Abstract
The effects of different levels of sodium hypochlorite (1–4 g/100 g dry solids active chlorine) on the physicochemical, pasting, and structural properties of tamarind kernel starches were investigated. The isolated starch had low traces of non-starch components, such as protein, fat, and ash, indicating its purity. Both the carboxyl and carbonyl contents in the oxidized starches increased significantly with the increase in chlorine concentration. The introduction of carboxyl and carbonyl groups resulted in significantly lower amylose content. The swelling power of oxidized starches was significantly lower than native starch and the solubility values increased when the chlorine concentration increased at all the measured temperatures. The percentage of light transmittance increased progressively after oxidation. Pasting properties showed that in treatments at high active chlorine concentrations, the peak viscosity decreased more drastically than in treatments at low chlorine concentration, indicating a greater degradation of starch. The morphology of the starches was not altered after oxidation. After oxidative treatment no change in the X-ray diffraction pattern was observed but intensity of the peaks increased. Therefore, tamarind kernel being underutilized raw material, has a great potential as a non-conventional source of starch and desirable properties of this starch can be enhanced by oxidation for applications in food industry.
INTRODUCTION
Tamarind (Tamarindus indica L.) grows naturally in tropical and subtropical regions and now is one of the most important plant resources as food materials.[Citation1] It grows in more than 50 countries of the world.[Citation2] Tamarind fruit is a brown pod-like legume which contains many hard-coated seeds (34%) embedded in a soft acidic pulp (about 55%).[Citation3] India produces about 0.3 million tons of tamarind yearly, of which the seed constitutes about 30–34% of the whole fruit.[Citation4] The seed, a by-product of the tamarind pulp industry is an underutilized or waste material. It comprises of seed coat or testa (20–30%) and the kernel or endosperm (70–80%). The mineral concentrations of seeds are high, especially those of potassium and magnesium.[Citation5] Tamarind seeds are used to obtain tamarind kernel powder (TKP) which is a rich source of protein, fat, and valuable amino acids and a good source of gelling polysaccharide (xyloglucan) called polyose which is a permitted gelling and stabilizing agent in the food industry.[Citation1,Citation5]
Starch is the main storage carbohydrate of several crops and is highly abundant in nature. The morphologies, chemical compositions, and the molecular structures of starches are unique for each particular plant species.[Citation6] Starch is an extensively used biopolymer both in food and non-food industries.[Citation7] Substantial progress has been made in studying the functional, rheological, and physicochemical properties of starches obtained from non-conventional sources.[Citation8–Citation10] Native starches have limited uses due to various drawbacks such as low paste clarity, low shear stress resistance, and high retrogradation. Therefore, starches are commonly tailored via physical and chemical modifications or both to improve native functional properties and thereby extending its applications in the food industries.[Citation7] Oxidized starch is produced by reacting starch with a specified amount of some oxidizing reagent under controlled conditions of temperature and pH.[Citation11] Oxidation causes depolymerization, which results in a lower dispersion viscosity and introduces carbonyl and carboxyl groups, which retard recrystallization.[Citation12] Such starches have been established to be whiter in color and have restricted retrogradation.[Citation13] The main use of oxidized starch is in paper and textile industries. Its applications in the food industry are increasing because of its high stability, clarity, low viscosity, and film forming properties.[Citation10] Although oxidative treatment of starches from different botanical sources like sorghum,[Citation7] breadfruit,[Citation14] cassava,[Citation15] cocoyam,[Citation16] corn,[Citation11] fieldpea,[Citation17] potato,[Citation18] and pinenut[Citation19] has been reported earlier but no work has, until this date, been done on the modification of tamarind kernel starch by oxidative treatment. Also, there is a growing tendency toward finding alternative sources of starch from novel and under-utilized starch sources rather than relying on known starch sources.[Citation14] Keeping this in mind, the present investigation was undertaken to isolate starch from decorticated tamarind kernels and modify it by oxidation using sodium hypochlorite at different levels of active chlorine concentration. Though many oxidizing reagents, such as hydrogen peroxide, chromic acid, permanganate, and sodium hypochlorite, have been used for oxidizing starch, sodium hypochlorite was chosen as the oxidizing reagent as it is the oldest and most popular commercial oxidant. Also, the physiochemical, pasting, and structural properties of native and oxidized starches were evaluated to aim at effective utilization of these starches.
MATERIAL AND METHODS
Tamarind seeds were purchased from local market of Amritsar, India. All the chemicals and reagent used were of analytical grade.
Starch Isolation
Starch was isolated from de-hulled tamarind kernels by steeping the kernels in water containing sodium metabisulphite (0.16%) at 50°C for 12 h. The steep water was then drained off, and the kernels, after grinding in a laboratory blender, were screened through nylon cloth (100 mesh). The filtrate slurry obtained was allowed to stand for 2 h and the settled starch layer was re-suspended in distilled water. It was then centrifuged in wide mouthed cups for 5 min at 3000 rpm. The upper non-white layer was scraped off and the white layer was re-centrifuged for 2–3 times in distilled water until a pure white starch was obtained. The starch slurry obtained was then collected and dried in an oven 12 h at 40°C.
Starch Oxidation
Starch was oxidized according to method described by Wang and Wang[Citation11] with slight modifications. In brief, 200 g starch (dry basis) was taken in a 1 L reaction vessel and to it 500 g of distilled water was added. The resultant starch slurry was maintained at 35°C with pH adjusted to 9.5 with 2N NaOH. Sodium hypochlorite (NaOCl) with different active chlorine (1, 2, 3, 4%) w/v was slowly added into the starch slurry over 30 min. The pH of slurry was maintained at 9.5 with 1N H2SO4 during NaOCl addition. Thereafter, the pH of slurry was maintained at 9.5 with 1N NaOH for an additional 50 min. Finally, the slurry was adjusted to pH 7.0 with 1N H2SO4 and passed to 1 L flask to precipitate. The reaction mixture was decanted and washed with several volumes of distilled water and dried in a convection oven (50°C) for 48 h.
Determination of Carbonyl Content
The carbonyl content was determined by following the titrimetric method of Smith.[Citation20] Starch (4 g) was suspended in 100 mL of distilled water in a 500 mL flask for 20 min, cooled to 40°C, adjusted to pH 3.2 with 0.1 N HCl, and mixed with 15 mL of hydroxylamine reagent. A stopper was placed in the flask and placed in a 40°C water bath for 4 h with slow stirring. The excess hydroxylamine was determined by rapidly titrating the reaction mixture to pH 3.2 with standardized 0.1 N HCl. A blank determination with only hydroxylamine reagent was performed by first dissolving 25 g hydroxylamine hydrochloride in 100 mL of 0.5 N NaOH before adjusting the final volume to 500 mL with distilled water. Carbonyl content was calculated as follows:
Determination of Carboxyl Content
The carboxyl content of oxidized starch was determined according to the modified procedure of Chattopadhyay et al.[Citation21] A starch sample (2 g) was mixed with 25 mL of 0.1 N HCl, and the slurry was stirred occasionally for 30 min with a magnetic stirrer. The slurry was then vacuum-filtered through a 150 mL medium porosity fritted glass funnel and washed with 400 mL of distilled water. The starch cake was then carefully transferred into a 500 mL beaker, and the volume was adjusted to 300 mL with distilled water. The starch slurry was heated in a boiling water bath with continuous stirring for 15 min to ensure complete gelatinization. The hot starch dispersion was then adjusted to 450 mL with distilled water and titrated to pH 8.3 with standardized 0.01 N NaOH. A blank test was performed with unmodified starch. Carboxyl content was calculated as follows:
Amylose Content
Apparent amylose content of native and oxidized starches was determined by the rapid colorimetric method given by Williams et al.[Citation22] A starch sample (20 mg) was taken and 10 mL of 0.5 N KOH was added to it. The suspension was thoroughly mixed. The dispersed sample was transferred to a 100 mL volumetric flask and diluted to the mark with distilled water. An aliquot of test starch solution (10 mL) was pipetted into a 50 mL volumetric flask and 5 mL of 0.1 N HCl was added followed by the 0.5 mL of iodine reagent. The volume was diluted to 50 mL and the absorbance was measured at 625 nm. The measurement of the amylose was determined from a standard curve developed using amylose and amylopectin blends.
Swelling Power and Solubility
Swelling power and solubility were determined using method of Leach et al.[Citation23] 1% aqueous suspension of starch (100 mL) was heated in a water bath at 50, 60, 70, 80, and 90°C for 1 h with constant stirring. The suspension was cooled for 30 min at 30°C. Sample was then poured into preweighed centrifuge tubes, centrifuged at 3000 × g for 10 min and weight of sediments determined. For the measurement of solubility, the supernatants were poured into aluminium dishes and evaporated at 110°C for 12 h and weight of dry solids was determined.
Light Transmittance
Transmittance of native and modified tamarind starches was measured as described by Perera and Hoover.[Citation24] A 1% aqueous suspension of starch was heated in a water bath at 90°C for 1 h with constant stirring. The suspension was cooled for 1 h at 30°C. The samples were stored for 5 days at 4°C in a refrigerator and transmittance was determined every 24 h by measuring absorbance at 640 nm against a water blank with a Shimadzu UV-1601 spectrophotometer (Shimadzu Corporation, Kyoto, Japan).
X-Ray Diffraction (XRD)
XRD allows the determination of crystallinity and composition of crystalline phases in starch samples. The samples were stored at room temperature before analysis. XRD analysis was conducted using an X-ray diffractometer (Shimadzu-7000, Japan) operated at 40 kV and 30 mA. Diffractographs were obtained from 4° (2θ) to 30° (2θ) at a scanning speed of 5°/min.
Morphological Properties
Scanning electron micrographs (SEM) were taken by a scanning electron microscope (Model EVOLS10 ZEISS, Oberkochen, Germany). Starch samples were suspended in ethanol to obtain 1% suspension and a drop of this solution was applied on an aluminum stub using double-sided adhesive tape. An accelerating potential of 15kV was used during micrography.
Pasting Properties
The pasting properties of starches were evaluated using starch cell of Modular Compact Rheometer (Model 52, Anton Paar Co. Ltd, Austria). Viscosity profiles of starches were recorded using starch suspensions (6%, w/w; 15 g total weight). The suspensions were held at 50°C for 1 min, heated to 95°C at the rate of 6°C/min and held at 95°C for 2.7 min. These were then cooled from 95 to 50°C at the rate of 6°C/min and held at 50°C for 2 min. Various pasting parameters, such as peak viscosity, trough viscosity, final viscosity, breakdown viscosity (BV), setback viscosity (SV), and pasting temperature (PT), were recorded.
Statistical Analysis
The data reported was subjected to one-way analysis of variance (ANOVA) using Minitab Statistical Software version 13 (Minitab Inc., State College, PA, USA).
RESULTS AND DISCUSSION
Carbonyl and Carboxyl Contents
The carbonyl and carboxyl contents of tamarind kernel starch oxidized at different levels are reported in . The oxidation grade in a modified starch is determined by carboxyl group concentration. Both the carbonyl and carboxyl groups content significantly increased from 0.016 to 0.082 and 0.069 to 0.373 g/100g, respectively when active chlorine concentration increased in the reactive solution. Similar increase in the carbonyl and carboxyl contents for oxidized field pea starch with an increase in the oxidant concentration from 0.89 to 3.28% has been reported.[Citation17] At all the active chlorine concentrations significantly higher carboxyl groups than carbonyl groups were detected. This may be due to the fact that carbonyl groups formed during the reaction are transformed to carboxyl groups. According to Wang and Wang,[Citation11] oxidation conducted in alkaline condition at pH 9.5 and by hypochlorite promoted the production of carboxyl groups in comparison with oxidation conducted in acidic condition.
TABLE 1 Carboxyl and carbonyl group contents at different active chlorine concentrations
Physicochemical Properties
The isolated tamarind kernel starch had moisture, ash, fat and protein contents of 10.9, 0.38, 0.47, and 0.69 g/100 g (dry weight basis, db), respectively. Functional properties of starches depend on the amylose content to a large extent.[Citation25] The amylose content of native and oxidized starches from tamarind kernel is reported in . A significant reduction in amylose content with increase in active chlorine concentration was observed; reducing the amylose content of starch from 13.5 to 8.96 g/100 g after oxidation with 4% active chlorine. These observations are in agreement with those reported earlier for reduction in amylose content after treatment with sodium hypochlorite for corn starch,[Citation26] bambarra groundnut starch,[Citation27] and pine nut starch.[Citation19] Amylose content is responsible for a number of factors that affect the swelling power, solubility, and gel forming property of the starch.[Citation28] The amylose content of oxidized starches decreased progressively with the increase in oxidant concentration. The decrease is not only due to decrease in molecular size of amylose but also the rupture of helical structure of amylose that reduces the ability of iodine to make a blue color inclusion complex with amylose. The reduction in amylose content after oxidation may be due to the reaction of amylose with NaOCl, thereby reducing NaOCl for the oxidation of amylopectin. Also the linear structure and random arrangement of amylose makes it more susceptible to oxidative degradation.[Citation26,Citation27]
TABLE 2 Amylose content of native and oxidized tamarind kernel starch
The swelling power of native and oxidized starches measured from 50 to 90°C at 10°C intervals and at different concentrations of active chlorine is reported in . The swelling power of oxidized starches was significantly lower in comparison to native starch, and it decreased progressively with the increase in level of oxidant concentration. A similar decrease in swelling power upon oxidation was reported for new cocoyam,[Citation16] banana,[Citation10] potato,[Citation29,Citation30] and black gram starches.[Citation31] Oxidation is also a depolymerization reaction. The reduction in the swelling power after oxidation may also be attributed to structural disintegration within the granules of the starch during the process of modification[Citation7,Citation16] resulting in less ability to hold water. Hypochlorite oxidation is also highly effective for weakening the internal structure of starch granules, thereby making starch more soluble causing a decrease in swelling power.[Citation27] The weakening of starch granule integrity is related to negative charge of starch polymer after introduction of carboxyl group but not related to carbonyl group (carbonyl group has no charge). As the temperature increased from 50 to 90°C there was a progressive increase in swelling power of both native and oxidized starches. Starch molecules can hardly be soluble in free water but imbibed water as the temperature increases above the critical value.[Citation28] These results are in agreement with the observations on increase in swelling power with temperature for breadfruit[Citation14] and mucuna bean starch.[Citation32] The solubility value of oxidized starches was higher as compared to its counterpart native starch and increased with the increase in chlorine concentration (). This is probably due to the weakening of the starch granules during hypochlorite oxidation which eventually results in less restricted leaching of amylose chains and subsequently leading to improved solubility.[Citation33,Citation34] Solubility of starches also followed a similar pattern as shown by swelling power; both showed an increase with the increase in temperature. An increase in temperature unfolds the granular structure of starch due to commotion of hydrogen bonds present between the starch chains.[Citation7] There is increase in mobility of the starch granules, which facilitated enhanced dispersion of starch molecules in water.[Citation14] Similar pattern of increase in solubility with increase in temperature and chlorine concentration was reported for common and waxy corn starch,[Citation11] potato and barley starch,[Citation30] and banana starch.[Citation10]
TABLE 3 Swelling power (g/g) of native and oxidized tamarind kernel starches at different temperatures
TABLE 4 Solubility (g/100 g) of native and oxidized tamarind kernel starches at different temperatures
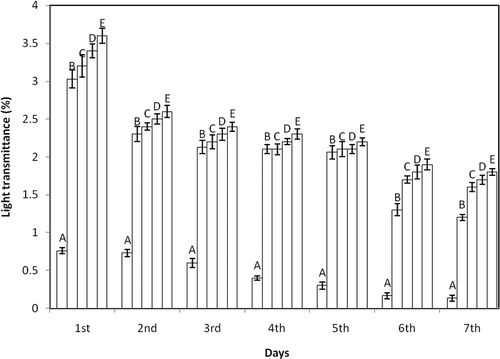
The light transmittance value of native and oxidized gelatinized starch pastes from tamarind kernel is shown in . Starch paste clarity is among the important attributes of starch, which is essential in foods, such as jellies and fruit pastes, to obtain the desired consistency.[Citation28] The light transmittance values of pastes from oxidized starches were observed to be higher than those from its counterpart native starch after similar storage intervals. Similar increase in clarity of oxidized starch pastes in comparison to native starch was observed by Conto et al.[Citation19] and Sangseethong et al.[Citation35] Substitution of carbonyl and carboxyl groups combined with depolymerization resulted in higher paste clarity of oxidized starches.[Citation7] Moreover, with the increase in active chlorine concentration a significant increase in transmittance value of oxidized starches was observed. The presence of hydrophilic groups in the oxidized starches, especially carboxyl groups, which hinder molecular reassociation, could be responsible for the increase in paste clarity.[Citation35] With the increase in duration of storage the percentage of light transmittance values decreased. Paste clarity decreases on refrigerated storage due to molecular realignment of solubilized starch chains.[Citation7]
Pasting Properties
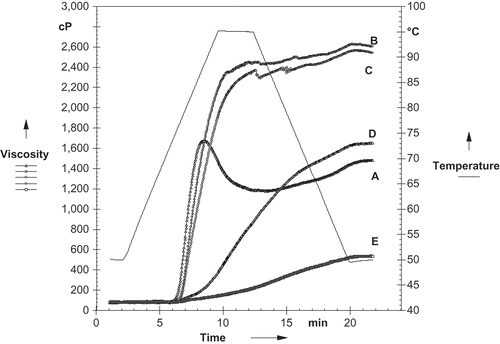
The pasting curves for native and oxidized starches are shown in . Pasting properties are representative of the intensity of changes that occur during starch modification.[Citation36] The oxidized starches with 1 and 2% of active chlorine, showed a peak viscosity which was higher than its native counterpart starch. This behavior may be attributed to the fact that the carbonyl and carboxyl groups introduced to the starch molecule promote granule swelling.[Citation13] However, when the active chlorine concentration increased to 3 and 4%, a reverse pattern was obtained and the peak viscosity decreased in comparison to native starch. This is probably due to a cleavage of the starch chains which might have occurred at high chlorine concentration resulting in decrease of peak viscosity. The decreased viscosity of hypochlorite oxidized starches is attributed to the oxidative cleavage of starch chains, resulting in starch of lower molecular size.[Citation12,Citation13] The existence of carboxyl group that has negative charge can weaken the molecular interactions that result in viscosity reduction. However, the introduction of uncharged carbonyl group has no effect on the viscosity. The oxidized starch with the highest concentration of active chlorine (4%) showed severe decrease in pasting properties and no peak viscosity was detected. In their study on effect of sodium hypochlorite oxidation on properties of banana starch Sanchez-Rivera et al.[Citation10] reported an increase in peak viscosity at low active chlorine concentrations, whereas the reverse was observed at high active chlorine concentrations. A similar trend was also reported by Guerra Dias et al.[Citation36] for oxidized fermented cassava starch. They reported that in treatments at low active chlorine concentrations, the peak viscosity was high which indicated that even small level of oxidation could promote molecular rearrangement of the granule surface. Native and oxidized starch suspensions (1 and 2% of active chlorine concentration) showed gradual increase in viscosity upon heating followed by a drop and then again increase in viscosity upon cooling. At active chlorine concentration higher than 1% there was a protective effect of the carbonyl and carboxyl groups where the solubilized amylose chains are longer and produce a more compact structure because viscosity did not increase during the cooling step.[Citation10] Oxidized starches showed significantly lower BV than normal starch. The reduction in the BV of starches resulted from the introduction of new substituent groups into the oxidized starches.[Citation25] SV of the starches reduced following oxidation suggesting minimizing of retrogradation. Oxidation causes depolymerization of starch, which results in lower gel viscosity and minimizes retrogradation of amylose by introducing carbonyl and carboxyl groups.[Citation12] At a higher degree of oxidation, the viscosity of paste was found to be constant during heating and cooling, indicating that the PV, BV, and SV were minimally affected by heating and cooling.[Citation17] PTs of starches slightly decreased after oxidation. The reduction of PT following oxidation is a consequence of structural weakening and disintegration during oxidation.[Citation7,Citation16,Citation37]
XRD Pattern
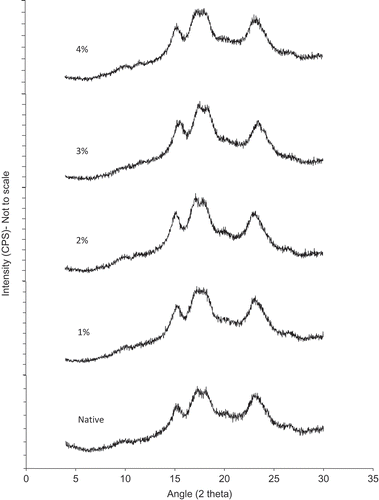
XRD was used to study the crystalline structure of native and oxidized starches. In the diffraction spectra, native starch showed strong diffraction peaks at 15.9° and 23.1° and a doublet between 17.2° and 18.8°(2θ; ). Thus, tamarind kernel starch has A-type XRD pattern. Oxidized starches showed X-ray patterns similar to native starch, as all starches showed diffraction patterns of typical A-type starch. Starch crystallites are due to sequential packing of double helices that are formed between the flexible A-chains of amylopectin.[Citation38] Several evidences indicate that amylopectin is the main contributor to crystalline order within the granule.[Citation6] Oxidation did not result in any significant change in the X-ray pattern of native starch because it took place mainly in the amorphous region.[Citation16] These observations are in agreement with the previous results reported by Kuakpetoon and Wang[Citation13] on potato starch and by Lawal[Citation16] on new cocoyam starch. After oxidative treatment, no change in diffraction pattern was observed but intensity of the peaks increased which suggests that starch crystallinity increased following oxidation. The increasing starch crystallinity is related to the cleavage of amylopectin chain (debranching of amylopectin) that makes amylopectin with lower branches and promotes crystallization.
SEM
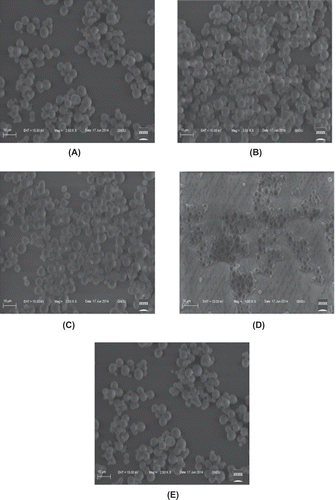
The SEM of native and oxidized starches are shown in . The micrographs revealed the presence of small, oval to round shaped granules for both native and oxidized starches with smooth surfaces. No noticeable difference was observed in the morphology of starch granules among native and the modified derivates. However, oxidation caused some rupture of the starch granules. Since starch granules are semi-crystalline with both amorphous and crystalline regions, the phenomenon observed is due to the fact that oxidation attacks the amorphous region preferentially, thereby removing the region from the surface of the starch, which consequently leads to rupture of the granules.[Citation39] Rutenberg and Solarek[Citation12] reported that the surfaces of corn and potato starch granules were unaffected by hypochlorite oxidation up to 6% active chlorine. No changes in granule morphology of potato, corn, and rice starches modified with sodium hypochlorite (0.8 and 2% active chlorine) has been reported by Kuakpetoon and Wang.[Citation13]
CONCLUSIONS
Modification by sodium hypochlorite at different levels of active chlorine concentration to starch isolated from tamarind kernel caused significant changes in physicochemical and pasting properties. Oxidative treatment resulted in a significant decrease in amylose content of the starches. The swelling power decreased, whereas solubility values increased progressively with the increase in level of oxidant concentration. Both swelling power and solubility were found to be temperature dependent. Oxidized starches showed higher peak viscosity at low active chlorine concentrations in comparison to native starch but at highest concentration a practically linear pasting behavior was observed. Oxidized starches showed improved paste clarity and had a lower tendency to retrograde as indicated by their lower SV in comparison to native counterpart starch. Both native and oxidized tamarind kernel starches showed similar XRD pattern. SEM revealed no noticeable difference between the appearance of the unmodified starch granule and the modified derivatives. There is currently a need in the food industry for starches that possess specific pasting characteristics for a wide range of products. Oxidation of starch with different levels of sodium hypochlorite has been shown to yield oxidized products with different rheological characteristics and could find applications in food products where high solid contents without excessive thickening and clarity are required.
FUNDING
The financial support from University Grants Commission (UGC), New Delhi in the form of a research grant to author M. Kaur is gratefully acknowledged.
Additional information
Funding
REFERENCES
- Siddhuraju, P. Antioxidant Activity of Polyphenolic Compounds Extracted from Defatted Raw and Dry Heated Tamarindus indica Seed Coat. LWT–Food Science and Technology 2007, 40, 982–990.
- Kaur, M.; Sandhu, K.S.; Kaur, J. Pasting Properties of Tamarind (Tamarindus indica) Kernel Powder in the Presence of Xanthan, Carboxymethylcellulose and Locust Bean Gum in Comparison to Rice and Potato Flour. Journal of Food Science and Technology 2013, 50(4), 809–814.
- Rao, P.S.; Srivastava, H.C. Tamarind. In Industrial Gums; Whistler, R.L.; Ed.; Academic Press Inc.; New York, NY, 1974, 370–411.
- Kumar, C.S.; Bhattacharya, S. Tamarind Seed: Properties, Processing, and Utilization. Critical Reviews in Food Science and Nutrition 2008, 48, 1–20.
- Ajayi, I.A.; Oderinde, R.A.; Kajogbola, D.O.; Uponi, J.I. Oil Content and Fatty Acid Composition of Some Underutilized Legumes from Nigeria. Food Chemistry 2006, 99, 115–120.
- French, D. Organization of Starch Granules. In Starch Chemistry and Technology; Whistler, R.L.; BeMiller, J.N.; Paschall, E.F.; Eds.; Academic Press: New York, NY, 1984, 183–247.
- Ali, T.M.; Hasnain, A. Morphological, Physicochemical, and Pasting Properties of Modified White Sorghum (Sorghum Bicolor) Starch. International Journal of Food Properties 2014, 17, 523–535.
- Hoover, R. Composition, Molecular Structure, and Physicochemical Properties of Tuber and Root Starches: A Review. Carbohydrate Polymers 2001, 45, 253–267.
- Moorthy, S.N. Physicochemical and Functional Properties of Tropical Tuber Starches: A Review. Starch/Stärke 2002, 54, 559–592.
- Sanchez-Rivera, M.M.; Garcia-Suarez, F.J.L.; Velazquez del Valle, M.; Gutierrez-Meraz, F.; Bello-Perez, L.A. Partial Characterization of Banana Starches Oxidized by Different Levels of Sodium Hypochlorite. Carbohydrate Polymers 2005, 62, 50–56.
- Wang, Y.J.; Wang, L. Physicochemical Properties of Common and Waxy Corn Starches Oxidized by Different Levels of Sodium Hypochlorite. Carbohydrate Polymers 2003, 52, 207–217.
- Rutenberg, M.W.; Solarek, D. Starch Derivatives: Production and Uses. In Starch Chemistry and Technology; Whistler, R.L.; BeMiller, J.N.; Paschall, E.F.; Eds.; Academic Press: New York, NY, 1984; 311–323.
- Kuakpetoon, D.; Wang, Y. Characterization of Different Starches Oxidized by Hypochlorite. Starch/Stärke 2001, 53, 211–218.
- Adebowale, K.O.; Olu-Owolabi, B.I.; Olawumi, E.K.; Lawal, O.S. Functional Properties of Native, Physically, and Chemically Modified Breadfruit (Artocarpus Artilis) Starch. Industrial Crops Products 2005, 21, 343–351.
- Sangseethong, K.; Termvejsayanon, N.; Sriroth, K. Characterization of Physicochemical Properties of Hypochlorite and Peroxide-Oxidized Cassava Starches. Carbohydrate Polymers 2010, 82, 446–453.
- Lawal, O.S. Composition, Physicochemical Properties and Retrogradation Characteristics of Native, Oxidized, Acetylated, and Acid-Thinned New Cocoyam (Xanthosoma Sagittifolium) Starch. Food Chemistry 2004, 87, 205–218.
- Li, J.H.; Vasanthan, T. Hypochlorite Oxidation of Field Pea Starch and Its Suitability for Noodle Making Using An Extrusion Cooker. Food Research International 2003, 36, 381–386.
- Zhu, Q.; Sjoholm, R.; Nurmi, K.; Bertoft, E. Structural Characterization of Oxidized Potato Starch. Carbohydrate Research 1998, 309, 213–218.
- Conto, L.C.D.; Plata-Oviedo, M.S.V.; Steel, C.J.; Chang, Y.K. Physico-Chemical, Morphological, and Pasting Properties of Pine Nut (Araucaria Angustifolia) Starch Oxidized with Different Levels of Sodium Hypochlorite. Starch/Stärke 2011, 63, 198–208.
- Smith, R.J. Production and Uses Of Hypochlorite Oxidized Starches (Vol. II). In Starch Chemistry and Technology; Whistler, R.L.; BeMiller, J.N.; Paschall, E.F.; Eds.; Academic Press: New York, NY, 1967, 620–625.
- Chattopadhyay, S.; Singhal, R.S.; Kulkarni, P.R. Optimization of Conditions of Synthesis of Oxidized Starch from Corn and Amaranth for Use in Film-Forming Applications. Carbohydrate Polymers 1997, 34, 203–212.
- Williams, P.C.; Kuzina, F.D.; Hlynka, I. A Rapid Calorimetric Procedure for Estimating the Amylose Content of Starches. Cereal Chemistry 1970, 47, 411–420.
- Leach, H.W.; Mc Cowen, L.D.; Schoch, T.J. Stucture of the Starch Granule. I Swelling and Solubility Patterns of Various Starches. Cereal Chemistry 1959, 36, 535–544.
- Perera, C.; Hoover, K. Influence of Hydroxypropylation on Retrogradation Properties of Native, Defatted, and Heat Moisture Treated Potato Starches. Food Chemistry 1999, 64, 361–375.
- Adebowale, K.O.; Lawal, O.S. Microstructure, Functional Properties, and Retrogradation Behavior of Mucuna Bean (Mucuna Pruriens) Starch on Heat Moisture Treatments. Food Hydrocolloids 2003, 17, 265–272.
- Sandhu, K.S.; Kaur, M.; Singh, N.; Lim, S.T. A Comparison of Native and Oxidized Normal and Waxy Corn Starches: Physicochemical, Thermal, Morphological, and Pasting Properties. LWT–Food Science and Technology 2008, 41, 1000–1010.
- Adebowale, K.O.; Afolabi, T.A.; Lawal, O.S. Isolation, Chemical Modification, and Physicochemical Characterization of Bambarra Groundnut (Voandzeia Subterranean) Starch and Flour. Food Chemistry 2002, 78, 305–311.
- Wu, Y.; Lin, Q.; Cui, T.; Xiao, H. Structural and Physical Properties of Starches Isolated from Six Varieties of Millet Grown in China. International Journal of Food Properties 2014, 17, 2344–2360.
- Lim, S.T.; Kasemsuwan, T.; Jane, J.L. Characterisation of Phosphorus in Starch By 31P Nuclear Magnetic Spectroscopy. Cereal Chemistry 1994, 71, 468–472.
- Forssell, P.; Hamunen, A.; Autio, K.; Suortti, T.; Poutanan, K. Hypochlorite Oxidation of Barley and Potato Starch. Starch/Stärke 1995, 47, 371–377.
- Deshpande, S.S.; Sathe, S.K.; Rangnekar, P.D.; Salunkhe, D.K. Functional Properties of Modified Black Gram (Phaseolus Mungo L.) Starch. Journal of Food Science 1982, 47, 1528–1533.
- Adebowale, K.O.; Lawal, O.S. Effects of Annealing and Heat Moisture Conditioning on the Physicochemical Characteristics of Bambarra Groundnut (Voandzeia Subterranea) Starch. Nahrung/Food 2002, 46, 311–316.
- Shieldneck, P.; Smith, C.E. Production and Uses of Acid Modified Starch. In Starch Chemistry and Technology; Whistler, R.L.; BeMiller, J.N.; Paschall, E.F.; Eds.; Academic Press: New York, NY, 1976, 106–124.
- Hodge, J.E.; Osman, E.M. Carbohydrates. In Food Chemistry, Fennema, O.R.; Ed.; Marcel Dekker: New York, NY, 1996; 47.
- Sangseethong, K.; Lertphanich, S.; Sriroth, K. Physicochemical Properties of Oxidized Cassava Starch Prepared under Various Alkalinity Levels. Starch/Stärke 2009, 61, 92–100.
- Guerra Dias, A.R.; Zavareze, E.R.; Elias, M.C.; Helbig, E.; Oliveira da Silva, D.; Ciacco, C.F. Pasting, Expansion, and Textural Properties of Fermented Cassava Starch Oxidised with Sodium Hypochlorite. Carbohydrate Polymers 2011, 84, 268–275.
- Kuakpetoon, D.; Wang, Y.J. Structural Characteristics and Physicochemical Properties of Oxidized Corn Starches Varying in Amylose Content. Carbohydrate Research 2006, 341, 1896–1915.
- Wu, H.C.H.; Sarko, A. The Double Helical Molecular Structure of Crystalline B-Amylose. Carbohydrate Research 1978, 61, 7–40.
- Adebowale, K.O.; Afolabi, T.A.; Olu-Owolabi, B.I. Functional, Physicochemical, and Retrogradation Properties of Sword Bean (Canavalia Gladiata) Acetylated and Oxidized Starches. Carbohydrate Polymers 2006, 65, 93–101.