Abstract
The purified phenolic extracts of Artocarpus heterophyllus, Oldenlandia corymbosa, Cyclosorus extensa, and Alpinia malaccensis were tested for the presence of various polyphenols and antioxidant activities. The polyphenols were extracted with ethanol and purified by stepwise dialysis and column elution. High-performance liquid chromatography was performed to detect and quantitate the polyphenols and 10 different in vitro methodologies were used to measure antioxidant properties. Quercetin, ferulic acid, and salicylic acid were present in all the species. Chlorogenic and p-coumaric acid in A. heterophyllus; p-coumaric acid, caffeic acid, naringin, catechol, and resorcinol in C. extensa; chlorogenic, caffeic, and quinic acid in O. corymbosa and naringin, quinic acid and catechol in A. malaccensis were detected with varied concentrations. The 2-deoxyribose, 1,1-diphenyl-2-picryl-hydrazyl and 2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) assays exhibited high radical scavenging activities and they were also able to combat •OH radicals and H2O2 to an extent of 25.53 and 71.23% respectively. Superoxide anions and nitric oxide radicals were scavenged up to 63.85 and 56.24%, respectively, and the extracts exhibited ferric ions chelation and ferrous ions reduction capacity. A reduction in the level of lipid peroxidation was also observed. All four species were found to be potent sources of polyphenols having antioxidative property. The results fully justified the use of these plants in the beer making process of Assam, India.
INTRODUCTION
Free radicals and reactive oxygen species (ROS) are formed continuously as normal by-products of oxygen metabolism during mitochondrial oxidative phosphorylation in living organisms and results in cell death and tissue damage.[Citation1] It correlates with diseases like cancer, coronary heart diseases, diabetes, neurodegenerative diseases, neural disorders, atherosclerosis, inflammatory injury, and aging.[Citation2] Antioxidants can delay or inhibit the oxidation of lipids or other molecules by inhibiting the initiation or propagation of oxidative chain reactions.[Citation3] The phenolic compounds in plants have antioxidant properties and can act as reducing agents, hydrogen donors, singlet oxygen quenchers, and metal chelating agents.[Citation4]
Rice beer is a traditional alcoholic beverage prepared in Assam, India and the people of this region believe this drink to possess many medicinal properties. Starters in the form of dry powder or hard ball cakes are prepared from various plants and are used in the preparation of rice beer.[Citation5,Citation6] In an initial test of total phenolic compounds content and antioxidant activity (2-deoxyribose, 1,1-diphenyl-2-picryl-hydrazyl; DPPH, radical scavenging activity) conducted by us, Artocarpus heterophyllus, Cyclosorus extensa, Oldenlandia corymbosa, and Alpinia malaccensis were found to have the highest content and activity, and hence, were selected from among the 60 plants screened.
A. heterophyllus grows in many tropical countries of Southeast Asia and is a large evergreen tree which is usually 10–15 m in height.[Citation7] C. extensa is found in India, Sri Lanka, Indo-China, Malaysia, and Australia. In Assam the leaves are traditionally used in the treatment of pneumonia,[Citation8] herpes, and skin infections.[Citation9] O. corymbosa is a prostrate to decumbent, annual herb and grows in areas such as roadsides, lawns, open forests, stream sides, etc.[Citation10] A. malaccensis is a perennial plant growing widely in the subtropical and tropical regions of Asia. It is a large ginger (2 to 4 m tall) in which leafy stems arise from stout rhizomes just below the ground.[Citation11]
These four plant species traditionally used in the making of rice beer in Assam have not been studied vis-à-vis their antioxidant properties. Although certain works have been reported[Citation12–Citation19] on the bioactivities of the extracts of these plants, detailed work on the antioxidant activities of the constituent polyphenols in the extracts has not been reported so far. The present study was aimed to determine content of various polyphenols and compare the antioxidant potentials of the purified phenolic extracts (PPEs) of these plant leaves. Altogether 10 different in vitro methodologies of determining antioxidant properties with distinctive mode of operation were employed and helped in determining the efficacy of various assays.
MATERIALS AND METHODS
Materials
Young and tender leaves of Artocarpus heterophyllus Lam., Cyclosorus extensa (Blume) Ching, Oldenlandia corymbosa L., and Alpinia malaccensis (Burm.f.) Roscoe were collected from the botanical garden of Tezpur University campus during the summer of 2013. The high-performance liquid chromatography (HPLC) standards and the chemicals viz., α tocopherol, 3,5-di-tert-4-butylhydroxytoluene (BHT), DPPH, 2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid; ABTS), nitro blue tetrazoliumchloride (NBT), ethylenediaminetetraacetic acid (EDTA), nicotinamide adenine dinucleotide reduced (NADH), phenazine methosulfate (PMS), thiobarbituric acid (TBA), linoleic acid, and ferrozine were procured from Sigma-Aldrich Corporation (USA). Other chemicals and solvents used for analysis were obtained from E. Merck (Germany). The instruments which were used during the study were a freeze dryer (LDF 5512, Daihan Labtech, Korea), HPLC system (Ultimate 3000, Dionex, Germany), UV-Vis spectrophotometer (Spectrascan UV2600, Thermo Scientific, USA), incubator (LBI-150M, Labtech, Korea), incubator shaker (Excella E24R, NBS, USA), water bath (BW-20G, Jeiotech, Korea), rotary vacuum evaporator (N-1200AS, Eyela, Japan), and ultrasonic bath (RK 510H, Bandelin, Germany).
Extraction and Purification of Phenolic Compounds
Extraction and purification of phenolic compounds from the plants were done as per the method described by Tsujita et al.[Citation20] The freshly plucked plant leaves were freeze dried until a final moisture content of 2% was obtained and then ground in a laboratory blender to a fine powder and passed through a mesh of 50 US size. Dried powdered samples (500 g) were extracted with 1 L of 75% ethanol in an incubator shaker at 100 rpm (37ºC for 48 h). The mixture was filtered through four layers of muslin cloth and extracts were centrifuged at 10,000 × g for 20 min and the supernatant was filtered through Whatman No. 1 filter paper in a vacuum assisted filtration unit. The extract was washed twice with hexane and dried in a rotary vacuum evaporator under reduced pressure and lyophilized. The lyophilized powder was redissolved in 30 mL of deionized water (DW) in an ultrasonic bath to enhance the dissolution process. This solution was dialyzed against 2 L of DW using a 12,000 Da MWCO dialysis membrane (D6066, Sigma-Aldrich, USA). The inner dialyzed materials were concentrated, lyophilized, and dissolved with DW (100 mg/10 mL) and applied to a column (300 mm × 20 mm i.d.) prepared with 20-60 mesh Amberite XAD-2 resin (RM9218-100G, Himedia, India) equilibrated with DW. The column was washed twice with DW and then eluted with 60% aqueous ethanol. The ethanolic eluate was concentrated, lyophilized, and again dissolved in DW (100 mg/10 mL) and applied to a column (300 mm × 20 mm i.d.) prepared with Sephadex® LH-20 (LH20100, Sigma-Aldrich, USA) equilibrated with DW. This column was washed with DW and eluted with 70% aqueous acetone to obtain the PPEs. The PPEs were again concentrated and lyophilized, and various concentrations of the dry powder were made in DW.
Estimation of Phenolic Compounds in the PPEs by HPLC
The eluate from the Sephadex® column were subjected to hydrolysis with 2 N HCl at 100°C for 1 h and neutralized by the addition of 2 mL of 2 N NaOH. The volume of the mixture was made up to 50 mL and the pH was adjusted to 7.0. This was passed through two C18 Sep Pak® cartridges in series which were pre-conditioned with methanol and 20 µL of filtrate was injected for estimation. The quantitative analysis of polyphenols was carried out in a HPLC system with an UV detector at 265 nm. The column used was Acclaim 120® C18 column (5 µm beads size; 120 Å; 4.0 × 250 mm, Thermo Scientific) and the column oven temperature was maintained at 30ºC. The mobile phase used was a mixture of the following two solvents; Eluent A: acidified DW (pH adjusted to 2.64 with dilute HCl) and Eluent B: Acidified DW: acetonitrile (20:80). A gradient run at a constant flow rate of 1.5 mL/min was used for elution.[Citation21]
Estimation of Antioxidant Activities
The lyophilized PPEs were separated to two sets of varying concentrations in DW. The first set (S1) contained concentrations in the series of 20, 40, 60, 80, 100, and 120 µg/mL and the second set (S2) contained concentrations in the series of 50, 100, 150, 200, 250, and 300 μg/mL. Both the sets were made based on some preliminary study in relation to their antioxidant activities, keeping in mind that a correlation is maintained between the concentrations used and the activity shown under various assay conditions. A total of 10 different techniques for evaluation of antioxidant activities were employed. BHT, α-tocopherol, or EDTA were used as reference standards. The reduction in the intensity of color was measured in a spectrophotometer and the antioxidant activities were calculated according to the Eq. (1).
where A0 is the absorbance of the control and A1 is the absorbance in presence of the antioxidant compound. All the absorbance readings were taken in a UV-Vis spectrophotometer.
DPPH Free Radical Scavenging Activity Assay
DPPH reacts with an antioxidant compound that can donate hydrogen and gets reduced to become a stable diamagnetic molecule. It loses this absorption when accepting an electron or a free radical species, which results in a discoloration from purple to yellow. To 4 mL of S1, 2 mL of 0.1 mM DPPH solution in ethanol was mixed. The tubes were incubated for 10 min in the dark and the absorbance was taken at 517 nm and for blank, PPEs and ethanol were used in place of DPPH solution. The absorbance of the DPPH solution incubated with DW was taken as control.[Citation22]
ABTS Radical Cation Decolorization Assay
This technique is involved in the production of the blue/green ABTS+ chromophore through the reaction between ABTS+ and potassium persulphate. The presence of antioxidant compounds compete with ABTS+ and diminish the color formation. To 4 mL of S1, 0.54 mL of ABTS solution (7 mM ABTS and 2.6 mM potassium persulphate in 1:1 ratio, allowed to stand in the dark at room temperature for 16 h before use) and 0.5 mL of 100 mM phosphate buffer (pH 7.4) were added and allowed to stand in the dark for 2 h and the absorbance was taken at 734 nm. For the control, DW was used instead of the PPEs and the blank contained different concentrations of the sample in buffer and DW.[Citation23]
Hydroxyl Radical (•OH) Scavenging Assay
Hydroxyl radicals are generated by direct addition of iron (II) salts to a reaction mixture containing ascorbic acid and the radicals in turn caused degradation of 2-deoxyribose to products with pink color. To 0.6 mL of S2, 0.2 mL of phosphate buffer (0.2 M, pH 7.4), 0.4 mL of 500 μM ferric chloride solution, 0.2 mL of 1 mM ascorbic acid solution, 0.2 mL of 1 mM EDTA, 0.2 mL of 10 mM hydrogen peroxide solution, and 0.4 mL of 15 mM 2-deoxyribose were added and vortexed. The contents were incubated at room temperature for 60 min and 2 mL of 1% TBA in 0.05 N NaOH and 2 mL of 2.8% TCA were added and followed by keeping all the tubes in a boiling water bath for 30 min. The absorbance of the resulting mixture was read at 535 nm and the mixture containing DW instead of the PPEs was used as control.[Citation24]
Hydrogen Peroxide (H2O2) Scavenging Activity Assay
To 3.5 mL of S2, 0.6 mL of 40 mM H2O2 solution in phosphate buffer (pH 7.4) was mixed and the mixture was incubated in the dark for 10 min and absorbance of the mixture was read at 230 nm. The absorbance of the H2O2 solution was taken as a control and phosphate buffer without H2O2 was used as a blank.[Citation25]
Superoxide Anion (O2•-) Scavenging Activity Assay or NBT Assay
Superoxide reduces NBT to form blue colored formazon. Competition for superoxide occurs when an antioxidative substance is added with NBT and decrease in the formation of color occurs which is a measure of its superoxide scavenging activity. To 2 mL of S2, 1 mL of NBT (100 μM NBT in 100 mM phosphate buffer, pH 7.4) solution and 1 mL of NADH (468 μM in 100 mM phosphate buffer, pH 7.4) solution were mixed, followed by the addition of 100 μL of PMS solution (60 μM PMS in 100 mM phosphate buffer, pH 7.4). The reaction mixture was incubated at 30ºC for 15 min and the absorbance of the resulting mixture was measured at 560 nm and incubation with DW instead of the PPEs was used as a control.[Citation26]
Nitric Oxide Radical (NO•) Scavenging Activity Assay
Nitric oxide (NO) which interacts with oxygen to produce nitrite ions is estimated using Griess reagent. Scavengers of NO compete with oxygen, leading to less production of nitrite ions. To 2 mL of S2, 2 mL of 5 mM sodium nitroprusside in 0.5 M phosphate buffer (pH 7.4) and 0.5 mL of chloroform were mixed and the mixture was incubated at 25ºC for 3 h in presence of a visible polychromatic light source. Incubation mixture (2 mL) was mixed with 2 mL of Griess reagent (1% sulfanilamide in 5% phosphoric acid and 0.1% naphthylethylenediaminedihydrochloride, 1:1 ratio) and incubated at room temperature for another 30 min, after which the absorbance of the reaction mixture was read at 546 nm and incubation with DW instead of the PPEs was used as a control.[Citation27]
Ferrous Ion (Fe2+) Chelating Assay
The antioxidants compete with ferrozine for Fe2+ ions in free solution and bivalent transition metal ions like Fe2+ either acts as catalysts of oxidative processes, leading to the formation of hydroxyl radicals or causes hydrogen peroxide decomposition reactions via Fenton reaction. To 4 mL of S2, ferrous chloride (2 mM, 0.1 mL) and ferrozine (5 mM, 0.2 mL) were added and the absorbance was taken at 562 nm after 20 min room temperature incubation in the dark. DW instead of ferrozine was used as a blank and ferrous chloride and ferrozine were used as a control.[Citation2]
Ferric Reducing Antioxidant Power (FRAP) Assay
The amount of Fe (II) complex formed is monitored by measuring the formation of ferric-ferrous complex. Reduction of ferric to ferrous ion increases the absorbance indicating the reducing ability. To 2 mL of S2, 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of potassium ferricyanide (1% solution in DW) were added and incubated at 50ºC for 20 min. This was followed by addition of 1.5 mL of 10% TCA and centrifuged at 3000 × g for 10 min. Supernatant (1 mL) was mixed with 2 mL of DW and 1 mL of ferric chloride (0.1%) and the absorbance was measured at 700 nm and incubation with DW in place of PPE was used as the blank.[Citation28]
Ferric Thiocyanate (FTC) Assay
Ferric ions are formed upon reaction of peroxide with ferrous chloride and then unites with ammonium thiocyanate producing FTC, a red-colored substance. The darker the color the more the peroxidation value, and is reduced in presence of an antioxidant. A 0.02 M linoleic acid emulsion was prepared in phosphate buffer (0.05 M, pH 7.4) and emulsified by adding equal amount of Tween 20. To 0.2 mL of S2, 2.5 mL of linoleic acid emulsion and 2.3 mL of phosphate buffer (0.2 M, pH 7.0) were added and the mixture was placed in screw-capped glass vials, vortexed, and incubated at 37ºC in the dark. To 0.1 mL of reaction mixture, 4.7 mL of 75% ethanol, 0.1 mL 30% ammonium thiocyanate, and 0.1 mL of 0.02 M FeCl2 in 3.5% HCl were added and exactly after 3 min the absorbance was taken at 500 nm. This reading was taken at every 24 h until the absorbance of the control reached a maximum and incubation with DW instead of the PPEs was used as a control.[Citation29]
TBA Assay
The secondary product malonaldehyde is formed and binds at low pH and high temperature with TBA to form a red complex that gives an indication of peroxidation. To 0.2 mL of S2, 2.5 mL of 0.02 M linoleic acid emulsion and 2.3 mL of phosphate buffer (0.2 M, pH 7.0) were added. The mixture was placed in screw-capped glass vials, vortexed and incubated at 37ºC in the dark. To 2 mL of the mixture, 2 mL of 20% TCA and 2 mL of 0.67% TBA (prepared in 0.05 N NaOH) were added and the mixture was kept in a water bath at 100ºC for 10 min, cooled, and centrifuged at 3000 rpm for 20 min. Antioxidant activity was measured from the absorbance of the supernatant at 532 nm and DW instead of PPEs was used as a control.[Citation30]
IC50 Value Calculation
The PPEs’ concentration required to bring 50% of scavenging (IC50) was calculated from the interpolation of the curves plotted for percentage inhibition against the respective concentrations. The IC50 value specifies that the tested preparation contains 50% of the contents of the inhibitor which is enough to exhibit the activity under study. It is important to accurately measure the optimal concentration of the compounds which needs to be administered in order to obtain the desired function. These values might be useful in further processing of these polyphenols in the preparation of functional foods.
Statistical Analysis
Calculation of mean values, standard deviation, and plotting of curves were done using the statistical software OriginPro 8.0 (OriginLab Corporation, USA) and results were expressed as average of three replicates.
RESULTS
Content of Phenolic Compounds in the Leaves
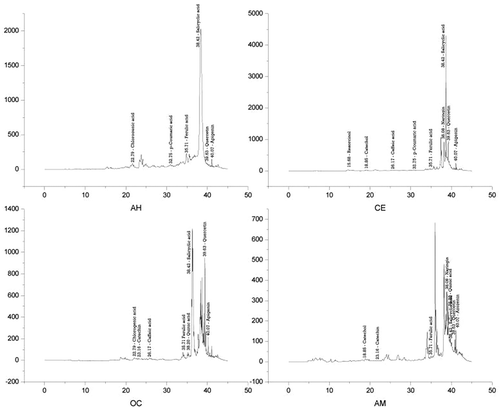
The HPLC chromatograms of phenolic compounds in PPEs are illustrated in and various concentrations are presented in . Quinic acid (C7H12O6) was present in O. corymbosa (2277.69 ppm) and A. malaccensis (1703.75 ppm); however, it was not detected in both A. heterophyllus and C. extensa. Caffeic acid (C9H8O4) was detected in C. extensa (47.63 ppm) and O. corymbosa (110.68 ppm), and not found in A. heterophyllus and A. malaccensis. Ferulic acid (C10H10O4) was found in C. extensa (912.75 ppm) and A. heterophyllus (400.48 ppm) and low content was observed in O. corymbosa (48.73 ppm) and A. malaccensis (15.06 ppm). The highest content of p-coumaric acid (C9H8O3) was detected in A. heterophyllus (90.05 ppm) and lowest in C. extensa (5.87 ppm) and not recorded in both O. corymbosa and A. malaccensis. The highest concentration of chlorogenic acid (C16H18O9) was found in O. corymbosa (259.15 ppm) and lowest in A. heterophyllus (24.69 ppm) and absent in both C. extensa and A. malaccensis. Quercetin (C15H10O7) was recorded in all the four plant species and the highest concentration was observed in O. corymbosa (2088.74 ppm), followed by A. heterophyllus (282.19 ppm), A. malaccensis (202.33 ppm), and C. extensa (150.26 ppm). Salicylic acid (C7H6O3) was t detected in all the four plant species in reasonably high content except in A. malaccensis and the highest content was recorded in A. heterophyllus (24164.93 ppm), followed by O. corymbosa (12534.11 ppm) and C. extensa (4412.78 ppm). Naringin (C27H32O14) content was found to be highest in C. extensa (16020.16 ppm) followed by A. malaccensis (4842.14 ppm) and absent in A. heterophyllus and O. corymbosa. Negligible content of catechin (C15H14O6) was recorded in O. corymbosa (8.94 ppm) and A. malaccensis (3.14 ppm) and absent in A. heterophyllus and C. extensa. Catechol (C6H4[OH]2) was detected only in C. extensa and A. malaccensis having concentrations of 74.91 ppm and 36.90 ppm, respectively. Resorcinol (C6H6O2) was detected only in C. extensa (30.91 ppm). Apigenin (C15H10O5) was detected in A. malaccensis in high concentration (17785.22 ppm), followed by A. heterophyllus (5394.60 ppm), C. extensa (4340.50 ppm), and O. corymbosa (1080.67 ppm). In the present study gallic acid, vanillic acid, syringic acid, and hydroquinone were not detected in all the tested four plant species.
TABLE 1 Phenolic compounds detected by HPLC in the PPE of the four plant species
DPPH and ABTS Scavenging Activity
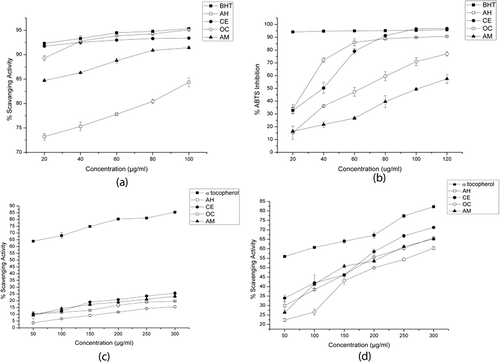
DPPH free radical scavenging activities of the PPEs are shown in and the highest activity was found in O. corymbosa (95.09% at 100 µg/mL) and C. extensa (93.39% at 100 µg/mL) and this result was close to the reference standard BHT (95.33% at 100 µg/mL) and followed by A. malaccensis (91.43% at 100 µg/mL) and A. heterophyllus (84.34% at 100 µg/mL). The IC50 values () of all the PPEs including BHT was below the lowest concentration of the PPEs (20 µg/mL) taken for analysis. ABTS radical decolorization activities of the PPEs against the respective concentrations are presented in . At the concentration of 100 µg/mL the highest activity was noted in C. extensa (96.8%) which was even higher than BHT (95.79%). The activity of A. heterophyllus (90.67% at 100 µg/mL) was also close to C. extensa throughout all concentrations. The IC50 values () of the PPEs were in the order of A. heterophyllus (28.64 µg/mL) > C. extensa (39.95 µg/mL) > O. corymbosa (64.86 µg/mL) > A. malaccensis (103.76 µg/mL).
TABLE 2 The IC50 values of the PPE under various assay conditions
FIGURE 2 A: DPPH free radical scavenging activity assay of the PPE; B: ABTS radical cation decolorization assay of the PPE; C: Hydroxyl radical scavenging assay of the PPE; D: Hydrogen peroxide scavenging activity assay of the PPE. The results are means ± SD (n = 3); (AH: A. heterophyllus; CE: C. extensa; OC: O. corymbosa; AM: A. malaccensis).
•OH Scavenging Activity
Hydroxyl radical scavenging activities of the PPEs are illustrated in . The scavenging activities of all the four PPEs were close; however, C. extensa showed the highest activity of 25.53% at concentration of 300 µg/mL. The IC50 value of α-tocopherol was lower than the lowest experimental concentration (50 µg/mL) and the IC50 values () of all the PPEs were not found within the highest concentration (300 µg/mL) taken for analysis.
H2O2 Scavenging Activity
H2O2 scavenging activities assay of the PPEs () revealed that at the highest concentration of 300 µg/mL, the activity of C. extensa (71.23%) was highest, followed by A. heterophyllus (65.76%), A. malaccensis (65.21%), and O. corymbosa (60.31%). However, their values were lower than the standard α-tocopherol (82.21%). The IC50 values () of the PPEs were in the order of A. malaccensis (146.50 µg/mL) > C. extensa (165.76 µg/mL) > A. heterophyllus (171.48 µg/mL) > O. corymbosa (201.04 µg/mL).
O2•- scavenging activity
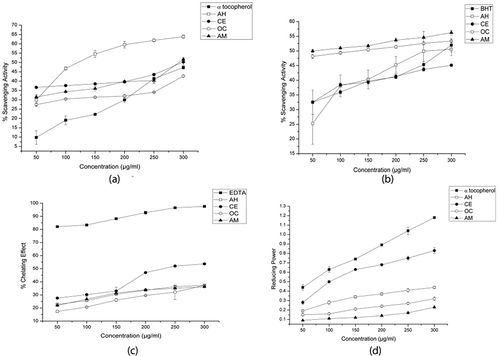
Superoxide anion scavenging activities of the PPE are illustrated in and A. heterophyllus, C. extensa and A. malaccensis evinced better results over α-tocopherol (47.30% at 300 µg/mL), and A. heterophyllus was found to be the most potent in the scavenging of superoxide anions. C. extensa and A. malaccensis also exhibited better results as compared to the reference standard. It was also observed that the IC50 values () of the three PPEs viz., A. heterophyllus (120.92 µg/mL), C. extensa (298.75 µg/mL) and A. malaccensis (293.84 µg/mL) were lower than α-tocopherol.
FIGURE 3 A: Superoxide anion scavenging activity assay of the PPE; B: Nitric oxide scavenging activity assay of the PPE; C: Ferrous-ion chelating assay of the PPE; D: Ferric reducing antioxidant power assay of the PPE. The results are means ± SD (n = 3); (AH: A. heterophyllus; CE: C. extensa; OC: O. corymbosa; AM: A. malaccensis).
NO• Scavenging Activity
NO scavenging activity of the PPE against various concentrations () revealed that the PPEs of A. malaccensis was the most effective with an activity of 56.24% at 300 µg/mL and an IC50 value of 52.51 µg/mL. Furthermore, O. corymbosa and A. heterophyllus also evinced substantial activity and both recorded IC50 values of 130.40 µg/mL and 256.93 µg/mL, respectively (), and was lesser than the reference standard BHT; however, C. extensa was found to have comparatively lesser activity.
Fe2+ Chelating Activity
Ferrous ion chelating activity of the PPEs against various concentrations () revealed that none of the PPEs were as effective as the positive control EDTA. However, among the PPEs, C. extensa produced the highest activity with an IC50 value of 230.38 µg/mL (), followed by A. malaccensis, O. corymbosa, and A. heterophyllus at the maximum concentration of 300 µg/mL.
FRAP Assay
FRAP assay of the PPEs () revealed that the reducing power of C. extensa was almost at par with α-tocopherol. A linear increase in the reducing power of all the PPEs with an increase in concentration was observed. In all the concentrations studied, the order of reducing power of the PPEs was C. extensa > A. heterophyllus > O. corymbosa > A. malaccensis.
FTC Assay
FTC assay of the PPEs () revealed that at 0 h all the PPEs exhibited low activity and their IC50 values () including α-tocopherol were higher than the highest concentration (300 µg/mL) taken for analysis and in addition the highest activity at all concentrations was that of O. corymbosa and lowest activity of inhibition was that of the positive control. At 24 and 48 h, all concentrations of various PPEs and α-tocopherol substantially inhibited the peroxidation of lipid at the highest level. The activity of all the PPE was above 68% and the highest inhibition activity at this period was that of A. heterophyllus (92.31% at 24 h and 84.5% at 48 h, at concentrations of 300 µg/mL). During this period, IC50 values of all the PPE and positive control were below the lowest concentration of the PPEs (50 µg/mL) taken for analysis. However, at 72 h the inhibition of all the PPEs decreased below 80%, and during this time the best activity was shown by C. extensa which had an IC50 value of 50.00 µg/mL and was followed by O. corymbosa (103.01 µg/mL), BHT (112.77 µg/mL), A. heterophyllus (146.72 µg/mL), and A. malaccensis (190.04 µg/mL).
TABLE 3 Ferric thiocyanate assay showing the inhibition of lipid peroxidation by PPE
TBA Assay
TBA assay of the PPEs () revealed that at both 0 and 24 h, the percentage of peroxidation inhibition was found to be low. At 0 h the IC50 values () of all the PPEs including α-tocopherol were higher than the highest concentration (300 µg/mL) taken for analysis. At 24 h, the inhibition activities all the PPE were below 48%. However, certain activity was recorded in O. corymbosa at 24 h, which had an IC50 value of 280.06 µg/mL, and this was close to the IC50 value of α-tocopherol (275 µg/mL). From 48 h onward, a high percentage of peroxidation inhibition was observed for all the PPEs. During 48, 72, and 96 h, IC50 values of all the PPEs and positive control was found below the lowest concentration of the PPEs (50 µg/mL) taken for analysis and the activity of all the PPE throughout all concentrations was almost at par with the reference standard (α-tocopherol).
TABLE 4 Thiobarbituric acid assay showing the inhibition of lipid peroxidation by the PPE
DISCUSSIONS
A wide array of polyphenols was detected in all the tested four plant species. Salicylic acid, a β-hydroxy phenolic acid was found in high amount in all the four species and it has the ability to ease aches, pains, and reduce fevers.[Citation31] From the flavonoid group, quercetin, naringin, apigenin, and catechin were detected in fairly good amounts. Quercetin is a potential anti-cancer agent, including cell cycle regulation, interaction with type II estrogen binding sites, tyrosine kinase inhibition, and has also reports of inhibiting tumor growth.[Citation32] Naringin’s beneficiary activities include anti-inflammatory, antioxidant, cardioprotective, lowering of blood glucose, and cholesterol concentrations and improved insulin signaling.[Citation33] Apigenin has been shown to possess remarkable anti-inflammatory, antioxidant, anticarcinogenic, antimutagenic, antiproliferative, and antiprogression properties.[Citation34] Catechin can exert vascular protective effects through multiple mechanisms like antioxidative, anti-hypertensive, anti-inflammatory, anti-proliferative, anti-thrombogenic, and lipid lowering effects.[Citation35] Caffeic acid and its biosynthesized form ferulic acid were present and they have many physiological functions like antioxidant, antimicrobial, anti-inflammatory, antithrombosis, and anticancer activities. They are also effective against coronary disease, lowers cholesterol, and increases sperm viability.[Citation36] The cinnamic acid derivatives p-coumaric acid and chlorogenic acid were also present at variable amounts. p-Coumaric acid has antioxidant properties, decreases peroxidation of low-density lipoprotein, and has potential to reduce the formation of carcinogenic nitrosamines.[Citation37] Chlorogenic acid has been associated with reduction in risk of cardiovascular disease, type 2 diabetes, Alzheimer’s disease, antibacterial and anti-inflammatory activities.[Citation38] Quinic acid was present and it possesses DNA repair, immune, and anti-inflammatory enhancing properties and versatile chiral starting material for the synthesis of new pharmaceuticals.[Citation39] Resorcinol and catechol were also present in small quantities.
Free radicals play an important role in the development of numerous chronic and degenerative diseases and have been implicated in premature ageing.[Citation40] The results of the DPPH free radical scavenging activity assay suggest a good potential of all the four PPEs. DPPH is a stable free radical which has an unpaired valence electron at one atom of nitrogen bridge and the ability to scavenge this free radical is considered an important antioxidant property.[Citation41]
The PPEs were found to scavenge hydroxyl radical, hydrogen peroxide, superoxide anion, and NO radical. Hydroxyl radical is the most reactive species among the oxygen radicals and induces severe damage to nearby biomolecules. It acts by abstracting hydrogen atoms from biological molecules, thereby leading to the formation of sulfur radicals. These radicals are capable of combining with oxygen to generate oxysulfur radicals, which are toxic to biomolecules.[Citation42] Hydrogen peroxide may give rise to hydroxyl radicals inside the cell which can be toxic. The scavenging of H2O2 may be attributed mainly to the phenolic compounds which can donate electrons to H2O2, thereby neutralizing it to water.[Citation43] A superoxide anion can be generated by a xanthine oxidase/hypoxanthine system. Superoxide radical acts as a precursor of the more ROS such as the hydroxyl radical (HO•), peroxynitrite (ONOO-), and singlet oxygen, which are very harmful to cellular components and causes tissue damage and various diseases in the body.[Citation1] NO radical is a ROS and is implicated in inflammation, cancer, and other pathological conditions in the body and can alter the structure and function of many cellular components.
The PPE also exhibited ferrous ions chelating and ferric reducing activities. The ferrous ions may be released in the breakdown of red blood cells, causing the levels of ferrous ion in the body to increase and it may also be implicated in human cardiovascular disease.[Citation45] The reducing power (electron donating capacity) of bioactive compounds is associated with antioxidant activity. Fe (III) reduction is an important mechanism of phenolic antioxidant action, and the transformation of Fe (III) to Fe (II) due to the reductive ability of the four PPEs can be a strong indicator of the antioxidant activity.
Fairly good inhibition of lipid peroxidation was exhibited by the PPEs. Membrane lipids are rich in unsaturated fatty acids and are most susceptible to oxidative processes. Lipids such as linoleic and arachidonic acids are easy targets of peroxidation. Lipid peroxidation is involved in the formation and propagation of lipid radicals, which eventually destroy membrane lipids.[Citation29] The FTC method determines the amount of peroxide at the initial stage of lipid peroxidation and hence, these PPEs can be beneficial in combating the threat of lipid peroxidation, both in the body and food materials. The TBA assay is used to measure the secondary product of oxidation such as aldehyde and ketone and this assay revealed that the PPE were found to have positive implications in the prevention of lipid peroxidation.
Some significant studies on these and other related species have also been reported earlier by other workers. Omar et al.[Citation12] examined the antioxidative, hypoglycemic, and hypolipidemic activities of A. heterophyllus leaf extracts and concluded that 70% ethanol and n-butanol extracts exert hypoglycemic and hypolipidemic effects in streptozotocin—diabetic rats through an antioxidative pathway that might be referred to their flavonoid contents. The antioxidant and antibacterial activities of A. heterophyllus leaf extracts was tested by Loizzo et al.[Citation13] They found that the MICs determined by agar dilution method against some foodborne pathogens ranged from 221.9 μg/mL for ethyl acetate fraction to 488.1 μg/mL for total extracts. Assays of the extracts revealed significant antioxidant activity through DPPH, ABTS, FRAP, and Fe2+ chelating activity. In another work Chandrika et al.[Citation14] found that the total flavonoid content of A. heterophyllus leaf exhibited a non-toxic and significant hypoglycaemic activity in male Wistar rats. Chen et al.[Citation15] on their work with Cyclosorus acuminatus found that its flavanone-rich extract had renoprotective properties in diabetic mice via modulating the peroxisome proliferator-activated receptors (PPAR) signaling pathway and eventually improving the extents of oxidative stress and inflammatory response. Recently, three new chalcone derivatives together with four known chalcones were isolated from the leaves of Cyclosorus parasiticus by Wei et al.[Citation16] Some of the compounds exhibited substantial cytotoxicity against six cell lines, especially toward HepG2. Pandey et al.[Citation17] found anticancer activity of the ethanolic extracts of O. corymbosa Lam leaves on K562 human leukemia cancer cell line by SRB assay. The ethanolic and aqueous extracts of O. corymbosa also revealed antioxidant activity on chromium induced oxidative stress in albino rats.[Citation18] In another study by Sahoo et al.[Citation19] the antioxidant activity of methanol extract of Alpinia malaccensis leaf was investigated and the IC50 values were found to be 22.5 μg/mL in DPPH, 72.38 μg/mL in NO, 26.23 μg/mL in ABTS, and 80 μg/mL in H2O2 radical scavenging assays, respectively.
CONCLUSION
All the four species are potentially rich sources of polyphenols and have high antioxidative properties. The PPEs were found to quench free radicals, act as reducing agents, and chelate transition metals to suppress the initiation of radical formation and inhibit lipid peroxidation. Based on the assay results, it was observed that all the PPEs exhibited varying levels of antioxidant activities. A. heterophyllus showed good activity in case of DPPH, ABTS, superoxide anion scavenging activity, and FTC assays. C. extensa showed good results for ABTS, hydroxyl radical scavenging, H2O2 scavenging activity, ferrous-ion chelating, FRAP, and FTC assays. O. corymbosa and A. malaccensis exhibited good results in case of TBA and NO scavenging activity assays, respectively. These extracts could effectively be used as natural antioxidants in food, nutraceuticals, cosmetic, and pharmaceutical industries thereby restricting the use of synthetic antioxidants. In addition, they can act as therapeutic or preventive agents against certain degenerative diseases or slowing the oxidative stress in body. All the four plant species are abundantly available throughout the entire Northeastern region of India and has high potential of industrial application. The results of the present study strongly justify the age old use of these plant species in preparation of rice beer of Northeast India and for traditional management of various ailments as well.
FUNDING
The authors are thankful to the Ministry of Food Processing Industries, Government of India, New Delhi for financial support of a project on functional attributes of rice beer of Assam and other Northeastern states of India.
Additional information
Funding
REFERENCES
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Clarendon, Oxford, UK, 1999.
- Halliwell, B. Antioxidants and Human Disease: A General Introduction. Nutrition Reviews 1997, 55(1 Pt 2), S44–S52.
- Huang. D.; Ou, B.; Prior, R.L. The Chemistry Behind Antioxidant Capacity Assays. Journal of Agricultural and Food Chemistry 2005, 53(6), 1841–1856.
- Rice-Evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The Relative Antioxidant Activities of Plant-Derived Polyphenolic Flavonoids. Free Radical Research 1995, 22(4), 375–383.
- Das, A.J.; Khawas, P.; Miyaji, T.; Deka, S.C. HPLC and GC-MS Analyses of Organic Acids, Carbohydrates, Amino Acids, and Volatile Aromatic Compounds in Some Varieties of Rice Beer from Northeast India. Journal of the Institute of Brewing 2014, 120(3), 244–252.
- Das, A.J.; Deka, S.C.; Miyaji, T. Methodology of Rice Beer Preparation and Various Plant Materials Used in Starter Culture Preparation by Some Tribal Communities of North-East India: A Survey. International Food Research Journal 2012, 19(1), 101–107.
- Ashrafuzzaman, M.; Kar, S.; Khanam, D.; Prodhan, S.H. In vitro Regeneration and Multiplication of Jackfruit (Artocarpus Heterophyllus L.). Research Journal of Biology 2012, 2(2), 59–65.
- Acharyya, B.K.; Sharma, H.K. Folklore Medicinal Plants of Mahmora Area, Sivasagar District, Assam. Indian Journal of Traditional Knowledge 2004, 3(4), 365–372.
- Rout, J.; Sajem, A.L.; Nath, M. Medicinal Plants of North Cachar Hills District of Assam Used by the Dimasa Tribe. Indian Journal of Traditional Knowledge 2012, 11(3), 520–527.
- Soerjani, M.; Kostermans, A.J.G.H.; Tjitrosoepom, G. Weeds of Rice in Indonesia; Jakarta: Balai Pustaka; 1987.
- Azah, M.A.N.; Sam, Y.Y.; Mailina, J.; Chua, L.S.L. (E)-Methyl Cinnamate: The Major Component of Essential Oils of Alpinia Malaccensis Var. Nobilis. Journal of Tropical Forest Science 2005, 17(4), 631–633.
- Omar, H.S.; El-Beshbishy, H.A.; Moussa, Z.; Taha, K.F.; Singab, A.N.B. Antioxidant Activity of Artocarpus Heterophyllus Lam. (Jack Fruit) Leaf Extracts: Remarkable Attenuations of Hyperglycemia and Hyperlipidemia in Streptozotocin-Diabetic Rats. The Scientific World Journal 2011, 11, 788–800.
- Loizzo, M.R.; Tundis, R.; Chandrika, U.G.; Abeysekera, A.M.; Menichini, F.; Frega, N.G. Antioxidant and Antibacterial Activities on Foodborne Pathogens of Artocarpus Heterophyllus Lam. (Moraceae) Leaves Extracts. Journal of Food Science 2010, 75(5), 291–295.
- Chandrika, U.G.; Wedage, W.S.; Wickramasinghe, S.M.D.N.; Fernando, W.S. Hypoglycaemic Action of the Flavonoid Fraction of Artocarpus Heterophyllus Leaf. African Journal of Traditional, Complementary, and Alternative Medicines 2006, 3(2), 42–50.
- Chen, J.; Lei, Y.; Liu, Y.; Xiong, C.; Fu, W.; Ruan. J. Extract of Cyclosorus Acuminatus Attenuates Diabetic Nephropathy in Mice Via Modifying Peroxisome Proliferators Activated Receptor Signalling Pathway. Food Chemistry 2011, 128, 659–666.
- Wei, H.; Zhang, X.; Wud, G.; Yang, X.; Pan, S.; Wang, Y.; Ruan, J. Chalcone Derivatives from the Fern Cyclosorus Parasiticus and Their Anti-Proliferative Activity. Food and Chemical Toxicology 2013, 60, 147–152.
- Pandey, K.; Sharma, P.K.; Dudhe, R. Anticancer Activity of Parthenium Hysterophorus Linn and Oldenlandia Corymbosa Lam by Srb Method. Scientific Reports 2012, 1(6), 1–3.
- Sethuramani, A.; Jegadeesan, M.; Kavimani, S. Evaluation of Antioxidant Activity of Ethanolic and Aqueous Extract of Oldenlandia Umbellata and Oldenlandia Corymbosa on Chromium (vi) Induced Oxidative Stress in Albino Rats. International Journal of Pharmacy and Therapeutics 2014, 5(4), 261–266.
- Sahoo, S.; Singh, S.; Nayak, S. Chemical Composition, Antioxidant, and Antimicrobial Activity of Essential Oil and Extract of Alpinia Malaccensis Roscoe (Zingiberaceae). International Journal of Pharmacy and Pharmaceutical Sciences 2014, 6(7), 183–188.
- Tsujita, T.; Yamada, M.; Takaku, T.; Shintani, T.; Teramoto, K.; Sato, T. Purification and Characterization of Polyphenols from Chestnut Astringent Skin. Journal of Agricultural and Food Chemistry 2011, 59(16), 8646–8654.
- Lee, H.S. HPLC Analysis of Phenolic Compounds. In Food Analysis by HPLC, 2nd Ed.; Nollet, L.M.L.; Ed.; Marcel Dekker Inc.: New York, NY, 2000; 775–824.
- Brand-Williams, W.; Cuverlier, M.E.; Berset, C. Use of Free Radical Method to Evaluate Antioxidant Activity. LWT–Food Science and Technology 1995, 28(1), 25–30.
- Wolfenden, B.S.; Willson, R.L. Radical-Cations As Reference Chromogens in Kinetic Studies of One-Electron Transfer Reactions; Pulse Radiolysis Studies of 2, 2,-azinobis-(3-ethylbenzothiazoline-6-sulfonate). Journal of the Chemical Society Perkin Trans 1982, 2, 805–812.
- Halliwell, B.; Gutteridge, J.M.C.; Aruoma, O.I. The Deoxyribose Method: A Simple “Test-Tube” Assay for Determination of Rate Constants for Reactions of Hydroxyl Radicals. Analytical Biochemistry 1987, 165(1), 215–219.
- Rosen, G.M.; Rauckman, E.J. Spin Trapping of Superoxide and Hydroxyl Radicals. Methods in Enzymology 1984, 105, 198–209.
- Nishimiki, M.; Rao, N.A.; Yagi, K. The Occurrence of Superoxide Anion in the Reaction of Reduced Phenazinemethosulphate and Molecular Oxygen. Biochemical and Biophysical Research Communications 1972, 46(2), 849–853.
- Marcocci, L.; Maguire, J.J.; Droy-Lefaix, M.T.; Packer, L. The Nitric Oxide Scavenging Properties of Ginkgo Biloba Extract EGb761. Biochemical and Biophysical Research Communications 1994, 201(2), 748–755.
- Benzie, I.F.F.; Strain, J.J. Ferric Reducing/Antioxidant Power Assay: Direct Measure of Total Antioxidant Activity of Biological Fluids and Modified Version for Simultaneous Measurement of Total Antioxidant Power and Ascorbic Acid Concentration. Methods in Enzymology 1999, 299, 15–27.
- Chang, L.W.; Yen, W.J.; Huang, S.C.; Duh, P.D. Antioxidant Activity of Sesame Coat. Food Chemistry 2002, 78(3), 347–354.
- Farag, R.S.; Badei, A.Z.M.A.; Hawed, F.M.; El-Baroty, G.S.A. Antioxidant Activity of Some Spice Essential Oils on Linoleic Acid Oxidation in Aqueous Media. Journal of the American Oil Chemists’ Society 1989, 66(6), 793–799.
- Mackowiak, P.A. Brief History of Antipyretic Therapy. Clinical Infectious Diseases 2000, 31(5), 154–156.
- Lamson, D.W.; Brignall, M.S. Antioxidants and Cancer III: Quercetin. Alternative Medicine Review 2000, 5(3), 196–208.
- Alam, M.A.; Kauter, K.; Brown, L. Naringin Improves Diet-Induced Cardiovascular Dysfunction and Obesity in High Carbohydrate, High Fat Diet-Fed Rats. Nutrition 2013, 5(3), 637–650.
- Patel, D.; Shukla, S.; Gupta, S. Apigenin and Cancer Chemoprevention: Progress, Potential and Promise (Review). International Journal of Oncology 2007, 30(1), 233–245.
- Babu, P.V.; Liu, D. Green Tea Catechins and Cardiovascular Health: An Update. Current Medicinal Chemistry 2008, 15(18), 1840–1850.
- Ou, S.; Kwok, K.C. Ferulic Acid: Pharmaceutical Functions, Preparation, and Applications in Foods. Journal of the Science of Food and Agriculture 2004, 84(11), 1261–1269.
- Kikugawa, K.; Hakamada, T.; Hasunuma, M.; Kurechi, T. Reaction of p-Hydroxycinnamic Acid Derivatives with Nitrite and Its Relevance to Nitrosamine Formation. Journal of Agricultural and Food Chemistry 1983, 31(4), 780–785.
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic Acids from Green Coffee Extract Are Highly Bioavailable in Humans. Journal of Nutrition 2008, 138(12), 2309–2315.
- Pero, R.W.; Lund, H. In Vivo Treatment of Humans with Quinic Acid Enhances DNA Repair and Reduces the Influence of Lifestyle Factors on Risk to Disease. International Journal of Biotechnology and Biochemistry 2009, 5(3), 293–305.
- Halliwell, B.; Gutteridge, J.M.C. Oxidative Stress: Adaptation Damage Repair and Death. In Free Radical in Biology and Medicine; Halliwell, B.; Gutteridge, J.M.C.; Eds.; Oxford University Press: Clarendon, Oxford, UK, 1989; 285–295.
- Eklund, P.C.; Langvik, O.K.; Warna, J.P.; Salmi, T.O.; Willfo, S.M.; Sjoholm, R.E. Chemical Studies on Antioxidant Mechanisms and Free Radical Scavenging Properties of Lignans. Organic and Biomolecular Chemistry 2005, 3(18), 3336–3347.
- Puppo, A. Effect of Flavonoids on Hydroxyl Radical Formation by Fenton-Type Reactions: Influence of the Iron Chelator. Phytochemistry 1992, 31(1), 85–88.
- Halliwell, B. The Biological Toxicity of Free Radicals and Other Reactive Species. In Free Radicals and Food Additives; Aruoma, O.I.; Halliwell, B.; Eds.; Taylor and Francis: London, UK, 1991; 33–57.
- Lorente, L.; Aller, M.A.; Arias, J.L.; Arias, J. Nitric Oxide (NO) Is a Highly Reactive Free Radical with a Multitude of Organ Specific Regulatory Functions. Annals of Surgery 1996, 224(5), 688–689.
- Halliwell, B.; Gutteridge, J.M.C. Role of Free Radicals and Catalytic Metal Ions in Human Disease: An Overview. Methods in Enzymology 1990, 186, 01–85.