Abstract
Gum karaya is a polysaccharide gum from Sterculia urens tree. It is used as an emulsifier and thickening agent in cosmetics and pharmaceuticals. However, it has very strong swelling properties, high viscosity, and low solubility, providing the restricted applications in the food industry. The main objective of this study was to investigate the effects of different heat treatment and microwave variables (i.e., time: 8, 10, and 12 min; power: 700 and 1000 W) on the functional properties of gum karaya in the aqueous system and oil-in-water emulsion. In this regard, the rheological properties, emulsifying activity, average droplet size, and surface morphology of the native- and microwave-treated gums were analyzed and compared. Dynamic oscillatory test indicated that the microwave-treated gum karaya had more gel-like behavior than viscous-like behavior (G′ > G″) at a relatively high concentration (20% or 20 g/100 g). When gum karaya was treated by microwave for 8–12 min, both elastic (G′) and viscous (G″) moduli were declined. The native- and microwave-treated gum karaya exhibited a shear-thinning (pseudoplastic) behavior in the aqueous system and oil-in-water emulsion. The results showed that the microwave-treated gum karaya had smaller particles than the native gum in the aqueous system. On the other hand, the emulsion containing the microwave-treated gum karaya had finer emulsion droplets than the control containing the native gum karaya. This confirmed that the application of microwave treatment led to significantly (p < 0.05) improve the emulsifying activity of gum karaya.
INTRODUCTION
Polysaccharide gums have a wide range of applications due to their hydrophilic properties. They have many applications as a gelling agent, encapsulating agent, thickener, emulsifier, and stabilizer.[Citation1,Citation2] In the food industry, they are mainly used to modify the physicochemical and functional properties (i.e., rheological properties, solubility, oil- and water-holding capacity) of food products.[Citation3,Citation4] Sourcing natural gums from botanical and plant sources has become an important focus to produce acceptable ingredients in liquids and semi-solid foods.[Citation5] This is mainly because of the positive attitude of consumers toward plant-based gums rather than other gums from animal and microbial sources.[Citation6]
Gum karaya (GK) is a commercially viable polysaccharide gum extracted from Sterculia urens tree which is extensively grown in India.[Citation7] It is an exudate comprised of 60% ordinary sugar dregs (galactose and rhamnose), 40% acidic dregs (galacturonic and glucuronic acid), and 8% acetyl groups.[Citation8] GK has very strong swelling properties, high viscosity, and very poor solubility because of its acetyl groups, therefore, it is mainly used in cosmetics and pharmaceuticals rather than in food processes.[Citation8–Citation11] It was hypothesized that there is a possibility to modify the functional properties of GK through microwave treatment.
Microwave treatment is a simple, relatively safe, and healthy process with many advantages such as energy savings and precise process control.[Citation12,Citation13] In fact, it is known as a cost-effective technique, as it consumes low levels of energy and is environmentally friendly.[Citation14] The use of microwave treatment has gained many interests and supports among researchers. Microwave heating is mostly applied as an alternative for a conventional drying. Furthermore, the microwave treatment can significantly affect the physicochemical properties and functional characteristics of many components. Currently, it is used to improve the physicochemical and functional properties of different plant gums.[Citation15] Electromagnetic spectrum microwaves are between infrared and radio waves with 0.3–30 GHz frequency range and 0.01–1 m wavelength. For industrial applications, it would typically be 915–2450 MHz over a short exposure. Microwave process in such frequency levels can provide rapid, uniform, and volumetric thermal process.[Citation16] In microwave heating, the sample begins to be heated from the center. Therefore, the moisture is driven outward as vapor, toward the surface where it condenses.[Citation17] Previous researchers described the significant impact of microwave treatment on functional properties of gums.[Citation18–Citation22]
The main objective of this study was to investigate the effects of heat treatment and microwave variables (i.e., time, 8, 10, and 12 min; power, 700 and 1000 W) on the functional properties of GK in the aqueous system and oil-in-water (O/W) emulsion.
MATERIALS AND METHODS
Materials
High pure GK (99.5%) was purchased from Nutriroma Co. (Hyderabad, Andhra Pradesh, India). The pH of GK solution (1% or 1 g/100 g) was 4.30. Canola oil was purchased from the local market (Selongor, Malaysia). Citric acid was supplied by Riedel-deHaen (Victoria, Austria). Sodium benzoate was supplied by Fisher Scientific (Pittsburgh, PA, USA).
Microwave Treatment of GK
For microwave treatment, 100 g of GK was placed in a porcelain bowl and heated in a sand bath at 120°C for 2 h according to the method reported earlier.[Citation23] Then, different aqueous solutions (5% or 5 g/100 g) containing the native- and microwave-treated gums were prepared. All gum solutions were heated to achieve full hydration. The gum solutions were transferred to different Petri dishes for cooling down at room temperature. Finally, they were subjected to different microwave treatments (i.e., 700 W and 1000 W for 8, 10, and 12 min; ). The treatment was carried out by means of a microwave (Model MS-1921HE, LG Electronics Inc., India). The microwave-treated gums were then passed through a sieve (No. 80) before storing at 4°C for further analysis.[Citation19] The microwave treatments were in duplicates.
FIGURE 1 Flow chart representing microwave treatment of gum karaya.
Preparation of O/W Emulsion
In this study, several emulsions containing the native- and microwave-treated gums were prepared according to the method reported by previous researchers.[Citation24,Citation25] Initially, sodium benzoate (0.1% or 0.1 g/100 g), citric acid (0.3% or 0.3 g/100 g), and GK (1% or 1 g/100 g) were added to the distilled water to prepare the gum solution. The mixture was stored at room temperature for one overnight to facilitate hydration before making a coarse emulsion. Then, the known amount (20% or 20 g/100 g) of canola oil was gradually added to the hydrated continuous phase. The mixture was stirred for 5 min to form the coarse emulsion. Finally, the coarse emulsion was homogenized by a high pressure homogenizer (APV, Crawley, UK) at 30 MPa for two cycles.[Citation26]
Analytical Tests
Surface morphology analysis
A scanning electron microscope (Quanta 200™ FEG, FEI Co., Hillsboro, OR, USA) was used to capture the topographical images of GK before and after applying microwave treatment. The powder specimens were mounted using a double-sided carbon adhesive tape onto aluminium strips (Electron Microscopy Sciences, Hatfield, PA, USA). Scancoat Six sputter coater (BOC Edwards, Wilmington, MA, USA) was used to coat the sample with a thin gold as a conductor. High-vacuum secondary-electron imaging mode with the voltage of 10 kV was applied for analysis. The images were taken in 250× magnification. This experiment was carried out in duplicate for each sample.[Citation27]
Determination of droplet size and distribution
The average droplet size and distribution of gum solutions and O/W emulsions were determined by using a Malvern particle size analyzer (Mastersizer 2000S, Malvern Instruments Inc., Westborough, MA, USA). The measurement of average droplet size was performed immediately after preparing samples in the fresh form. To avoid multiple scattering effects, the samples were diluted with deionized water prior to analysis. The droplet absorbance of 0 and refractive indices of 1.466 and 1.333 were considered for the dispersed phase and continuous phase, respectively. The surface weighted mean diameter (d32) and volume weighted mean diameter (d43) were measured to determine the average droplet size. In fact, d43 value was measured to monitor any changes in droplet-size distribution occurred during storage.[Citation28] The gum solution (0.5% or 0.5 g/100 g) was prepared with a magnetic stirrer at 25°C. The solution was then hydrated for 24 h before analysis.[Citation29] In this experiment, the span was measured as an indicator for the particle size distribution. The span and average droplet size were calculated based on the following formula:
where D(ʋ,0.1), D(ʋ, 0.5), and D(ʋ, 0.9) are diameters at 10, 50, and 90% cumulative volume, respectively;[Citation30]
where ni is the number of droplets with diameter Di.[Citation31]
Measurement of dynamic rheological properties
The rheological properties of gum solutions and O/W emulsions were measured by using a rheometer (RheoStress 600, Haake instruments, Karlsruhe, Germany) with the sensor C 60/2°. In this experiment, the apparent viscosity, elastic modulus (G′), and viscous modulus (G″) of gum solutions were measured under a controlled experimental condition. Initially, 1 mL continuous phase or gum solution was dispensed onto the rheometer plate and left to be equilibrated for 10 min at 25°C. Then, different shear rates (10–200 1/s) were applied to assess the apparent viscosity. For measurement of viscoelastic properties, the elastic modulus (G′) and viscous modulus (G″) of gum solutions were measured at a fixed stress level (0.5 Pa) at different frequency levels (0.1–10 Hz).[Citation32,Citation33] The dynamic viscoelastic modulus (G*) is dependent on both elastic and viscous modulus (G′ and G″) as a function of the oscillation frequency:[Citation32,Citation33]
where (σ), (γ), and phase shift (δ) are three rheological stress-strain oscillation variables. σ0 and γ0 are the amplitudes of stress and strain (t = 0), respectively.[Citation32,Citation33]
Emulsion stability (ES) test
A test tube was filled with 13 mL of the fresh emulsion. Then it was maintained at 25 ± 1 C for 30 days storage.[Citation34] The cream opaque layer at the surface was then separated from the translucent/turbid serum at the base. This experiment was carried out in triplicate for each sample. ES was calculated based on the following equation:
where HE is the overall height of the emulsion. HC and HS are the heights of the cream and serum layers, respectively.[Citation35]
Determination of moisture content
The moisture content of GK was determined in duplicate according to the previous method.[Citation36] Almost 2 g of GK was weighted in the crucible. Then, it was put into an oven at 105°C overnight. Once, the crucible was cooled, it was weighed regularly until it reached a constant weight.[Citation36]
Experimental Design and Data Analysis
A full factorial model was used to prepare different microwave treatments based on the applied factors or independent variables (, ). The native GK was considered as a control for all assessments. Minitab version 16 (Minitab Inc., State College, PA, USA) was applied to generate the experimental design and further data analysis.[Citation37]
TABLE 1 p-Value and F-ratio of microwave condition variables in the final reduced model (emulsion system)
TABLE 2 p-Value and f-ratio of microwave variables in the final reduced model (aqueous system)
RESULTS AND DISCUSSION
Surface Morphology of GK
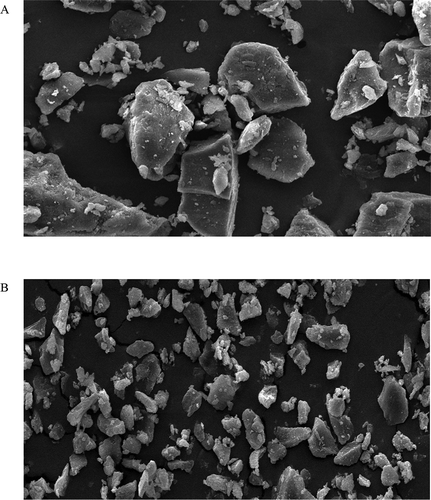
shows the morphology surface structure of the native GK (A) and microwave-treated gum (B) with the magnification of 250×. The results showed that the microwave-treated sample (B) had smaller particles than the native gum (control). This might be explained by the significant effect of the microwave treatment on the molecular weight of GK. The microwave-treated gum had higher solubility than the native gum. This could be because of the significant reduction in particle size, viscosity, and molecular weight of GK after applying microwave treatment. The improved solubility might be also because of the porosity and destruction induced by microwave treatment in the microstructure of GK. This observation was also reported by previous researchers.[Citation38] They reported that the porosity and destruction levels of dried berries were increased by applying microwave treatment. The reduction of particle size aggregation might be also responsible for improvement of the solubility.[Citation39]
Effect of Heat and Microwave Treatments on Functional Properties of GK
Effects of heat and microwave treatments on average droplet size and distribution
The results showed that the microwave variables had the most significant (p ˂ 0.05) effect on the volume weighted mean (d43) of GK in O/W emulsion. Conversely, they induced the least significant (p ˂ 0.05) effect on the ES as shown by its low F-ratio (, ). On the other hand, the microwave variables had the most and least significant (p < 0.05) on span (particle size distribution) and viscosity of GK in the aqueous system, respectively (). shows the droplet size distributions of all samples. The results showed that the average droplet size of O/W emulsions ranged from 0.4 to 5.2 μm, depending on the applied microwave condition. The current study revealed that the particle size of GK was considerably decreased after applying heat and microwave treatments. In most cases, the heated samples had smaller particles than the non-heated samples. The percentage of size reduction was increasing by enhancing thermal and microwave processing condition. In fact, the microwave-treated gum L′′ subjected to the harsh microwave process (heated gum, 1000 W, 12 min) had the smallest average droplet size (0.44 μm). Conversely, the unheated gum (Aʹ), which was mildly treated at 700 W for 8 min, had the largest droplet size (5.29 μm) among all microwave-treated samples ().
TABLE 3 Effect of different heat- and microwave treatments on average particle size and distribution of gum karaya in oil in water (O/W) emulsion
The emulsions containing different microwave-treated gums had smaller droplets then the emulsion containing the native (non-treated) gum (). This might confirm that the microwave-treatment substantially improved the emulsifying activity of GK, resulting in finer emulsion droplets. On the other hand, the microwave-treated gum had smaller particles than the native gum. This indicates the significant impact of microwave treatment on the particle size of GK. The hydration time of the gum is usually longer if its particle is bigger. More extensive hydration and droplet expansion may result from the smaller particles. In fact, the presence of smaller particles in the gum can facilitate its hydration, thereby contributing more efficiently in emulsifying oil droplets.[Citation40] In the aqueous system, the particle size distribution of the microwave-treated samples varied from ~0.72 to 1.94, while the control sample had relatively higher span (2.16) than the microwave-treated gum (). This confirmed that the microwave-treated gum induced lower polydispersity and more uniform particles than the native GK. This could be explained by the presence of various particles with different sizes, resulting in lower span (or more polydispersity).[Citation41] The same observation was also reported for the microwave-treated gellan gum.[Citation19]
FIGURE 3 Effect of different heat and microwave treatments on the particle size distribution of gum karaya; A: without heat treatment and B: with heat treatment (120°C, 2 h)
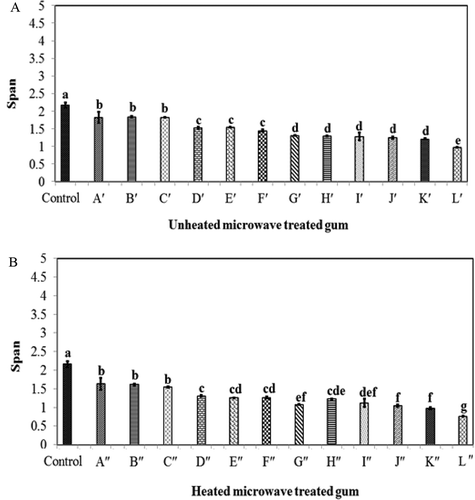
As shown in , the particle uniformity was improved and span was decreased by enhancing microwave treatment conditions (). The results showed that the particle uniformity was improved by prolonging microwave treatment from 8 to 12 min. The similar improvement was observed by increasing microwave power level from 700 to 1000 W. Extensive microwave-based tests was also applied for treating other polysaccharide gums such as sodium alginate, xanthan gum, and carrageenan.[Citation42] In most cases, the similar improvement in the particle uniformity was reported by the researchers.[Citation42]
Effects of heat and microwave treatments on rheological properties of GK
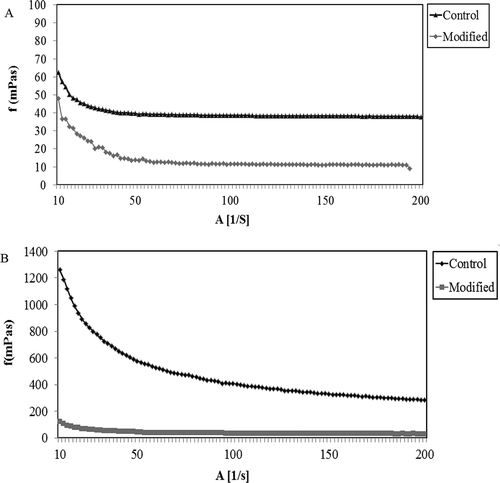
The viscosity and viscoelastic properties of polysaccharide gums depend on the type and condition of applied treatments.[Citation43] shows the viscosity of the aqueous system and O/W emulsions containing the native- and microwave-treated gums at a particular shear rate. The results indicated that the viscosity of GK was decreased by applying different heat and microwave treatments. In fact, the microwaved-treated gums showed lower viscosity than the native GK in the aqueous system and O/W emulsion. This might be explained by the significant effect of microwave treatment on the molecular structure of GK. On the other hand, the unheated microwave-treated gum induced higher viscosity than the heated-microwave-treated gum (). This might be explained by the possible degradation in the molecular structure of GK after applying thermal treatment. This might be explained by the fact that the application of heat and microwave treatments led to decrease particle size, thus lowering viscosity.[Citation32]
TABLE 4 Effect of different heat and microwave treatments on the apparent viscosity of gum karaya in the aqueous system and O/W emulsion
The viscosity of the microwave-treated gums in the unheated form ranged from 58.25 to 383.5 (mPa.s) and 21.78 to 37.81 (mPa.s) in the aqueous system and O/W emulsion, respectively. After applying heat treatment along with microwave treatment, the viscosity of the microwave-treated gums varied from 25.5 to 256.5 (mPa.s) and 10.51 to 31.14 (mPa.s) in the aqueous system and O/W emulsion, respectively. As mentioned earlier, all treated samples had lower viscosity than the native GK. This indicates the reduction of viscosity after applying heat and microwave treatment. On the other hand, this might be due to the presence of very large particles in the native GK; while the microwave-treated gums had smaller particles and more particle uniformity than the native gum (control). According to Goldstein,[Citation44] the viscosity of GK is directly proportional to its volatile acetyl content. In fact, the viscosity of GK is decreased by degrading its volatile acetyl group. This might be responsible for the viscosity reduction of GK after applying heat and microwave treatments. In this research, there was a possibility for degradation of volatile acetyl group when GK was heated in the sand bath. Previous researchers[Citation45–Citation47] also reported the viscosity reduction when the microwave treatment was prolonged at the elevated temperature. In fact, the longer exposure to microwave treatment at high temperature results in the advanced reduction of viscosity. The apparent viscosity of the native- and microwave-treated gum was decreased by increasing shear rate. This confirmed that GK exhibited non-Newtonian (shear-thinning) behavior before and after applying heat and microwave treatments (). Similar shear-thinning behaviors have been reported for seed gums from P. flexuosa DC,[Citation2] Alyssum homolocarpum,[Citation48] and flaxseed.[Citation49]
FIGURE 4 Steady shear rate dependence of viscosity of A: gum karaya control and microwave modified gum in emulsion system and B: gum karaya control and microwave modified gum in aqueous system.
The potential industrial uses of gum depend on the viscoelastic rheological behavior.[Citation50] The elastic (G′) and viscous (G″) moduli of GK were examined to test its viscoelastic behavior.[Citation51] shows the viscous modulus (Gʺ) and elastic modulus (Gʹ) of the native- and microwave-treated GK in the aqueous system and O/W emulsion. The results showed that the elastic (G′) and viscous (G″) moduli of GK were significant declined by applying heat and microwave treatment. This indicated the viscoelastic behavior of GK became weaker after exposure to heat and microwave treatments. There is sufficient time to disrupt reforming bonds at the applied frequencies (0.1–10 Hz) during one period of oscillation test.[Citation49] The microwave-treated gum (L′′) had weaker viscoelastic behavior (lower G′ and G″) than the native gum in the aqueous system and O/W emulsion ().
TABLE 5 Effect of different heat and microwave treatments on the viscous modulus (G”) and elastic modulus (G’) in the aqueous system and O/W emulsion
The native- and microwave-treated gums exhibited stronger elastic behavior than viscous behavior (G′ > G″) at the low frequency. This means that GK has consistent solid- or gel-like behavior at the low frequency (). Similar results was reported for flaxseed gum, where the elastic modulus (G′) was higher than the viscous modulus (G″) over the whole frequency range.[Citation49] Previous researchers[Citation52] also reported that the viscoelasticity and elastic behavior of gum Arabic, pectin, and hydroxypropyl methylcellulose were significantly declined by applying heat treatment. In the aqueous system, the elastic modulus of GK was 15 Pa at low frequency. This elastic behavior was stronger than the elasticity (0.55–0.92 Pa) reported for flaxseed gum by previous researchers.[Citation49] Under similar experimental conditions, GK has stronger viscoelastic behavior than flaxseed gum.
Effects of heat and microwave treatments on emulsifying activity of GK
The effectiveness of heat and microwave treatments on the emulsifying activity of GK was investigated by comparing the stability of O/W emulsions containing the native- and microwave-treated gums after 30 days storage. The emulsion can retain stability if the small droplets do not attract each others. When the droplet coalescence or collision period exceeds the time required for adsorption of the gum into the interface, small emulsion droplet retention is feasible.[Citation53] shows the effect of different microwave treatments on the emulsion activity of GK before and after applying heat treatment (120°C, 2 h). The current study revealed that the emulsifying activity of GK was significantly improved by applying heat and microwave treatments. In most cases, the emulsifying activity of GK was more considerably improved by prolonging microwave treatment. On the other hand, the microwave power level had also positive effect on the emulsifying activity of GK. In fact, the microwave-treated gum had stronger emulsion activity than the native GK ().
FIGURE 5 Effect of different heat and microwave treatments on the emulsion stability of gum karaya A: without heat treatment and B: with heat treatment (120°C, 2 h).
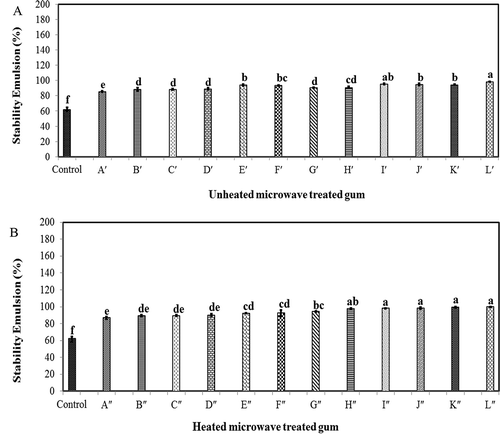
The results showed that the microwave-treated gum L′′ had the smallest droplet size and most desirable particle uniformity (the lowest span; 0.89 μm) among all microwave-treated gums. The emulsion containing the microwave-treated gum L′′ had the highest stability among all prepared emulsions (). On the other hand, the emulsion containing the native gum showed the lowest stability after 30 days storage. This might be explained by the significant reduction of particle size through microwave treatment. The phase separation occurred after 10 days storage when the emulsion had a relatively high average droplet (~22 μm). This might be explained by the reduction of repulsive forces among emulsion droplets, thus causing droplet collision and consequently lowering ES.
Effects of heat and microwave treatments on moisture content of GK
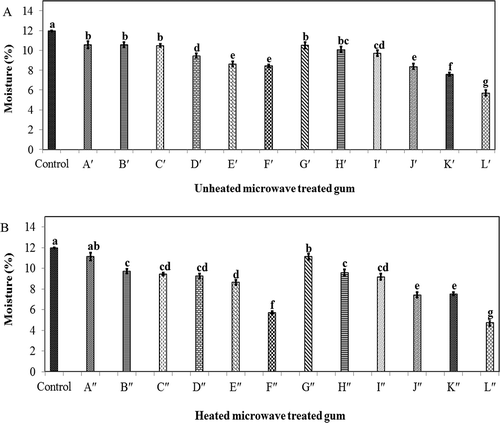
Moisture content of GK was determined by measuring the weight loss of the samples in a vacuum oven at 105°C for one overnight. The moisture content of GK ranged from ~5 to 12% (w/w) depending on the applied microwave treatment (). This was comparable with the moisture contents reported for locust bean gum (7.92%), guar gum (7.36%), and tragacanth (6.31%).[Citation54] The results showed that the moisture content of GK was decreased by prolonging microwave process (). This might be due to the effect of the microwave energy on the water molecules, which turns it to vapor. The microwave-treated gum L′′ had the lowest moisture content among all samples. As illustrated by pervious researchers, the sample with lower moisture content is more hygroscopic linking to the greater water gradient between the sample and the surrounding air.[Citation55] Therefore, the microwave-treated gum L′′ seems to be more hygroscopic than the control (native GK) and other microwave-treated gums. On the other hand, the native gum and mildly treated gum (Gʺ) had the highest moisture content among all samples. Therefore, they provided the least hygroscopic properties among all samples.
FIGURE 6 Effect of different heat and microwave treatments on the moisture content of gum karaya; A: without temperature and B: with temperature (120°C, 2 h).
CONCLUSION
The main objective of this study was to investigate the effect of different microwave variables on emulsifying activity and other functional characteristics of GK in aqueous system and O/W emulsion. In general, the application of heat and microwave treatments led to decrease moisture content, particle size and viscosity. On the other hand, the particle uniformity, porosity, and emulsifying activity of GK were significantly improved by microwave treatment. This might be explained by the significant effect of microwave treatment on the microstructure of GK. SEM analysis confirmed some changes in the morphology structure of GK as a result of microwave treatment. The current study revealed that the viscoelastic properties of GK became weaker through microwave treatment. The native and microwave-treated gum had a shear-thinning behavior in both aqueous and emulsion systems. Among all prepared sample, the microwaved treated gum L′′ (1000 W for 12 min) had the most desirable rheological and functional properties. It had the most desirable particle uniformity and emulsifying activity among all samples. Therefore, the current study suggests microwave treatment for the improvement of functional properties of GK. The optimization of microwave treatment for GK is highly recommended.
FUNDING
We gratefully appreciate the financial support provided for this research by the Ministry of Science, Technology, and Innovation Science (03-01-04-SF1884).
Additional information
Funding
REFERENCES
- Williams, P.A.; Phillips, G.O. Handbook of Hydrocolloids; Phillips, G.O.; Williams, P.A.; Eds.; CRC Press: New York, NY, 2000; 1‒14.
- Patel, S.; Goyal, A. Applications of Natural Polymer Gum Arabic: A Review. International Journal of Food Properties 2015, 18, 986‒998.
- Nussinovitch, A. Hydrocolloid Applications; Blackie Academic and Professional: London, 1997; 140‒151.
- Tabatabaee Amid, B.; Mirhosseini, H. Optimization of Aqueous Extraction of Gum From Durian (Durio Zibethinus) Seed: A Potential, Low Cost Source of Hydrocolloid. Food Chemistry 2012, 132, 1258–1268.
- Rana, V.; Rai, P.; Tiwary, A.K.; Singh, R.S.; Kennedy, J.F.; Knill, C.J. Modified Gums: Approaches and Applications in Drug Delivery. Carbohydrate Polymer 2011, 83, 1031‒1047.
- Nishinari, K.; Zhang, H.; Ikeda, S. Hydrocolloid Gels of Polysaccharides and Proteins. Current Opinion Colloid Interfacial 2000, 5, 195‒201.
- Verbeken, D.; Dierckx, S.; Dewettinck, K. Exudate Gums: Occurrence, Production, and Applications. Applied Microbiology Biotechnology 2003, 63, 10‒21.
- Morris, E.R. Food Polysaccharides and Their Applications; Stephens, A.M.; Ed.; Marcel Dekker: New York, NY, 1995; 341‒375.
- Bhardwaj, T.R.; Kanwar, M.; Lal, R.; Gupta, A. Natural Gums and Modified Natural Gums As Sustained-Release Carriers. Drug Development and Industrial Pharmacy 2000, 26, 1025‒1038.
- Weiping, W.; Phillips, G.; Williams, P. Tragacanth; Karaya. In: Handbook of Hydrocolloids. Philips, G.O. and Williams, P.A.; Eds., Woodhead Publishing Limited: New York, 2000, 231‒245.
- Silva, D.; Brito, A.; De Paula, R.; Feitosa, J.; Paula, H. Effect of Mono and Divalent Salts on Gelation of Native, Na and Deacetylated Sterculia Striata and Sterculia Urens Polysaccharide Gels. Carbohydrate Polymer 2003, 54, 229‒236.
- Biswas, G.R.; Maity, S. Solubility Enhancement of Poorly Water Soluble Drug Amoxycillin Trihydrate by Modified Gum Karaya Using Solid Dispersion Technique. Drug Formula Research 2011, 2, 235‒249.
- Izli, N.; Isik, E. Color and Microstructure Properties of Tomatoes Dried by Microwave, Convective, and Microwave-Convective Methods. International Journal of Food Properties 2015, 18, 241‒249.
- Li, Y.; Yang, W. Microwave Synthesis of Zeolite Membranes: A Review. Journal of Membrane Science 2008, 316, 3‒17.
- Uslu, T.; Atalay, Ü.; Arol, A.I. Effect of Microwave Heating on Magnetic Separation of Pyrite. Colloids and Surfaces A. Physicochemical and Engineering 2003, 225, 161‒167.
- Shogren, R.L.; Biswas, A. Preparation of Water-Soluble and Water-Swellable Starch Acetates Using Microwave Heating. Carbohydrate Polymer 2006, 64, 16‒21.
- Albert, Á.; Salvador, A.; Hough, G.; Fiszman, S. Influence of Outer Layer Formulation on the Sensory Properties of Microwaved Breaded Nuggets. International Journal of Food Properties 2014, 17, 829‒841.
- Singh, V.; Tiwari, A. Hydrolytic Fragmentation of Seed Gums under Microwave Irradiation. International Journal of Biological Macromolecules 2009, 44, 186–189.
- Shah, D.; Jani, G.A. Newer Application of Physically Modified Gellan Gum in Tablet Formulation Using Factorial Design. Ars Pharmaceutica 2010, 51, 28‒40.
- Rani, P.; Sen, G.; Mishra, S.; Jha, U. Microwave Assisted Synthesis of Polyacrylamide Grafted Gum Ghatti and Its Application As Flocculant. Carbohydrate Polymer 2012, 89, 275‒281.
- Kumar, A.; Singh, K.; Ahuja, M. Xanthan-g-Poly (Acrylamide): Microwave-Assisted Synthesis, Characterization and in Vitro Release Behaviour. Carbohydrate Polymer 2009, 76, 261–267.
- Likhitha, M.; Sailaja, R.R.N.; Priyambika, V.S.; Ravibabua, M.V. Microwave Assisted Synthesis of Guar Gum Grafted Sodium Acrylate/Cloisite Superabsorbent Nanocomposites: Reaction Parameters and Swelling Characteristics. International Journal of Biological Macromolecules 2014, 65, 500–508.
- Murali Mohan Babu, G.V.; Prasad, C.D.S.; Ramana Murthy, K.V. Evaluation of Modified Gum Karaya As Carrier for the Dissolution Enhancement of Poorly Water-Soluble Drug Nimodipine. International Journal of Pharmaceutics 2002, 234, 1‒17.
- Yadav, M.P.; Parris, N.; Johnston, D.B.; Onwulata, C.I.; Hicks, K.B. Corn Fiber Gum and Milk Protein Conjugates with Improved Emulsion Stability. Carbohydrate Polymer 2010, 81, 476‒483.
- Tabatabaee Amid, B.; Mirhosseini, H. Emulsifying Activity, Particle Uniformity, and Rheological Properties of a Natural Polysaccharide-Protein Biopolymer from Durian Seed. Food Biophysics 2012a, 7, 317‒328.
- Mirhosseini, H.; Tan, C.P.; Taherian, A.R.; Boo, H.C. Modeling the Physicochemical Properties of Orange Beverage Emulsion As Function of Main Emulsion Components Using Response Surface Methodology. Carbohydrate Polymer 2009, 75, 512‒520.
- Yadav, M.P.; Strahan, G.D.; Mukhopadhyay, S.; Hotchkiss, A.T.; Hicks, K.B. Formation of Corn Fiber Gum-Milk Protein Conjugates and Their Molecular Characterization. Food Hydrocolloid 2012, 26, 326‒333.
- Kasran, M.; Cui, S.W.; Goff, H.D. Emulsifying Properties of Soy Whey Protein Isolate-Fenugreek Gum Conjugates in Oil-in-Water Emulsion Model System. Food Hydrocolloid 2013, 30, 691‒697.
- León-Martínez, F.; Rodríguez-Ramírez, J.; Medina-Torres, L.; Méndez Lagunas, L.; Bernad-Bernad, M. Effects of Drying Conditions on the Rheological Properties of Reconstituted Mucilage Solutions (Opuntia Ficus-Indica). Carbohydrate Polymer 2011, 84, 439‒445.
- Kováčová, R.; Synytsya, A.; Stetina, J. Characterisation of Whey Proteins–Pectin Interaction in Relation to Emulsifying Properties of Whey Proteins. Czech Journal of Food Sciences 2009, 27, S4‒S8.
- Samavati, V.; Emam-Djomeh, Z.; Mohammadifar, M.A. Physical and Rheological Characteristics of Emulsion Model Structures Containing Iranian Tragacanth Gum and Oleic Acid. Dispersion Science and Technology 2013, 34, 1635–1645.
- Tabatabaee Amid, B.; Mirhosseini, H. Influence of Different Purification and Drying Methods on Rheological Properties and Viscoelastic Behaviour of Durian Seed Gum. Carbohydrate Polymer 2012b, 90, 452‒461.
- Tabatabaee Amid, B.; Mirhosseini, H. Shear Flow Behaviour and Emulsion-Stabilizing Effect of Natural Polysaccharide-Protein Gum in Aqueous System and Oil/Water (O/W) Emulsion. Colloids and Surfaces B 2013, 103, 430–440.
- Golkar, A.; Nasirpour, A.; Keramat, J.; Desobry, S. Emulsifying Properties of Angum Gum (Amygdalus Scoparia Spach) Conjugated to β-Lactoglobulin Through Maillard-Type Reaction. International Journal of Food Properties 2015, 18, 2042–2055.
- Huang, X.; Kakuda, Y.; Cui, W. Hydrocolloids in Emulsions: Particle Size Distribution and Interfacial Activity. Food Hydrocolloid 2001, 15, 533‒542.
- Amin, A.M.; Ahmad, A.S.; Yin, Y.Y.; Yahya, N.; Ibrahim, N. Extraction, Purification, and Characterization of Durian (Durio Zibethinus) Seed Gum. Food Hydrocolloid 2007, 21, 273‒279.
- Mirhosseini, H.; Tan, C.P.; Taherian, A.R. Effect of Glycerol and Vegetable Oil on Physicochemical Properties of Arabic Gum-Based Beverage Emulsion. European Food Research and Technology 2008, 228, 19‒28.
- Nagalakshmi, S.A.; Mitra, P.; Meda, V. Color, Mechanical, and Microstructural Properties of Vacuum Assisted Microwave Dried Saskatoon Berries. International Journal of Food Properties 2014, 17, 2142‒2156.
- Sadar, L.N. Rheological and textural characteristics of copolymerized hydrocolloidal solutions containing curdlan gum. Master Thesis, University of Maryland, 2004. http://hdl.handle.net/1903/1850.
- Márquez, A.L.; Palazolo, G.G.; Wagner, J.R. Water in Oil (W/O) and Double (W/O/W) Emulsions Prepared with Spans: Microstructure, Stability, and Rheology. Colloid and Polymer Science 2007, 285, 1119‒1128.
- Samavati, V.; Emam‐Djomeh, Z.; Mohammadifar, M.A.; Omid, M.; Mehdinia, A. Influence of Tragacanth Gum Exudates from Specie of Astragalus Gossypinus on Rheological and Physical Properties of Whey Protein Isolate Stabilised Emulsions. International Journal of Food Science and Technology 2011, 46, 1636‒1645.
- Knudsen, K.E.B. Dietary Fibre in Nutrition and Health of Piglets, 2009. http://www.pig333.com/nutrition/dietary-fibre-in-nutrition-and-health-of-piglets/ (accessed May 27, 2009).
- Jaya, S.; Durance, T.D. Compressive Characteristics of Cellular Solids Produced Using Vacuum-Microwave, Freeze, Vacuum, and Hot Air Dehydration Methods. Journal of Porous Materials 2009, 16, 47‒58.
- Goldstein, A.M.; Alter, E.W. Gum Karaya. In Industrial Gums; Whistler, R.L.; Be Miller, J.N.; Eds.; Academic Press: New York, NY, 1973; 273‒288.
- Moreira, R.; Chenlo, F.; Torres, M.; Glazer, J. Rheological Properties of Gelatinized Chestnut Starch Dispersions: Effect of Concentration and Temperature. Journal of Food Engineering 2012, 112, 94‒99.
- Staroszczyk, H.; Fiedorowicz, M.; Opalińska-Piskorz, J.; Tylingo, R. Rheology of Potato Starch Chemically Modified with Microwave-Assisted Reactions. LWT–Food Science and Technology 2013, 53, 249‒254.
- Varatharajan, V.; Hoover, R.; Liu, Q.; Seetharaman, K. The Impact of Heat-Moisture Treatment on the Molecular Structure and Physicochemical Properties of Normal and Waxy Potato Starches. Carbohydrate Polymer 2010, 8, 466‒475.
- Koocheki, A.; Razavi, S.M. Effect of Concentration and Temperature on Flow Properties of Alyssum Homolocarpum Seed Gum Solutions: Assessment of Time Dependency and Thixotropy. Food Biophysics 2009, 4, 353‒364.
- Wang, Y.; Wang, L.J.; Li, D.; Xue, J.; Mao, Z.H. Effects of Drying Methods on Rheological Properties of Flaxseed Gum. Carbohydrate Polymer 2009, 78, 213‒219.
- Medina-Torres, L.; Brito-De La Fuente, E.; Torrestiana-Sánchez, B.; Katthain, R. Rheological Properties of the Mucilage Gum (Opuntia Ficus Indica). Food Hydrocolloid 2000, 14, 417‒424.
- Xie, Y.; Yan, M.; Yuan, S.; Sun, S.; Huo, Q. Effect of Microwave Treatment on the Physicochemical Properties of Potato Starch Granules. Chemistry Central Journal 2013, 7, 113.
- Bárcenas, M.E.; De la O-Keller, J.; Rosell, C.M. Influence of Different Hydrocolloids on Major Wheat Dough Components (Gluten and Starch). Journal of Food Engineering 2009, 94, 241‒247.
- Dickinson, E. Hydrocolloids at Interfaces and the Influence on the Properties of Dispersed Systems. Food Hydrocolloid 2003, 17, 25‒39.
- King, K.; Gray, R. The Effect of Gamma Irradiation on Guar Gum, Locust Bean Gum, Gum Tragacanth, and Gum Karaya. Food Hydrocolloid 1993, 6, 559‒569.
- Tonon, R.V.; Brabet, C.; Hubinger, M.D.J. Influence of Process Conditions on the Physicochemical Properties of Açai (Euterpe Oleraceae Mart.) Powder Produced by Spray Drying. Journal of Food Engineering 2008, 88, 411‒418.