Abstract
This study characterized the phenolic, aroma compositions, and antioxidant capacities of four Artemisia herba alba accessions collected from north and center regions in Tunisia in order to select valuable origin with optimal bioactive compounds production. The total polyphenol and flavonoid contents varied between accessions with maxima contents observed in center regions. Kaempherol, apigenin, naringenin, p-coumaric, trans-cinnamic, and caffeic acids were the most abundant compounds with percentage varying depending on the considered accession. Significant changes on essential oil yields (0.4–1.2%) were found between accessions and three different oil chemotypes were distinguished: camphor, fenchol/α-thujone, and α-thujone/camphor. Cluster analysis of volatile and phenolic compositions grouped the accessions on different groups. Antioxidant activities of extracts were found to be higher than essential oils for the four provenances. The results suggested that the center regions have high potential for selecting varieties rich on essential oil, antioxidant phenolic acids, and flavonoids.
INTRODUCTION
Increased interest in the beneficial effects of variety of plants in particular aromatic and medicinal ones, applied as herbal remedies to treat or prevent diseases or to promote health, has resulted in researches aimed to determine their specific health benefit and the levels of phytochemicals they contained.[Citation1] Phytochemicals have been emerged as a potential source of natural antioxidants.[Citation2] Most antioxidants isolated from higher plants are phenolic compounds. The goal is to use them in foods and pharmaceutical preparations to replace synthetic antioxidants. In addition, they are involved in the prevention of various diseases associated with oxidative stress, such as cancers and cardiovascular diseases. On the other hand, aromatic plants are rich on essential oils which are extensively used in the fragrance, flavor, and aromatherapy industries. Essential oils are applied in the fields of food preservation and the pharmaceutical industry where they are employed as medicines to promote medicine absorption, increasing penetration in the epidermis due to their lipophilic characteristics.[Citation3]
Artemisia herba-alba (A. herba-alba) is an aromatic and medicinal herb of the Asteraceae family, typical for the Middle East and North Africa, where it is common and sometimes stand-forming growing in arid and semi-arid climates. In Tunisia, this wild plant is commonly known as “Chih” and is used as aromatizing for tea and in folk medicine for treatment of coughing, colds, intestinal disturbances, and neurological diseases, as well as an antidiabetic.[Citation4] Phytochemical investigations have proven that Artemisia genus is rich on tannins, polyphenols, flavonoid, and essential oil. The occurrence of flavonoids, in particular those conjugated with one or more sugar moieties, as well as phenolic acids has been previously reported in some Artemisia species such as Artemisia vulgaris and Artemisia campestris.[Citation5] However, very limited studies were carried out on the identification of polyphenolic compounds of A. herba-alba.[Citation6] Furthermore, data concerning the antioxidant power of A. herba-alba are scarce.[Citation7] In Tunisia, studies are limited to the one of Khlifi et al.[Citation8] who showed that A. herba-alba soxhlet extracts possess antioxidant activity.
On the other hand, A. herba-alba is rich on essential oil which has been isolated and investigated for its chemical composition. Studies revealed a high chemical polymorphism according to the origin and led to the definition of several chemotypes such as thujone one from Jordan oil[Citation9] and camphor from Palestinian and Morrocan oil.[Citation10] Moreover, several chemotypes could be encountered even in the same origin; four chemotypes including p-cymene, camphor, 1,8-cineole, and chrysanthenone were found in Spanich A. herba alba[Citation11] and three different ones were described in Algeria.[Citation12] In Tunisia, the essential oil obtained from A. herba-alba collected from south domains showed high chemical polymorphism, being dominated by monoterpenes or sesquiterpenes or equal percentages between the two classes.[Citation13]
On the other hand, among volatiles that characterize A. herba alba, the oil could contain high percentage of thujones reaching 65%.[Citation13] Recently, there were mitigated opinions about the use of these compounds as a food additive.[Citation14] Although, there are no restrictions of food-containing thujones,[Citation14] these ketones have been regarded as a severe neurotoxicants when used as a food additive.[Citation15] Therefore, finding A. herba alba oils with little thujones levels appear to be of high importance to increase the value of the oil in livestock diets. Thus, the present study aimed to (1) assess for the first time the phenolic composition of four accessions of A. herba-alba obtained from semi arid and arid regions, (2) investigate the volatile composition of the four accessions in comparison with literature data in order to valorize these origins as sources of valuable aroma and bioactive compounds, and (3) study the antioxidant activity of extract and essential oils. Additionally, we used principal component analysis (PCA) technique to discriminate between A. herba-alba samples in order to found optimized origin to produce bioactive compounds.
MATERIAL AND METHODS
Plant Material
The aerial parts of four accessions of Tunisian A. herba alba were collected from plants growing wild in different Tunisian regions; Kef (northwest), Boukornine (northeast), Kairouan (center), and Kasserine (west center). Geographical characteristics of bioclimatic collection sites were described in . Plant identification was carried by Professor Abderrzek Smaoui (Biotechnology Center in Borj-Cedria Technopole, Tunisia). A voucher specimen was deposited at the herbarium of the Laboratory of Bioactive Substances, Biotechnology Center in Borj-Cedria Technopole under the number “TC2011-2405.” After that, the aerial part samples were freeze-dried and then ground to fine powder by an electric mill and conserved in a desiccator at room temperature (~25°C) in darkness for further extractions.
TABLE 1 Geographical characteristics of the four sites of collect of A. herba-alba
Polyphenols Extraction
Extracts were obtained by stirring 1 g of dry A. herba-alba aerial parts powder from the four origins with 10 mL of pure methanol for 30 min. The extracts were then kept for 24 h at 4°C, filtered through a Whatman No 4 filter paper, evaporated under vacuum to dryness, and stored at 4°C until analyzed.
Total Phenolic Content
Total phenolic contents were assayed using the Folin-Ciocalteu reagent, following the slightly modified Singleton’s method.[Citation16] An aliquot (0.125 mL) of a suitable diluted methanolic extract was added to 0.5 mL of deionized water and 0.125 mL of the Folin-Ciocalteu reagent. The mixture was shaken and allowed to stand for 6 min, before adding 1.25 mL of 7% Na2CO3 solution. The solution was then adjusted with deionized water to a final volume of 3 mL and mixed thoroughly. After incubation for 90 min at 23°C, the absorbance versus prepared blank was read at 760 nm. Total phenolic contents of aerial parts (three replicates per treatment) were expressed as mg gallic acid equivalents per gram (mg GAE/g) through the calibration curve with gallic acid. The calibration curve range was 50–400 mg/mL (RCitation2 = 0.99). All samples were performed in triplicate.
Total Flavonoids Content
The methanolic aerial part extract appropriately diluted was mixed with NaNO2 (5%). After 6 min, 150 µL of 10% AlCl3 and 500 µL of NaOH (1 M) were added to the mixture. Finally, the mixture was adjusted to 2.5 mL with distilled water.[Citation17] The absorbance versus prepared blank was read at 510 nm. Total flavonoids contents of aerial parts (three replicates per treatment) were expressed as mg catechin equivalents per gram (mg CE/g) through the calibration curve with catechin. The calibration curve range was 50–500 mg/mL.
Analysis of Phenolic Compounds by Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC)
Dried samples from aerial parts of A. alba alba were hydrolyzed.[Citation18] The acidic hydrolysis was used to release the aglycones in order to simplify the identification process since the free forms of phenolic compounds are rarely present in plants and they occur as esters, glycosides, or are bound to the cell wall. Twenty milliliters of methanol containing butylated hydroxytoluene (BHT) (1 g/L) were added to 0.5 g of dried sample. Then, 10 mL of 1 M HCl were added. The mixture was stirred carefully and sonicated for 15 min and refluxed in a water bath at 90°C for 2 h. The obtained mixture was injected to high-performance liquid chromatography (HPLC). The phenolic compound analysis was carried out using an Agilent Technologies 1100 series liquid chromatograph (RP-HPLC) coupled with an ultraviolet (UV)-vis multiwavelength detector. The separation was carried out on a 250 × 4.6-mm, 4 [m Hypersil ODS C18 reversed phase column at ambient temperature. The mobile phase consisted of acetonitrile (solvent A) and water with 0.2% sulphuric acid (solvent B). The flow rate was kept at 0.5 mL/min. The gradient program was as follows: 15% A/85% B 0–12 min, 40% A/60% B 12–14 min, 60% A/40% B 14–18 min, 80% A/20% B 18–20 min, 90% A/10% B 20–24 min, and 100% A 24–28 min. The injected volume was 20 µL, and peaks were monitored at 280 nm. Samples were filtered through a 0.45 µm membrane filter before injection. Phenolic compounds were identified according to their retention times and spectral characteristics of their peaks against those of standards, as well as by spiking the sample with standards. Analyses were performed in triplicate.
Validation of Quantitative Analysis
Standard stock solutions of p-coumaric acid, caffeic acid, trans-cinnamic acid, kaempferol, and apigenin were prepared by dissolving 0.01 g in 10 mL methanol (1 mg/mL) and further diluted to appropriate concentrations for standard curve preparation. The peak areas were automatically measured by an integrator of HPLC instrument. Calibration curve was obtained by plotting the peak area against concentration. The limit of detection (LOD) and quantification (LOQ) under the chromatographic conditions were determined by injecting a series of standard solutions until the signal-to-noise (S/N) ratio for each compound was 3 for LOD and 10 for LOQ.
Essential Oil Extraction
Dried aerial parts (100 g) were subjected to hydrodistillation in a clevenger type apparatus for 90 min. This time was fixed after a kinetic survey during 30, 60, 90, 120, and 180 min. The resulting essential oil was subsequently analyzed. Essential oil extractions were done in triplicate. Yield percentage was calculated as weight (mg) of essential oil per 100 g of plant dry matter.
Gas Chromatography (GC) and GC/mass spectrometry (MS) Analysis of Essential Oil
Analysis of volatile compounds by GC was carried out on a Hewlett-Packard 6890 gas chromatograph (Palo Alto, CA, USA) equipped with a flame ionization detector (FID) and an electronic pressure control (EPC) injector. A polar polyethylene glycol (PEG) HP Innowax and a 5% diphenyl, 95% dimethylpolysiloxane apolar HP-5 capillary columns (30 m × 0.25 mm, 0.25 mm film thickness; Hewlett-Packard, CA,127 USA) were used. The flow of the carrier gas (N2) was 1.6 mL/min. The split ratio was 60:1. The analysis was performed using the following temperature program: oven temperature kept isothermally at 35°C for 10 min, increased from 35 to 205°C at the rate of 3°C/min and kept isothermally at 205°C for 10 min. Injector and detector temperatures were held, at 250 and 300°C, respectively. The injected volume was 1 µL of essential oil. The individual peaks were identified by comparison of their retention indices relative to (C6-C22) n-alkanes with those of literature and/or with those authentic compounds available in our laboratory. Percentage compositions of samples were calculated according to the area of the chromatographic peaks using the total ion current.
Volatile aroma compounds analysis by GC/MS was performed on a gas chromatograph HP 5890 (II) interfaced with a HP 5972 mass spectrometer (Palo Alto, CA, USA) with electron impact ionization (70 eV). A HP-5 MS capillary column (30 m × 0.25 mm, coated with 5% phenyl methyl silicone, 95% dimethylpolysiloxane, 0.25 mm film thickness; Hewlett-Packard, CA, USA) was used. The column temperature was programmed to rise from 50 to 240°C at a rate of 5°C/min. The carrier gas was helium with a flow rate of 1.2 mL/min; split ratio was 60:1. Scan time and mass range were 1 s and 40–300 m/z, respectively. Identification of aroma compounds was made by matching their recorded mass spectra with those stored in the Wiley/NBS mass spectral library of the GC/MS data system and other published mass spectra.
1, 1-Diphenyl-2-Picrylhydrazyl Radical (DPPH) Radical Scavenging Assay
The electron donation ability of the extracts was measured by bleaching of the purple-colored solution of DPPH.[Citation19] One-half milliliter of 0.2 mM DPPH methanolic solution was added to extracts of (2 mL, 10–1000 μg/mL). After an incubation period of 30 min at room temperature, the absorbance was read against a blank at 517 nm. The inhibition percentage of free radical DPPH (IP%) was calculated as follows:
where Ablank is the absorbance of the control reaction and Asample is the absorbance in the presence of plant extract. Extract concentration providing 50% inhibition (IC50) was calculated from the regression equation prepared from the concentration of the extracts and the inhibition percentage.
Reducing Power Assay
The method of Oyaizu[Citation20] was used to assess the reducing power of extracts. These extracts (1 mL) were mixed with 2.5 mL of a 0.2 M sodium phosphate buffer (pH = 6.6) and 2.5mL of 1% potassium ferricyanide (K3Fe [CN]6) and incubated in a water bath at 50°C for 20 min. Then, 2.5 mL of 10% trichloroacetic acid was added to the mixture that was centrifuged at 650 g for 10 min. The supernatant (2.5 mL) was then mixed with 2.5 mL of distilled water and 0.5 mL of 0.1% iron (III) chloride solution. The intensity of the blue green color was measured at 700 nm. The extract concentration at which the absorbance was 0.5 for the reducing power (EC50) was obtained from the linear regression equation prepared from the concentrations of the extracts and the absorbance values. High absorbance indicates high reducing power.
Statistical Analysis
All analyses were performed in triplicate, and the results are expressed as mean values standard deviation (SD). The data were subjected to statistical analysis using statistical program package STATISTICA. The one-way analysis of variance (ANOVA) followed by the Duncan multiple range test was employed and the differences between individual means and each solvent used were deemed to be significant at P < 0.05. A cluster analysis (CA) was performed in order to discriminate between different regions of collection on the basis of their essential oils and phenolic composition.
RESULTS AND DISCUSSION
Total Polyphenols and Flavonoids Contents
The aerial parts of A. herba-alba were collected from different localities from north (Kef and Boukornine) and center (Kairouan and Kasserine) in Tunisia, characterized by diverse geographic and climate conditions (). Depending on its geographical origin, total polyphenols content of A. herba-alba extracts is demonstrated in . The results showed that the plant is a valuable source of phenolics with content ranging from 9 to 18 mg GAE/g (). The highest amount was encountered in Kasserine methanol extract. Besides, extracts were rich on flavonoids where Kairouan displayed the highest amount of 44 mg CE/g followed by Kasserine (40 mg CE/g) and Boukarnine (33 mg CE/g). Kef location displayed the lowest flavonoids amount of 16 mg CE/g. Our results are in accordance with those of literature which indicate that Asteraceae, especially Artemisia genus are a valuable source of phenolic compounds.[Citation7,Citation21] Recently, compared to our results, Khlifi et al.[Citation8] reported higher total phenolics amount in Tunisian A. herba-alba grown in the centre but lower total flavonoids amount (19.17 g CE/Kg). Such difference could be due to the fact that these authors chosen leaves as plant material while we studied the whole aerial part in our experiment.
TABLE 2 Essential oil yields and total polyphenols and flavonoids contents from four A. herba-alba accessions
On the other hand, the amounts of polyphenols and flavonoids, increased considerably from north to centre of Tunisia (). This could be explained by the different climatic features between these regions. In fact the centre is characterized by increased temperature and light, and lower rain in comparison to the north, which may favour polyphenols biosynthesis. Phenolics accumulation has been found to be enhanced in plants tissues exposed to environmental factors including water deficit and thermal stress.[Citation22]
Phenolic Composition of A. Herba-Alba Methanol Extracts
HPLC was used to analyse the phenolic composition of A. herba-alba extracts (). In our study, acid hydrolysis was employed to obtain aglycones form. In total, 17 compounds including 10 phenolic acids and 7 flavonoids were identified. The phenolic acids represented the major class with percentages of 65, 51, 49 and 45 in Kasserine, Kef, Boukarnine and Kairouan, respectively. Flavonoids class was also present with appreciate percentages varying from 24% in Kasserine to 42% in Kairouan. Moreover, the accessions differed in qualitative point of view. Kaempherol (12%) and trans-cinnamic acid (11%) were the most abundant in Kef while apigenin (11%) encountered mainly in Boukarnine. Besides, p-coumaric acid (23%) and naringenin (20%) were the most present in Kairouan. The Kasserine region was characterized by the presence of the greatest proportions of caffeic (14%), chlorogenic (9%) and syringic acids (9%). Among the phenolic compounds, vanillic acid was only present in Kairouan (2%). The summary of linear equations, squared correlation coefficients, limit of determination (LOD) and LOQ of the major phenolics identified in A. herba-alba are presented in .
TABLE 3 Analysis of variance analysis and phenolic composition (expressed in%, w/w, and in µg/g dry weight) of methanolic extracts from four A. herba-alba accessions
TABLE 4 Calibration curves, correlation coefficients (R2), detection limits, and quantification limits for high-performance liquid chromatography analysis for major phenolics identified from A. herba-alba
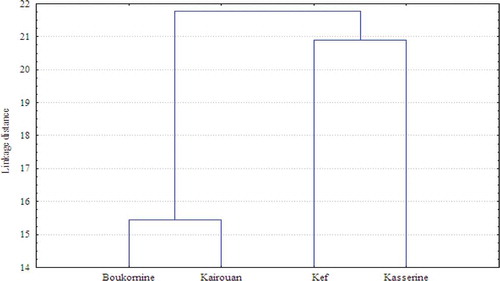
There have been few reports on phenolic composition of A. herba-alba; in the concordance of our results, apigenin was already reported in leaves and stems of A. herba-alba collected from Egypt.[Citation6] On the other hand, this flavonone was recovered from the aerial parts of Artemisia vulgaris and Artemisia campestris.[Citation5] The data obtained for the 17 phenolic compounds were subjected to CA to outline the phenolic profile differences among the accessions (). Samples will be grouped in clusters in terms of their nearness or similarity. The established dendrogram showed two distinct population groups (). The first subcluster includes the accessions of Boukarnine and Kairouan. The compounds most associated with this cluster were flavonoids. In fact, the highest epicatechin and naringenin levels were established in these two accessions (). In addition, the cluster was characterized by the highest p-coumaric acid. In this cluster, a correlation in the occurrence of these compounds can be found, and this relationship could be explained by the fact that p-coumaric acid, a common precursor of flavonoids in the biosynthetic pathway. The second cluster consists on the populations belonging to Kasserine and Kef. These accessions were clearly separated from the two others by their highest levels of phenolic acids (tannic, gallic, syringic and ferulic acids) and quercetin and kaempferol percentages ().
The growing evidence suggests that phenolics production is highly regulated by abiotic factors effects (e.g. altitude temperature, precipitation, soil characteristics, wind speed or snow cover). In our study, the composition of the two found groups could be at least in part influenced by altitude variation. In fact, Boukarnine and Kairouan belong to low altitude zones while Kasserine and Kef were highest altitude localities. In accordance with this, the later regions were characterized by the highest kampferol percentage (). Neugart et al.[Citation23] examined the effect of varying environmental factors on the levels of glycosides and aglycones flavonoids and reported a positive correlation between increasing altitude and increasing kamperol concentration. At high altitude, variations of phenolic compounds have been related to the natural changes in UV-B radiation of the environment in which plants grows. It has been suggested that flavonoids including kampferol behave mostly as antioxidants in photoprotection as well as UV-absorbing compounds in UV protection.[Citation24]
Essential Oils Yields
The results showed that A. herba-alba populations were rich on essential oils with yields reaching 1.2% (). Moreover, yields vary significantly (P < 0.05) depending on the region; the highest oil yields were obtained from Boukarnine and Kef accessions (1.2 and 1.1%) while Kasserine displayed lower content (0.8%) followed by Kairouan (0.4%). These results are in accordance of those of Haouari and Ferchichi[Citation13] who reported great variations on the essential oil yields obtained from the aerial parts of A. herba-alba grown in different localities in the south of Tunisia. In our study, variation on essential oil content according to the geographic origin could be linked to the changes in environmental factors between the four regions. Although the essential oil biosynthesis is known to be under strong genetic control, it is greatly modulated by abiotic factors. In fact, terpenoids is a developmentally regulated processes and can be affected by several conditions including humidity, light, temperature as well as water and soil minerals availability.[Citation25] In our study, Boukarnine and Kef accessions belong to the semi arid superior climatic zone while kasserine and Kairouan belong to the semi arid inferior and aride ones. Since the temperature and humidity are known to be the major different parameters between the localities, the reported high oil yields seems to be correlated to the more temperate north regions than the south ones characterized by drought and high temperature.
Chemical Composition of Essential Oils by GC and GC/MS
The identified components from the aerial parts of the four accessions of A. herba alba studied, their retention indices and their percentage composition are listed in . Fourty six compounds were identified in Kef and Kasserine and 44 in Boukarnine and Kairouan. The four oils were dominated by monoterpenes class mainly oxygenated monoterpenes which represented 70.3, 66.6, 65.8, and 65.5% in Boukornine, Kef, Kairouan, and Kasserine oils, respectively. Monoterpenes hydrocarbons were also well represented (11.2-17.4%) with the highest percentage found in Boukarnine oil. Sesquiterpnes hydrocarbons were present with appreciable level in Kairouan (7.5%) followed by Boukarnine (5.8%) while the main oxygenated sesquiterpenes fraction was found in Kasserine (8.6%) and Kef (7%) oils.
TABLE 5 Analysis of variance analysis and essential oils composition (%) from four A. herba-alba accessions
Moreover, the results showed the presence of high intra chemical polymorphism among Tunisian A. alba alba accessions. Boukarnine and Kairouan essential oils were camphor chemotypes since this compound represented the major one with values of 32 and 25%, respectively. However, Kasserine oil presented a fenchol/α-thujone chemotype with percentages of 13 and 12%, respectively, while Kef oil chemotype was α-thujone/camphor (14%/12%).
Camphanes derivatives are characteristics of A. alba alba oils and are chemotaxonomic markers of Aretemisia genus. Haouari and Ferchichi[Citation13] found that five oils of A. herba alba collected from several localities in south of Tunisia, presented a camphor chemotype, nevertheless, camphor percentages (12.9-17.8%) were lower than that obtained in our study. Camphor-type oils have been described in Algerian[Citation12] A herba-alba. Camphor is a valuable compound with several biological uses; it is commonly applied to the skin for its antipruritic, analgesic and counterirritant properties.[Citation26]
On the other hand, the results of our study showed that fenchol was present in the four oils with great percentage. This oxygenated monoterpene is not a common compound in the oils obtained from Artemisia genus. Tilaoui et al.[Citation17] reported small amount of fenchol in the oil of A. herba alba from Marroco, however, this compound has not been found either in Tunisian A. herba alba oils before. Haouari and Ferchichi[Citation13] reported high levels of its isomer borneol in A. herba-alba from south Tunisia. Since borneol was weakly represented in the samples collected in our study from north and center of Tunisia, the biosynthesis of borneol seem to be inhibited in favour to its isomer fenchol in these regions. Other main monoterpenes compounds in our work included nordavanone (1-9%), limonene (5-9%), chrysanthenone (4-7%) and camphene (0.3-5%). Chrysanthenone has been found to be the major constituent in some populations of A. herba-alba from Spain[Citation11] and Marocco.[Citation13] Moreover the sesquiterpene germacrene was present in our study with appreciable percentages (2-6%).
On the other hand, the significant differences on the chemotypes according to the origin, found in our study, suggest a significant impact on the aroma impression of the oils. In fact, the high camphor content in Boukarnine and Kairouan could contribute to a characteristic odor of these oils as typical fresh, warm-minty aroma impression.[Citation27] Kasserine oil may exhibit herbal and minty aroma notes attributed to fenchol[Citation27] as well as woody, earthy, green, burnt notes due to thujones.[Citation28] Finally, the mixture of all these latter compounds impressions (camphor, thujone and fenchol) will be responsible of Kef oil odor.
Interestingly, compared to Boukarnine and Kairouan, the accessions of Kef and Kasserine presented lower percentages of camphor and camphene and higher levels of β-thujone. This is in accordance with the biosynthetic known pathway of these compounds in which α-terpinyl is the common precursor. In fact, α-terpinyl cation was converted to bornyl cation which gives camphene and camphor in Boukarnine and Kairouan while in Kasserine and Kef, α-terpinyl cation provide thujyl cation which produce thujones.[Citation28] Such conversions results from terpenes synthases activities. The later are known to be modulated by environmental conditions such as light, temperature and moisture.[Citation25]
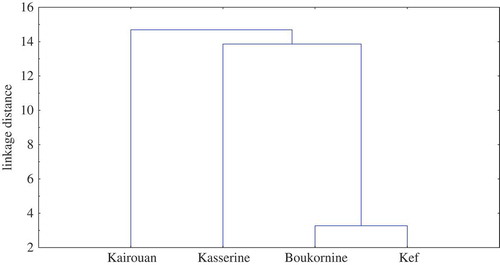
To evaluate the possibility of differentiating the samples on the basis of their volatiles composition we applied CA (). Results showed the existence of three defined groups. The first cluster group Kef and Boukarine accessions indicating thus a higher similarity. Camphor (12 -32%), α-thujone (12-14%), fenchol (9 -14%), limonene (7-9%) and nordavanone (2-7%) were major components of these acessions. The second cluster group includes Kasserine where α-thujone (12%), fenchol (13%), nordavanone (9%), β-thujone (8%) and chrysanthenone (6%) are main constituents of the oil. The third cluster comprises Kairoun accession characterized by camphor (25%), α-thujone (13%), fenchol (8%), chrysanthenone (7%), germacrene-D (6%) and limonene (6%). Since the three groups belong to three different bioclimatic zones (), the analysis suggested that variation in the compositions of essential oils among populations can be attributed at least in part to environmental factors.
Antioxidant Activities of Methanol Extracts and Essential Oils
The results for antioxidant activities from the different accessions measured by two different methods; DPPH scavenging activity and reducing power, are displayed in . For each origin, methanol extract and the essential oil were considered. ANOVA showed that the antioxidant property of A. herba-alba was affected by location. Moreover, for the different accessions, methanol extracts exhibit higher antioxidant activity than essential oils. In fact, solvent extracts obtained from the four accessions exhibit similar and high antiradical capacities with IC50 values by about 3 µg/mL. This activity was more potent than that of the well-known synthetic antioxidant BHT (IC50 = 12 µg/mL). Moreover, methanol extracts exhibit high reducing power with CE50 varying from 100 to 360 µg/mL. In agreement with our finding, solvent extracts recovered from Artemisia species have been reported as exhibiting high antioxidant activities in particular antiradical one.[Citation7] Recently, Khlifi et al.[Citation8] investigated the antioxidant power of methanol/water extracts from A. herba-alba grown in Tunisia and reported lower antiradical activity (IC50 = 20 µg/mL) than that obtained in our study. Phenolic compounds have been reported to be responsible for the antioxidant activities of botanical extracts. In our study, activity of extracts appears to be associated with the majors identified flavonoids and phenolic acids. Flavonols (kaempherol), flavone (apigenin), and flavanones (naringenin) exerts high efficiency and reactivity to scavenge free radicals. This activity is due to their structure, characterized by the presence of several hydroxyl substituents, namely 3-OH group and a conjugated double bond system. These characteristics are related to the stability of the aroxyl radical formed after the flavonoid donates a hydrogen atom to the radical. In addition, the hydroxycinnamic acids; trans-cinnamic, p-coumaric, caffeic, and chlorogenic acids have been reported to exhibit potent antioxidant activity which is linked to the number and substitution pattern of phenolic hydroxyl groups. Moreover, the double-bond characteristic of cinnamic derivatives is able to participate in the radical stabilization by resonance of the unpaired electron.[Citation29] On the other hand, essential oils of A. herba-alba assayed in this experiment showed moderate antiradical activity and weak reducing power. This could be explained by the absence of phenolic monoterpenes such as thymol, carvacrol which are known to be associated to a strong antioxidant power.
TABLE 6 Antiradical activity IC50 (µg/mL) and reducing power CE50 (mg/mL) of A. herba-alba extracts
CONCLUSIONS
The results obtained in this study revealed that A. herba-alba chemical composition is considerably affected by the geographical origin. North regions have high potential for selecting varieties rich on essential oil while centre regions are suitable to produce antioxidants including polyphenols and flavonoids. The chemical polymorphism in the oils and antioxidants indicated that environmental factors should be considered to ensure consistent chemical quality of A. herba-alba. Since consumers are aware of the need for a constant supply of phytochemical-containing foods for antioxidant and flavoring support and disease prevention, this study could supply a basis for the selection of plants with adequate levels of volatiles and/or polyphenolics and high antioxidant properties.
REFERENCES
- Adams, M.; Gmünder, F.; Hamburger, M. Plants Traditionally Used in Age Related Brain Disorders—A Survey of Ethnobotanical Literature. Journal of Ethnopharmacology 2007, 113, 363–381.
- Szabo, M.R.; Radu, D.; Gavrilas, S.; Chambre, D.; Iditoiu, C. Antioxidant and Antimicrobial Properties of Selected Spice Extracts. International Journal of Food Properties 2010, 13, 535–545.
- Burdock, G.A.; Carabin, I.G. Safety Assessment of Coriander (Coriandrum Sativum L,) Essential Oil As a Food Ingredient. Food and Chemical Toxicology 2009, 47, 22–34.
- Bailey, C.; Danin, A. Bedouin Plant Utilization in Sinai and Negev. Economic Botany 1981, 35, 145–162.
- Karabegovic, I.; Nikolova, M.; Velickovic, D.; Stojicevic, S.; Veljkovic, V.; Lazic, M. Comparison of Antioxidant and Antimicrobial Activities of Methanolic Extracts of the Artemisia Sp, Recovered Different Extraction Techniques. Chinese Journal of Chemical Engineering 2011, 19, 504–519.
- Saleh, M.A.M.; El-Negoumy, S.I.; Abou-Zaid, M.M. Flavonoids of Artemisia judaica, A. monosperma and A. herba-alba. Phytochemistry 1987, 11, 3059–3064.
- Orhan, I.E.; Belhattab, R.; Senol, F.S.; Gülpinarc, A.R.; Hosbas, S.; Kartal, M. Profiling of Cholinesterase Inhibitory and Antioxidant Activities of Artemisia Absinthium, A. Herba-Alba, A. Fragrans, Marrubium Vulgare, M. Astranicum, Origanum Vulgare Subsp. Glandulossum, and Essential Oil Analysis of Two Artemisia Species. Industrial Crops and Products 2010, 32, 566–571.
- Khlifi, D.; Sghaier, R.M.; Amouri, S.; Laouini, D.; Hamdi, M.; Bouajila, J. Composition and Anti-Oxidant Anti-Cancer and Anti-Inflammatory Activities of Artemisia Herba-Alba, Ruta Chalpensis L, and Peganum Harmala L. Food and Chemical Toxicology 2013, 55, 202–208.
- Hudaib, M.; Aburjai, T. Composition of the Essential Oil from Artemisia Herba-Alba Grown in Jordan. Journal of Essential Oil Research 2006, 18, 301–304.
- Lamiri, A.; Belanger, A.; Berrada, M.; Zrira, S.; Benjilali, B. Chemical Polymorphism of Artemisia Herba-Alba Asso from Morocco (in French) Rabat: Morocco, 1997; 69–79 pp.
- Salido, S.; Valenzuela, L.R.; Altarejos, J.; Nogueras, M.; Sanchez, A.; Cano, E. Composition and Infraspecific Variability of Artemisia Herba-Alba from Southern Spain. Biochemical and Systematic Ecology 2004, 32, 265–277.
- Belhattab, R.; Amor, L.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C. Essential Oil from Artemisia Herba-Alba Asso Grown Wild In Algeria: Variability Assessment and Comparison with An Updated Literature Survey. Arabian Journal of Chemistry 2014, 7, 243–251.
- Haouari, M.; Ferchichi, A. Essential Oil Composition of Artemisia Herba-Alba from Southern Tunisia. Molecules 2009, 14, 1585–1594.
- Lachenmeier, D.W.; Uebelacker, M. Risk Assessment of Thujone in Foods and Medicines Containing Sage and Wormwood—Evidence for a Need of Regulatory Changes? Regulatory Toxicology and Pharmacology 2010, 58, 437–443.
- Pelkonen, O.; Abass, K.; Wiesner, J. Thujone and Thujone-Containing Herbal Medicinal and Botanical Products: Toxicological Assessment. Regulatory Toxicology and Pharmacology 2013, 65, 100–107.
- Dewanto, V.; Wu, X.; Adom, K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. Journal of Agricultural and Food Chemistry 2002, 50, 3010–3014.
- Tilaoui, M.; Mouse, H.A.; Jaafari, A.; Aboufatima, R.; Chait, A.; Zyad, A. Chemical Composition and Antiproliferative Activity of Essential Oil from Aerial Parts of a Medicinal Herb Artemisia Herba-Alba. Revista Brasileira De Farmacognosia 2001, 21, 781–785.
- Proestos, C.; Boziaris, I.S.; Nychas, G.J.E.; Komaitis, M. Analysis of Flavonoids and Phenolic Acids in Greek Aromatic Plants: Investigation of Their Antioxidant Capacity and Antimicrobial Activity. Food Chemistry 2006, 95, 664–671.
- Hanato, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two New Flavonoids and Other Constituents in Licorice Root: Their Relative Astringency and Radical Scavenging Effect. Chemical and Pharmaceutical Bulletin 1988, 36, 1090–1097.
- Oyaizu, M. Studies on Products of Browning Reaction: Antioxidative Activity of Products of Browning Reaction. Japanese Journal of Nutrition 1986, 44, 307–315.
- Akrout, A.; Gonzalez, L.A.; El Jani, H.; Madrid, P.C. Antioxidant and Antitumor Activities of Artemisia Campestris and Thymelaea Hirsuta from Southern Tunisia. Food and Chemical Toxicology 2011, 49, 342–347.
- Dixon, R.A.; Paiva, N.L. Stress-Induced Phenylpropanoid Metabolism. The Plant Cell 1995, 7, 1085–1097.
- Neugart, S.; Kläring, H.P.; Zietz, M.; Schreiner, M.; Rohn, S.; Kroh, L.W.; Krumbein, A. The Effect of Temperature and Radiation on Flavonol Aglycones and Flavonol Glycosides of Kale (Brassica Oleracea Var, Sabellica). Food Chemistry 2012, 133, 1456–1465.
- Winkel-Shirley, B. Biosynthesis of Flavonoids and Effects of Stress. Current Opinion in Plant Biology. 2002, 5, 218–223.
- Sangwan, N.S.; Farooqi, A.H.A.; Shabih, F.; Sangwan, R.S. Regulation of Essential Oil Production in Plants. Plant Growth Regulation 2001, 34, 3–21.
- Burkhart, C.G.; Burkhart, H.R. Contact Irritant Dermatitis and Antipruritic Agents: The Need to Address the Itch. Journal of Drugs in Dermatology 2003, 2, 143–146.
- Mahattanatawee, K.; Perez-Cacho, P.R.; Davenport, T.; Rouseff, R. Comparison of Three Lychee Cultivar Odor Profiles Using Gas Chromatography−Olfactometry and Gas Chromatography−Sulfur Detection. Journal of Agricultural and Food Chemistry 2007, 7, 1939–1944.
- Degenhardt, J.; Kollner, T.G.; Gershenzon, J. Monoterpene and Sesquiterpene Synthases and the Origin of Terpene Skeletal Diversity in Plants. Phytochemistry 2009, 70, 1621–1637.
- Soobrattee, M.A.; Neergheen, V.S.; Luximon, R.A.; Aruoma, O.I.; Bahorun, T. Phenolics As Potential Antioxidant Therapeutic Agents: Mechanism and Actions. Mutation Research 2005, 579, 200–213.