71.2% (42) liver samples were having residual concentration above MRL. Results of our study are also confirmed by Horstkotter[Citation18] with similar findings. The concentrations of antibiotic residues were more in the liver sample than muscles because ENRO is mainly metabolized in liver. Our results were also consistent with other studies[Citation18–Citation20] regarding the highest mean of ENRO residues in poultry liver samples than meat. This may be because ENRO is easily distributed from plasma into tissues.[Citation20]
According to Jelena[Citation21] the level of residues is 2–4 levels greater in the liver than the muscles after treatment with ENRO. ENRO and ciprofloxacin occurrence in liver and muscle of breast, were identified through HPLC method and microbial inhibition assay. Thus, this is also in accordance with our results showing more residues concentration in the liver than in muscles. Bailac[Citation22] showed that the MRL recognized for quinolones in muscle tissue of chicken consist of 100 µg/kg for ENRO and ciprofloxacin and 400 µg/kg for flumequine, the suggested technique is adequately practical for the investigation of these quinolones in chicken tissues. Since the values of limit of quantification and limit of detection achieved were lower than MRL recognized for these drugs. Similarly in our study chicken meat has 43% smaller values than the MRL of liver samples.
Chen[Citation23] also performed the residual analysis of ENRO using quantum dot-based fluoroimmunoassay technique. According to their study, the average concentration of ENRO in chicken muscle was 46 µg/mL, which is lower than the values obtained in our study. It may differ due to variation in frequency of drug administration or sample collection time. Martin[Citation24] stated that for confirmation of supply of harmless animal products to consumers, the time of removal of drugs should also be considered. For this purpose, he used 234 broilers, and ENRO preparations were administered to birds by oral route on a daily basis at 10 mg/kg body weight. Liver and muscle samples were gathered, and investigated by HPLC mass spectroscopy. According to European Union and Chile, the time of removal of drug should not go above 5 days while according to Japan, the time of withdrawal of drug should be greater, up to 8 days. Their outcomes showed that there was a difference in the withdrawal time of the drug along with the MRL. Their results coincide with the present study which reveals that drug residues above MRL are a serious matter and there is a need of extensive work to quantify residues in the food producing animals particularly poultry.
CONCLUSIONS AND RECOMMENDATIONS
The results indicate the presence of residues, whereas the usage of this contaminated meat causes resistance in consumers and seems to be a public health threat. Thus, there is a need to educate the farmers about the ill effects of residual drugs on human health and withdrawal time in poultry birds. National authorities should also adopt more judicious approaches to ensure prudent use of antibiotics in food animals.
REFERENCES
- Johnston, A.M. Animals and Antibiotics. International Journal of Antimicrobial Agents 2001, 18, 291–294.
- Abedullah; Maqbooli, A.; Bukhsh, K. Issues and Economics of Poultry Production: A Case Study of Faisalabad Pakistan. Pakistan Veterinary Journal 2007, 27, 25–28.
- Èlanjak, E.; Smajlovi, M.; Èaklovica, F.; Alagi, D.; Èaklovica, K.; Smajlovi, A. Animal Protection and Welfare. Svibanj–lipanj 2011, 13, 198–205.
- Yan, Z.; Junbo, X.; Yanqing, Z.; Mingchun, Z. Degradation Kinetics of Jujuboside a by Rat Intestinal Flora and Identification of the Metabolites by HPLC-MS/MS. International Journal of Food Properties 2013, 17(8), 1841–1849.
- Wengui, L.; Kunlong, X.; Rong, X.; Gefen, Y.; Wenwen. L. Development of an HPLC-Based Method for the Detection of Aflatoxins in Pu-Erh Tea. International Journal of Food Properties 2014, 18, 842–848.
- Haritova, A.M.; Petrova, D.K.; Stanilova, S.A. A Simple HPLC Method for Detection of Fluoroquinolones in Serum of Avian Species. Journal of Liquid Chromatography & Related Technologies 2012, 35, 1130–1139.
- FAO/WHO. A Second Meeting of the Joint FAO/WHO Expert Committee on Food Additives. Code of Federal Regulations 1992, 21, 365.
- Barbosa, J.; Freitas, A.; Moura, S.; Mour~ao, J.L.; da Silveira, M.I.N.; Ramos, F. Detection, Accumulation, Distribution, and Depletion of Furaltadone and Nifursol Residues in Poultry Muscle, Liver, and Gizzard. Journal of Agricultural and Food Chemistry 2011, 59, 11927–11934.
- Naeem, M.; Khan, K.; Rafiq, S. Determination of Residues of Quinolones in Poultry Products by High Performance Liquid Chromatography. Journal of Applied Sciences 2006, 6, 373–379.
- Mehtabuddin, A.A.M.; Ahmed, T.; Nadeem, S.; Tanveer, Z.I.; Arshad, J. Sulfonamide Residues Determination in Commercial Poultry Meat and Eggs. Journal of Animal and Plant Sciences 2012, 22, 473–478.
- Numan, M.; Zahoor, M.A.; Khan, H.A.; Siddique, M. Serologic Status of Newcastle Disease in Broilers and Layers in Faisalabad and Surrounding Districts. Pakistan Veterinary Journal 2005, 25, 55–58.
- Shankar, B.P.; Prabhu, B.H.M.; Chandanm, S.; Ranjith, D.; Shivakumar, V. Rapid Methods for Detection of Veterinary Drug Residues in Meat. Veterinary World 2010, 3, 241–246.
- Yorke, J.C.; Froc, P. Quantitation of Nine Quinolones in Chicken Tissues by High-Performance Liquid Chromatography with Fluorescence Detection. Journal of Chromatography A 2000, 882, 63–77.
- Seri, H.I. Introduction to Veterinary Drug Residues: Hazards and Risks. Veterinary Drug Residues in Food Derived From Animals. The National Medicinal and Poisons Board, Khartoum, Sudan, May 26–27, 2013, 1–7 pp.
- Shareef, A.M.; James, Z.T.; Yonis, K.M. Detection of Antibiotic Residues in Stored Poultry Products. Iraqi Journal of Veterinary Science 2009, 23, 45–48.
- Kozarova, I.; Mate, D.; Hussein, K.; Raschmanova, K.; Marcincak, S.; Jevinova, P. High-Performance Liquid Chromatographic Determination of Sulfadimidine Residues in Eggs. Acta Veterinaria 2004, 54, 427–435.
- Rao, G.S.; Ramesh, S.; Ahmad, A.H.; Tripathi, H.C.; Sharma, L.D.; Malik, J.K. Pharmacokinetics of Enrofloxacin and Its Metabolite Ciprofloxacin after Intramuscular Administration of Enrofloxacin in Goats. Veterinary Research Communications 2001, 25, 197–204.
- Horstkötter, C.; Jimenez-Lozano, E.; Barron, D.; Barbosa, J.; Blaschke, G. Determination of Residues of Enrofloxacin and Its Metabolite Ciprofloxacin in Chicken Muscle by Capillary Electrophoresis Using Laser-Induced Fluorescence Detection. Electrophoresis 2002, 23, 3078–3083.
- Farahmand, S.; Aref, S.; Nordehr, R.; Rahim, M.; Fariba, G. Enrofloxacin Residue in Chicken Tissues from Tehran Slaughterhouses in Iran. Pakistan Journal of Nutrition 2007, 6, 409–413.
- Iqbal, A.S. Detection of Enrofloxacin Residue in Livers of Livestock Animals Obtained from a Slaughterhouse in Mosul City. Journal of Veterinary Science & Technology 2014, 5, 2.
- Jelena, P.; Balti, M.; Upi, V.; Stefanovim, S.; Dragica, S. Residues of Enrofloxacin and Its Main Metabolite Ciprofloxacin in Broiler Chickens. Acta Vet-Beograd 2006, 56, 497–506.
- Bailac, S.; Barron, D.; Barbosa, J. New Extraction Procedure to Improve the Determination of Quinolones in Poultry Muscle by Liquid Chromatography with Ultraviolet and Mass Spectrometric Detection. Analytica Chimica Acta 2006, 580, 163–169.
- Chen, J.; Xu, F.; Jiang, H.; Hou, Y.; Rao, Q.; Guo, P.; Ding, S. A Novel Quantum Dot-Based Fluoroimmunoassay Method for Detection of Enrofloxacin Residue in Chicken Muscle Tissue. Food Chemistry 2009, 113, 1197–1201.
- Martin, S.B.; Cornejo, J.; Lapierre, L.; Iraguen, D.; Perez, F.; Hidalgo, H.; Andre, F. Withdrawal Time of Four Pharmaceutical Formulations of Enrofloxacin in Poultry According to Different Maximum Residues Limits. Journal of Veterinary Pharmacology and Therapeutics 2009, 33, 246–251.