Abstract
Douchi is a fermented soybean food, which is popular in China. Composition and angiotensin I-converting enzyme-inhibitory activity of Douchi fermented with Mucor wutungkiao were studied in this work. The results showed that the nutritional potency of protein, fat, and soluble polysaccharide was improved in Douchi fermentation. The angiotensin I-converting enzyme-inhibitory activity of Douchi that ripening for 2 weeks showed the highest angiotensin I-converting enzyme-inhibitory activity. Then this sample was purified with Sephadex G-25 gel chromatography, and the amino acids and angiotensin I-converting enzyme-inhibitory activity of the purified fractions were studied. The results suggested that hydrophobic amino acids were assumed to cause the main increase of the angiotensin I-converting enzyme-inhibitory activity in Douchi.
Keywords:
Introduction
Douchi, generally used as a condiment, is a traditional Chinese fermented food. It is mainly fermented by Bacillus subtilis and mold.[Citation1] The soybean is used as the nutrition source by the fermentation microorganism during the fermentation, and many enzymes were produced in this process.[Citation2] Biochemical change was observed in the traditional soybean condiments.[Citation3] It was reported that nutrient digestibility had been improved during the fermentation.[Citation4] It was also reported that not only the nutritive value but also the functionality of fermented food were affected by the fermentation process.[Citation5–Citation9] Pan’s study showed fermentation conditions affect the production of angiotensin I-converting enzyme (ACE)-inhibitory peptides in sour milk.[Citation10] Zhang reported that hypertension can be lowered by peptides purified from Douchi, which was fermented by Aspergillus Egyptiacus.[Citation11]
Hypertension is a cardiovascular disease that can lead to brain, heart, and blood vessel damage.[Citation12] ACE is the key enzyme that controls hypertension in human body.[Citation13] Because of the safety of polypeptides, more and more people begin to pay attention to food source polypeptides, which can affect the physiological and biochemical reactions in human body. Many ACE-inhibitory peptides were separated from food proteins,[Citation14–Citation16] such as natto, tempeh,[Citation17] soy sauce,[Citation18] and so on. Although there are some reports relating to Douchi manufacturing, there are few that have paid attention to composition changes and ACE-inhibitors of Douchi fermented with Mucor wutungkiao during the fermentation. In the present study, we determined the protein content, fat content, soluble polysaccharide content, and we also analyzed the ACE-inhibitors in Douchi fermented with Mucor wutungkiao.
Materials and Methods
Materials
Soybeans were purchased from the Jilin Academy of Agricultural Sciences, China. Mucor wutungkiao was donated by China Center of Industrial Culture Collection (Beijing, China). Hippuryl-His-Leu (HHL), ACE (from rabbit lung), and O-phthaldialdehyde (OPA) were purchased from Sigma Chemical Company.
Douchi Preparation
Soybeans were washed and soaked in distilled water at 40ºC for 3 h. After the water had been drained, the soybeans were steamed at 121ºC for 30 min in an automatic autoclave (YXQ-LS-50, ShangHai BoXun Industry & Commerce Co., Ltd.), and then the surface of the sterilized soybeans was sprayed with conidia of Mucor wutungkiao spores (107 spores/mL, 1 mL conidia/100 g soybeans). The mixture was fermented at 28ºC and approximately 90% relative humidity for 60 h, and the semi-finished product was called Douchi qu which is the product of this pre-fermentation stage. Douchi qu was washed and put into a series of glass jars with the addition of NaCl, and the NaCl content was 12.5% (w/w, based on the weight of the soybeans). These samples were ripened for 1, 2, 3, and 4 weeks at 28ºC.
Determination of Crude Protein, Crude Fat, and Soluble Polysaccharide Content
Soaked soybean after soaking and Douchi qu after pre-fermentation were sampled for analysis. And Douchi ripening for 1, 2, 3, and 4 weeks (R1, R2, R3, R4) were also sampled for analysis. All the samples are lyophilized samples. Protein content was determined according to the Kjeldahl method.[Citation19] Fat was extracted in a Soxhlet apparatus according to the following way: 3.50 g sample wrapped with filter paper was dried to a constant weight at 105ºC. Then they were placed in a Soxhlet-extractor. Anhydrous diethyl ether was added to the extraction flask which was dried to constant weight. The extraction flask was heated in the water bath at 60ºC. The fat was extracted by the way of siphoning for 6 h. Then recycling the diethyl ether and evaporating the remaining diethyl ether (about 1~2 mL) within the extraction flask to dryness in the water bath. And then the extraction flask with fat in it was placed in a drying oven for 1 h before they were removed into a desiccator to cool to room temperature. The drying and cooling process was repeated until the weight was constant. And the increase in the weight of the flask with fat was the fat weight, which is calculated in the following formula:
where W is the weight of the sample; W0 is the weight of the extraction flask; W1 is the weight of the extraction flask and fat.
Determination of soluble polysaccharide content of the samples was according to the phenol-sulfuric acid method.[Citation20] Phenol solution (1.6 mL; w/v, 5%) was added into 1 mL of water extract of the sample. It was mixed and 7 mL of concentrated sulfuric acid was added into the water extract. Then the liquid mixture was added to a volume of 10 mL. The mixed solution was left at room temperature for 20 min. The absorbance of the liquid was measured at 490 nm. The standard curve was constructed with glucose.
Preparation of Water Extract
Lyophilized Douchi samples (1 g) were suspended in 10 mL of distilled water. The mixture was homogenized for 1 min using a T25BS4 homogenizer (IKA Labortechnik, Staufen, Germany) before it was sonicated for 30 min. The solution was then extracted in an orbital shaker (WSZ-100A, Yiheng Technical Co., Ltd., Shanghai, China) for 60 min at room temperature and under continuously stirring (150 rpm). After boiling for 15 min, the mixture was centrifuged at 12,000 × g for 20 min. The resulting supernatant was then filtered using a 0.45-μm membrane filter, and the filtrate was Douchi extract.
ACE-Inhibitory Activity In Vitro
ACE activity was measured by the modified method of Horie.[Citation21] ACE-inhibitory activity was calculated as follows:
where a is the fluorescence intensity of the ACE solution with ACE inhibitors; b is the fluorescence intensity of ACE solution without ACE inhibitors; c is the fluorescence intensity of the ACE inhibitors; and d is the fluorescence intensity of the buffer.
Purification and Characterization of ACE-Inhibitory Peptides
Polypeptide solution prepared from Douchi extract was purified by a Sephadex G-25 gel column (Pharmacia Co., Ltd, Ф 0.9 × 30 cm), which was eluted with distilled water at a flow rate of 0.2 mL/min. Absorbance of the elution that from the column was measured at 220 nm by using a UV spectrophotometer (UV mini 1240, Shimadzu, Kyoto). Fractions with ACE-inhibitory activity were collected and lyophilized.
Amino Acid Analysis
Amino acids of Douchi ACE-inhibitors were analyzed by an amino acid analyzer (L-8500A, Hitachi, Ltd.) according to the modified method of Sanni.[Citation22] Concentrated hydrochloric acid (0.3 mL) was added into 0.3 mL of purified fractions in a hydrolysis tube. Then the hydrolysis tube was flushed with nitrogen before sealing. The purified fraction was then hydrolyzed at 110ºC for 24 h. After cooling, the hydrolysate was filtrated, and 0.4 mL of the filtrate was lyophilized. Then citrate buffer (pH 2.2) was used to dissolve the lyophilized sample. Fifty microliters of the solution was used to analyze the amino acid composition.
Statistical Analysis
Experiments were performed in triplicate and repeated at least three times. Results are expressed as mean ± SD. Analysis of variance was performed using the Statistical Analysis System (Version 9.1, SAS Institute, Inc., Cary, NC, USA) to examine the differences among treatments at p < 0.05.
Result and discussion
Changes of Crude Protein Content During Douchi Fermentation
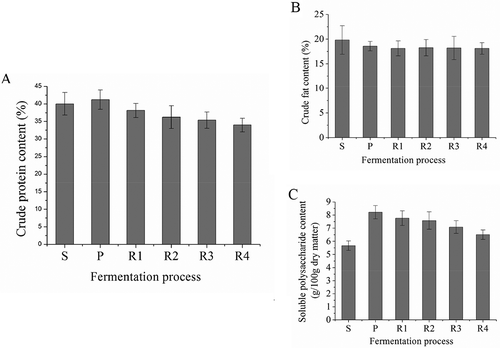
The crude protein content of soaked soybean is 40.02%, then it increased to 41.23% at the end of the pre-fermentation (). The protein content of Douchi samples ripening for 1 to 4 weeks was 38.16, 36.23, 35.36, and 33.98%, respectively. The analysis of variance showed that the change of protein was not significant during ripening. The reason for the decrease of crude protein content is that polypeptides and amino acids from the protein hydrolysis are used by the microorganisms.[Citation23] Proteins were broken down into polypeptides and amino acids by microbial enzymes during Douchi fermentation.[Citation24,Citation25] Our previous study results shows that the peptide content of Douchi fermented with Mucor wutungkiao had been increasing during the fermentation.[Citation26] Consequently, we can concluded that Douchi ripening accompanied with nutrient decomposition under the action of microbial enzyme, and the increase of peptide and amino acid content contributed to protein digestibility.[Citation27]
FIGURE 1 A: Changes of crude protein content during Douchi fermentation; B: Changes of crude fat content during Douchi fermentation; C: Changes of soluble polysaccharide content during Douchi fermentation. “S” is the abbreviation of “soaking,” and the sample of “soaking” was “soaked soybean;” “P” is the abbreviation of “pre-fermentation,” and the sample of “pre-fermentation” was Douchi qu; R is the abbreviation of “ripening,” and “R1” to “R4” were “ripening” for 1, 2, 3, and 4 weeks, respectively. Samples of R1 to R4 were Douchi samples after ripening for 1, 2, 3, and 4 weeks, respectively.
Crude Fat Content
Changes of crude fat content during Douchi fermentation was shown in . The analysis of variance suggested that there was no significant change in crude fat content during Douchi fermentation. The crude fat content of the soaked soybean is 19.81%, and the content decreased to 18.11% after 4 weeks ripening (R4). Similar results were observed in an early study,[Citation28] in which the crude fat content varied from 23.08 to 21.57% during Douchi fermentation. Fang[Citation29] also reported the similar results in Douchi fermentation. Factors affect fat hydrolysis are pH, temperature, substrate specificity, and so on.[Citation30] The change in crude fat content shows that the processing conditions of Douchi are not the best conditions for Douchi lipase.
Soluble Polysaccharide Content
The soluble polysaccharide content of soaked soybean was 5.66 g/100 g dry matter, as was shown in . The soluble polysaccharide content increased to 8.21 g/100 g dry matter at the end of Douchi pre-fermentation, then it decreased to 6.5 g/100 g dry matter after four weeks of ripening. The increase in soluble polysaccharide content was because of the hydrolysis of carbohydrate in these samples. Then the soluble polysaccharides were used by the microorganisms,[Citation31] and this phenomenon led to the slow decrease of soluble polysaccharide content at the ripening stage.[Citation32] The analysis of variance showed that the change of soluble polysaccharide content was not significant. However, the former results[Citation33] showed that the hardness of Douchi declined significantly during ripening (p < 0.05). Therefore, we can conclude that Douchi was hydrolyzed in the fermentation, and the nutritional potency was improved because more micro molecular polysaccharides which improved the nutritional potency were obtained by the way of ripening.
ACE-Inhibitory Activity
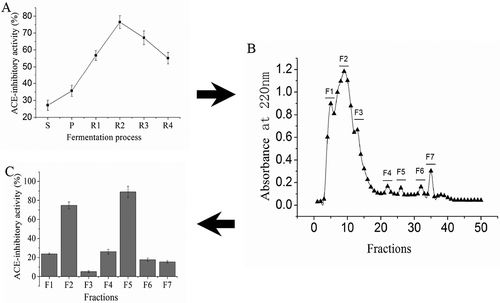
ACE-inhibitory activity of Douchi during the fermentation was shown in . The ACE-inhibitory activity increased at the pre-fermentation stage and the first two weeks of ripening. Douchi sample ripening for 2 weeks showed the highest ACE-inhibitory activity, which was 76.48%. Then the ACE-inhibitory activity declined. This result showed that the content of peptide with ACE-inhibitory activity decreased at the last 2 weeks of ripening.
FIGURE 2 A: Changes of ACE-inhibitory activity in Douchi fermentation, “S” is the abbreviation of “soaking,” and the sample of “soaking” was “soaked soybean;” “P” is the abbreviation of “pre-fermentation,” and the sample of “pre-fermentation” was Douchi qu; R is the abbreviation of “ripening,” and “R1” to “R4” were “ripening” for 1, 2, 3, and 4 weeks, respectively. Samples of R1 to R4 were Douchi samples after ripening for 1, 2, 3, and 4 weeks, respectively, B: Elution profile of R2 sample on a Sephadex G-25 gel filtration column, C: ACE-inhibitory activity of fractions purified from gel filtration.
Purification of ACE-Inhibitory Peptide
Extract of Douchi sample ripening for 2 weeks was purified by Sephadex G-25 gel column because of the highest ACE-inhibitory activity. The absorbance of each fraction at 220 nm was measured according to the method of Zhang.[Citation11] Seven peaks were observed in our study (). We collected the extracts at the same peak, and these samples were named F1, F2, F3, F4, F5, F6, and F7, respectively. Then the ACE-inhibitory activity was determined, the result was shown in . Every fraction in our study has the ACE-inhibitory activity, which varied from each other. The ACE-inhibitory activity of F2 and F5 was 74.78 and 89.04%, respectively. However, the ACE-inhibitory activity of F1, F3, F4, F6, and F7 was less than 30%. Consequently, F2 and F5 were selected for further study. The time interval of the elution time for F2 and F5 was 85 min. This result showed that the molecular weight of F2 and F5 is different, and the ACE-inhibitory activity of peptide with small molecular weight was higher than that of high molecular weight peptide in the extracts.
Amino Acid Analysis
In order to determine the composition, we analyzed the amino acids of F2 and F5. The result was shown in . The content of hydrophobic amino acid was 27.97% in F2. It was reported that the structure of C-terminal tripeptide in the peptide chain is in favor of the combination between ACE-inhibitory peptides and ACE.[Citation34,Citation35] Moreover, aromatic amino acid content is 16.22% in F2. When the dipeptide and tripeptide of the C-terminal group are aromatic amino acids, the polypeptide will show strong ACE-inhibitory activity.[Citation36–Citation38] Many peptides with high ACE-inhibitory activity contain proline.[Citation39] The proline content of F2 is 14.54%.
TABLE 1 Amino acid content of F2 and F5
In F5, the content of hydrophobic amino acid, aromatic amino acid and proline was 34.84, 11.85, and 10.39%, respectively. Hydrophobic peptides are easy to combine with the active site of ACE, as is the reason for ACE inactivation.[Citation40] The content of Val, Met, and Ala that belong to hydrophobic amino acid in F5 is higher than that in F2. We can conclude that these hydrophobic amino acids played an important role in the ACE-inhibitory activity.
Conclusion
The nutritional potency of Douchi was improved in the fermentation, and the ACE-inhibitory activity also changed during Douchi fermentation. Change of composition affected the ACE-inhibitory activity in Douchi, as was because that the proteins were hydrolyzed in to peptides, which are the main reasons for Douchi ACE-inhibitory activity. And peptides reacted with other hydrolysates. Consequently, finding the best time point for nutritional potency and ACE-inhibitory activity in Douchi fermentation is a good way to produce Douchi with high nutrient content and functionality. And hydrophobic amino acids contribute to the ACE-inhibitory activity of Douchi extract.
FUNDING
The authors thank the financial supports of the National Natural Science Foundation of China under Project No. 31401294 and the Specialized Research Fund for the Doctoral Program of Higher Education under Project No. 20130008120013.
Additional information
Funding
References
- Li, L.T.; Li, Z.G.; Yin, L.J. Soybean Processing and Utilization; Chemical Industry: Beijing, China, 2003; 240–245.
- Lu, L.; Zheng, X.Y. Research Advance on Distribution and Function of Microorganism in Douchi Fermentation Process. Cereal and Food Industry 2011, 18, 42–45.
- Azokpota, P.; Hounhouigan, D.J.; Nago, M.C. Microbiological and Chemical Changes During the Fermentation of African Locust Bean (Parkia Biglobosa) to Produce Afitin, Iru, and Sonru, Three Traditional Condiments Produced in Benin. International Journal of Food Microbiology 2006, 107, 304–309.
- Addo, K.; Lykins, S.; Cotton, C. Indigenous Fermentation and Soy Fortification: Effects on Protein Quality and Carbohydrate Digestibility of a Traditional Ghanaian Corn Meal. Food Chemistry 1996, 57, 377–380.
- Katina, K.; Laitila, A.; Juvonen, R.; Liukkonen, K.H.; Kariluoto, S.; Piironen, V. Bran Fermentation As a Means to Enhance Technological Properties and Bioactivity of Rye. Food Microbiology 2007, 24, 175–186.
- Jakubczyk, A.; Karaś, M.; Baraniak, B.; Pietrzak, M. The Impact of Fermentation and in Vitro Digestion on Formation Angiotensin Converting Enzyme (ACE) Inhibitory Peptides from Pea Proteins. Food Chemistry 2013, 141, 3774–3780.
- Ma, Y.L.; Wang, J.H.; Cheng, Y.Q.; Yin, L.J. Selected Quality Properties and Angiotensin I-Converting Enzyme Inhibitory Activity of Low-Salt Sufu, a New Type of Chinese Fermented Tofu. International Journal of Food Properties 2014, 17, 2025–2038.
- Amadou, I.; Le, G.W.; Shi, Y.H. Evaluation of Antimicrobial, Antioxidant Activities, and Nutritional Values of Fermented Foxtail Millet Extracts by Lactobacillus Paracasei Fn032. International Journal of Food Properties 2013, 16, 1179–1190.
- Zhang, Y.W.; Liu, J.; Lu, X.; Zhang, H.; Wang, L.; Guo, X.N.; Qi, X.G.; Qian, H.F. Isolation and Identification of An Antioxidant Peptide Prepared from Fermented Peanut Meal Using Bacillus Subtilis Fermentation. International Journal of Food Properties 2014, 17, 1237–1253.
- Pan, D.; Guo, Y. Optimization of Sour Milk Fermentation for the Production of ACE-Inhibitory Peptides and Purification of a Novel Peptide from Whey Protein Hydrolysate. International Dairy Journal 2010, 20, 472–479.
- Zhang, J.H.; Tatsumi, E.; Ding, C.H.; Li, L.T. Angiotensin I-Converting Enzyme Inhibitory Peptides in Douchi, a Chinese Traditional Fermented Soybean Product. Food Chemistry 2006, 98, 551–557.
- Matchar, D.B.; McCrory, D.C.; Orlando, L.A.; Patel, M.R.; Patel, U.D.; Patwardhan, M.B. Systematic Review: Comparative Effectiveness of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers for Treating Essential Hypertension. Annals of Internal Medicine 2008, 148, 16–29.
- Murray, B.A.; FitzGerald, R.J. Angiotensin Converting Enzyme Inhibitory Peptides Derived from Food Proteins: Biochemistry, Bioactivity, and Production. Current Pharmaceutical Design 2007, 13, 773–791.
- Chen, J.; Liu, S.; Ye, R.; Cai, G.; Ji, B.; Wu, Y. Angiotensin-I Converting Enzyme (ACE) Inhibitory Tripeptides from Rice Protein Hydrolysate: Purification and Characterization. Journal of Functional Foods 2013, 5, 1684–1692.
- Kind, T.; Meissen, J.K.; Yang, D.; Nocito, F.; Vaniya, A.; Cheng, Y.S. Qualitative Analysis of Algal Secretions with Multiple Mass Spectrometric Platforms. Journal of Chromatography A 2012, 1244, 139–147.
- Zhang, F.; Wang, Z.; Xu, S. Macroporous Resin Purification of Grass Carp Fish (Ctenopharyngodon Idella) Scale Peptides with in Vitro Angiotensin-I Converting Enzyme (ACE) Inhibitory Ability. Food Chemistry 2009, 117, 387–392.
- Gibbs, B.F.; Zougman, A.; Masse, R.; Mulligan, C. Production and Characterization of Bioactive Peptides from Soy Hydrolysate and Soy-Fermented Food. Food Research International 2004, 37, 123–131.
- Zhu, X.L.; Watanabe, K.; Shiraishi, K.; Ueki, T.; Noda, Y.; Matsui, T. Identification of ACE-Inhibitory Peptides in Salt-Free Soy Sauce That Are Transportable Across Caco-2 Cell Monolayers. Peptides 2008, 29, 338–344.
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th Ed.; Associaton of Official Analytical Chemists: Washington, DC, 1990; 807.
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Analytical Chemistry 1956, 28, 350–356.
- Horie, H. Handbook of Functional Evaluation of Food; Japanese Ministry of Agriculture, Forestry and Fisheries: Tokyo, 1999; 117–121.
- Sanni, A.I.; Onilude, A.A.; Ibidapo, O.T. Biochemical Composition of Infant Weaning Food Fabricated from Fermented Blends of Cereal and Soybean. Food Chemistry 1999, 65, 35–37.
- Li, H.; Feng, F.Q.; Shen, L.R.; Li, D. Identification of Predominant Strains from Dandouchi and Their Effects on Decomposition of Soybean Protein. Food and Fermentation Industries 2011, 37, 1–6.
- Zhao, G.H.; Fang, C.L. Change of Proximate Composition and in Vitro Digestibility of Protein Associated with Ripening Process of Douchi. Food Science 2009, 30, 59–62.
- Zhang, Y.H.; Ma, L.; Wang, X.M. Correlation Between Protein Hydrolysates and Color During Fermentation of Mucor-Type Douchi. International Journal of Food Properties 2015, 18, 2800–2812.
- Wang, H.; Li, Y.Y.; Cheng, Y.Q.; Yin, L.J.; Li, L.T. Effect of the Maillard Reaction on Angiotensin I-Converting Enzyme (ACE)-Inhibitory Activity of Douchi During Fermentation. Food and Bioprocess Technology 2013, 6, 297–301.
- Kao, C.; Robinson, R.J. Nutritional Aspects of Fermented Foods from Chickpea, Horsebean, and Soybeans. Cereal Chemistry 1978, 55, 512–517.
- Qin., K.L. Study on the Probiotics, Flavor Matter, and Atrament; Jiangnan University: Wuxi, China, 2006; 81–98.
- Fang, C.L. Studies on Biological Changes of Soybean Protein and Dietary Fiber During the Production of Douchi; Southwest University: Chongqing, China, 2007; 35–62.
- Okino-Delgado, C.H.; Fleuri, L.F. Obtaining Lipases from Byproducts of Orange Juice Processing. Food Chemistry 2014, 163, 103–107.
- Saldivar, X.; Wang, Y.J.; Chen, P.; Mauromoustakos, A. Effects of Blanching and Storage Conditions on Soluble Sugar Contents in Vegetable Soybean. LWT–Food Science and Technology 2010, 43, 1368–1372.
- Yang, H.L.; Zhang, L.; Xiao, G.N.; Feng, J.; Zhou, H.; Huang, F. Changes in Some Nutritional Components of Soymilk During Fermentation by the Culinary and Medicinal Mushroom Grifola Frondosa. LWT–Food Science and Technology 2015, 62, 468–473.
- Wang, H.; Yin, L.J.; Cheng, Y.Q.; Li, L.T. Effect of Sodium Chloride on the Color, Texture, and Sensory Attributes of Douchi During Post-Fermentation. International Journal of Food Engineering 2012, 8, 1556–3758.
- Ondetti, M.; Rubin, B.; Cushman, D. Design of Specific Inhibitors of Angiotensin-Converting Enzyme: New Class of Orally Active Antihypertensive Agents. Science 1977, 196, 441–444.
- Denti, P.; Sharp, S.K.; Kröger, W.L.; Schwager, S.L.; Mahajan, A.; Njoroge, M. Pharmacokinetic Evaluation of Lisinopril-Tryptophan, a Novel C-Domain ACE Inhibitor. European Journal of Pharmaceutical Sciences 2014, 56, 113–119.
- Kohmura, M.; Nio, N.; Kubo, K.; Ariyoshi, Y. Inhibition of Angiotensin-Converting Enzyme by Synthetic Peptide Fragments of Various β-Caseins. Agricultural and Biological Chemistry 1990, 54, 1101–1102.
- Matsui, T.; Li, C.H.; Osajima, Y. Preparation and Characterization of Novel Bioactive Peptides Responsible for Angiotensin I-Converting Enzyme Inhibition from Wheat Germ. Journal of Peptide Science 1999, 5, 289–297.
- Iroyukifujita, H.; Eiichiyokoyama, K.; Yoshikawa, M. Classification and Antihypertensive Activity of Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Food Proteins. Journal of Food Science 2000, 65, 564–569.
- Nakamura, Y.; Yamamoto, N.; Sakai, K.; Okubo, A.; Yamazaki, S.; Takano, T. Purification and Characterization of Angiotensin I-Converting Enzyme Inhibitors from Sour Milk. Journal of Dairy Science 1995, 78, 777–783.
- Li, G.H.; Le, G.W.; Shi, Y.H.; Shrestha, S. Angiotensin I-Converting Enzyme Inhibitory Peptides Derived from Food Proteins and Their Physiological and Pharmacological Effects. Nutrition Research 2004, 24, 469–486.
