Abstract
The effects of ultrasound-assisted, supercritical CO2 and solvent extraction techniques on antioxidant activity of Eriobotrya japonica (Lindl.) skin extracts were investigated using 2, 2-diphenyl-1-picrylhydrazyl, β-carotene, and Rancimat assays. Solvent extract of skin at 400 ppm had the highest antioxidant activity. Solvent extraction was the most effective method on extraction of phenols and tocopherols. Protective effects of extracts in stabilization of soybean oil during frying were determined by measuring its peroxide value, total polar compounds, carbonyl value, free fatty acids, conjugated dienes, and trienes. Results indicated that solvent extract of skin at 400 ppm exhibited stronger antioxidant activity in oil than tertiary butylhydroquinone.
INTRODUCTION
Deep-fat frying is a cooking process in which food is immersed into an edible oil or fat that serves as a heat-transfer medium, and in addition, such frying oil is an important ingredient of the fried food. Oils during deep frying in the presence of moisture and air will undergo reactions such as oxidation, hydrolysis, polymerization, isomerization, or cyclization. These reactions reduce the shelf life and quality of fried products.[Citation1]
Volatile and non-volatile compounds are formed in vegetable oils during deep frying. Volatile compounds are removed from oil and non-volatile compounds accumulate in the oil. Non-volatile compounds are produced primarily by thermal oxidation and polymerization of unsaturated fatty acids. The non-volatile compounds are often polar and of high molecular weight. These compounds have toxic effects on humans and animals.[Citation2]
Therefore, the synthetic antioxidants, such as butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), and tertiary butylhydroquinone (TBHQ), are often used to increase the oxidative stability of oils.[Citation3] Investigations have demonstrated that these antioxidants are toxic and carcinogenic for humans[Citation4] due to this consumers are more willing to use oils with natural antioxidants. In recent years, extensive researches have been conducted on plants, because plants are rich sources of antioxidant compounds.[Citation5]
Loquat (Eriobotrya japonica) is an Asian fruit and a member of the Rosaceae family.[Citation6] The species is native to southeastern China and mainly grows on subtropical and mild temperate. Currently it is also cultivated in other areas namely in South Africa, South America, Australia, and California.[Citation7] Loquat is an evergreen large shrub or small fruit tree with a rounded crown and short trunk. Loquats are unusual among other fruits such that they flower in autumn or early winter, and the fruits ripen in late winter or early spring.[Citation8] Loquat fruit is eaten as a fresh fruit and also recently is used for making jam, jelly, and chutney. Phenolic compounds of loquat fruits have been characterized in few studies which described the antioxidant activity of loquat fruits as being due to the presence of hydroxycinnamic and benzoic acids derivatives and cyanidine glycoside.[Citation9]
Recently, various methods such as microwave-assisted, ultrasound-assisted, and supercritical CO2 extraction are used for the extraction of bioactive compounds of plants, because different methods have different abilities in extraction.[Citation10] The aim of this research was to compare the effect of solvent extraction, ultrasound-assisted and supercritical CO2 extraction process on antioxidant activity of loquat fruit skin extracts. Furthermore, in this study the effect of loquat fruit skin extracts on the stability of soybean oil during deep frying at 180°C compared to that of TBHQ.
MATERIALS AND METHODS
Materials
Loquat (Eriobotrya japonica Lindl.) fruits were collected from fields in Sari in the Mazandaran province, Iran. Refined, bleached, and deodorized soybean oil with no added antioxidant was supplied by Rana (Gorgan, Iran) and stored at –20°C until analysis. All reagents used in the experiments were of analytical grade and obtained mostly from Sigma Chemical Co. (St. Louis, MO, USA). Solvents used for extraction of plant samples were purchased from Merck (Darmstadt, Germany).
Solvent Extraction
The loquat fruits skin were manually removed, sun-dried, powdered in a grinder to reach 40-mesh, and then were packed and stored at –20°C until extraction of antioxidants. In solvent extraction, dried powders of skin (20 g) were mixed with 100 mL of ethanol. The mixture was stirred in a shaker at 160 rpm away from light at room temperature for 48 h. After extraction, the extracts were filtered and solvent evaporated using a rotary evaporator (Heidolph, Germany) at 50°C. The concentrated extract was stored until testing at –20°C.[Citation11]
Supercritical Fluid Extraction
A Suprex MPS/225 Multipurpose system (Pittsburg, PA, USA) in the supercritical CO2 extraction mode was used for the extraction of phenolic compounds. In this method, extractions of 20 g of dried powder from the skin were accomplished with a 100 mL of ethanol at 35°C, 100 bar, for 30 min. The mixture was filtered and solvent evaporated and extract stored at –20°C until testing.[Citation12]
Ultrasound-Assisted Extraction
An Elma Transsonic (1000 W) model 690/H ultrasonic bath (Germany) was used for extraction of phenolic compounds from mixture of ethanol (100 mL) and powdered skin (20 g). The mixture was sonicated for 30 min at 35°C. The mixture was filtered and solvent evaporated and extract stored in a freezer.[Citation13]
Extraction Yield
The extraction yield (%) according to the method described by Tian et al.[Citation14] is calculated as follows:
Total Phenolic (TP) Content
The TP content of extracts was determined using Folin-Ciocalteu reagent according to the procedure reported by McDonald et al.[Citation15] Briefly, 0.5 mL of extract was mixed with 2.5 mL of 10-fold-diluted Folin-Ciocalteu reagent and 2 mL of 7.5% sodium carbonate. The mixture was shaken for 1 min and allowed to stand at room temperature for 15 min and spectrophotometric measurements were carried out (Cintra 20; GBC, Dandenong, Australia). The estimation of phenolic compounds was done at 760 nm and calculated by a calibration curve plotted with gallic acid. TP content was expressed as µg gallic acid per gram dry sample. Each test was repeated three times, and the results were averaged.
Total Tocopherol (TT) Content
The TT content was determined according to the colorimetric method described by Wong et al.[Citation16] A calibration curve of pure α-tocopherol in toluene was performed in a concentration range of 0–240 µg/mL. Extract (0.2 g) was dissolved in 5 mL of toluene and then 3.5 mL of 2, 2´-bipyridine (0.07% w/v in 95% aqueous ethanol) and 0.5 mL of FeCl3-6H2O (0.2% w/v in 95% aqueous ethanol) were added. The solution was made up to 10 mL with 95% aqueous ethanol. After standing for 1 min, the absorption at 520 nm was determined by using a spectrophotometer. The results were expressed as microgram of α-tocopherol equivalents per gram dry sample.
Free Radical Scavenging Activity
2, 2-diphenyl-1-picrylhydrazyl (DPPH•) scavenging capacity of the extracts was evaluated according to Burits and Bucar[Citation17] with some modifications. Briefly, 50 µL of various dilutions of the test materials (pure antioxidants or loquat extracts) were mixed with 5 mL of a 0.004% methanolic DPPH• solution. The reaction mixtures were shaken vigorously and incubated in the dark for 30 min. The absorbance of the solution was measured at 517 nm against a blank. The radical scavenging activity of each solution was calculated as percent inhibition according to the following equation:
β-Carotene-Linoleic Acid Assay
Antioxidant activities of loquat extracts were determined using the β-carotene bleaching method, as described by Amarowicz et al.[Citation18] Five milligrams of β-carotene were dissolved into 10 mL of chloroform and 600 µL β-carotene solution was mixed with 40 mg of linoleic acid and 400 mg of Tween 40 emulsifier in a round-bottom flask. Then chloroform was removed in a rotary vacuum evaporator. Oxygenated distilled water (100 mL) was added to the flask and the resulting mixture was stirred vigorously. Five milliliters of the emulsion was transferred to tubes containing 200 µL of different concentrations of extracts. After mixing, absorbance of the samples was measured at 470 nm at initial time (T = 0) against a blank. The remaining samples were placed in dark for 24 h. Then, the absorbance of each sample was measured at 470 nm (Abs24). Antioxidant activity was calculated as percent inhibition according to the following equation:
where Abss(24) is the absorbance of the antioxidant at 24 h, Absc(24) is the absorbance of the blank at 24 h, Abss(0) is the absorbance of the antioxidant at 0 times and Absc(0) is the absorbance of the blank at 0 time.
Rancimat Analysis
A Metrohm 743 Rancimat instrument (Herisan, Switzerland) was used in the experiment. Air supply was maintained at 15 L/h and the temperature was kept at 110°C.[Citation19]
Frying Conditions
The loquat skin extracts in levels of 400 and 1000 ppm and TBHQ in level of 100 ppm were added to soybean oil. Soybean oil without antioxidant addition was used as a negative control. Oil sample (2.5 L) was placed in a fryer oven of 2.5 L capacity (Tefal model 1250, France) and heated at 185 ± 5°C for 24 h. A batch of 20 g of potato pieces (7.0 cm × 0.5 cm × 0.3 cm) was fried for 7 min. After time of heating (0, 4, 8, 12, 16, 20, 24), 20 g of frying oil samples was stored until testing at –20°C.[Citation20]
Peroxide Value (PV)
The spectrophotometric method described by Shantha and Decker[Citation21] was used to determine PV. The oil samples (0.2 g) was dissolved in 9.8 mL chloroform–methanol (7:3 v/v). Then, 50 µL of ammonium thiocyanate solution (30% w/v) and 50 µL of iron (II) chloride solution ([0.5 g barium chloride dihydrate dissolved in 50 mL dH2O] + [0.5 g FeSO4-7H2O dissolved in 50 mL dH2O] + [2 mL 10 M HCl, with the precipitate barium sulphate, filtered off to produce a clear solution]) were added, and the sample was mixed on a vortex mixer for 2–4 s. After 5 min incubation at room temperature, the absorbance of reaction mixture was measured at 500 nm against a blank. Results were expressed in millequivalents of oxygen per kilogram of oil.
Total Polar Compounds (TPC)
The TPC was determined according to the method described by Schulte[Citation22] Silica gel 60 was dried at 160°C for 24 h and mixed with water at the ratio of 95:5. The solution was shaken vigorously for about 1 min to disperse lumps and after standing overnight the material was ready for use. One gram of the silica gel inside a plastic column (15 cm long) was compressed between two cotton wool balls. The oil sample (0.5 g) was introduced into a 5-mL volumetric flask, and the flask was filled by toluene. One milliliter of the solution was pipetted on top of the column. After the solution was soaked in, the column was washed with 1 mL eluent and after soaking, 7 mL (2 × 3.5 mL) of eluent was added. After elution (15 min), the end of the tip was washed with 500 µL of toluene. The eluate was evaporated at 50°C and thus, non-polar components were isolated. The TPC content was calculated by the formula of 100 (W – W1)/W, in which W and W1 are the sample weight and the weight of non-polar components in milligrams, respectively.
Carbonyl Value (CV)
The CV was determined according to the method described by Endo et al.[Citation23] A calibration curve of standard aldehyde (2, 4-decadienal) was performed in a concentration range of 50–500 µM. Sodium borohydride (0.5 g) of was added to 1000 g of 2-propanol and the mixture was refluxed for 30 min. 2, 4-Dinitrophenylhydrazine (DNPH) solution was prepared by dissolving 50 mg DNPH in a 100 mL solvent containing 3.5 mL concentrated HCl (37%). The oil sample (0.15 g) was pipetted into a 10 mL volumetric flask, dissolved in refluxed solvent, and then filled with the refluxed solvent. Oil solution (1 mL) was introduced into a 15 mL test tube and then mixed with 1 mL DNPH solution. The test tube heated at 40°C for 20 min, cooled for 10 min and then 8 mL of potassium hydroxide (KOH) solution (2%) was added. The test tube was centrifuged at 2000 × g for 5 min. The absorbance of the upper layer was measured at 420 nm by using a spectrophotometer (GBC, Cintra 20) against a blank. The results were expressed in micromoles of 2, 4-decadienal per gram of oil.
Free Fatty Acids (FFA) Content
The FFA content of the oils were determined according to official method Cd 3a-63 of the American Oil Chemists’ Society.[Citation24] The results were expressed in percentages (as oleic acid).
Conjugated Dienes (CDV) and Conjugated Trienes Value (CTV)
The CDV and CTV were calculated according to the method described by Fathi et al.[Citation25] which is based on the measurement of absorbance solution (5 mg of the oil sample dissolved in 10 mL cyclohexane) at 234 nm and 270 nm for CDV and CTV, respectively.
Statistical Analysis
All experiments were conducted in three levels measurements and results reported as mean ± standard deviation (SD). The SPSS version 19.0 software was used to analyze data for determining analysis of variance (ANOVA) and Duncan’s multiple range test for significance at 5% level.
RESULTS AND DISCUSSION
Extraction Yield
show the extraction yield of loquat fruit skin which range from 10.5 to 14.75%. The extraction yields of different methods in descending order were: solvent extraction > supercritical CO2 > ultrasound-assisted extraction method. This shows that solvent extraction was the best techniques for the extraction of compounds from the loquat fruit skin. Our results concurred with previously published results of Plánder et al.[Citation26] and Sánchez-Vioque et al.[Citation27] that reported the classic extraction method was more effective in extraction of plant material compared to other techniques.
TABLE 1 Extraction yield, total phenolic and tocopherol compounds of loquat fruit skin extracts
Total Phenolic Content
Polyphenolic compounds are widely distributed in different parts of plants[Citation28] and reports showed that there is a positive relation between TP content and antioxidant activity in many plant species.[Citation10] Phenolic compounds in plants are known as potent in vitro antioxidants due to their ability to donate hydrogen or electrons and to form stable radical intermediates.[Citation29] Concentration of phenolics in the extracts, expressed in µg GAE/g dry extract depends on the method used for extraction. There were significant differences (p < 0.05) between phenolic compounds of extracts of three mentioned methods (). The amounts of phenolic compounds in ultrasound-assisted extract (UAE), supercritical CO2 extract (SCE), and solvent extract (SE) were 394.67, 425.02, and 664.53 (µg/g), respectively. Luengthanaphol et al.[Citation12] and Plánder et al.[Citation26] reported similar results that the solvent extract was the most effective in extraction of phenolic compounds in comparison with ultrasound-assisted and supercritical CO2 extraction techniques. Ferreres et al.[Citation30] calculated that the TP content of various varieties of loquat fruit skin range from 13.1 to 1349.8 (µg/g). The phenolic content and composition of fruits depend on the genetic and environmental factors as well as post-harvest processing conditions.[Citation31,Citation32]
Total Tocopherol Content
Tocopherols are a group of antioxidant compounds that prevent oxidation progress by scavenging peroxyl radicals without reacting in further chain-propagating steps.[Citation33] shows TT compounds found in skin extracts of loquat which range from 228.41 to 486.53 (µg/g). The results indicated solvent extract had the highest effect on extraction of tocopherol compounds. We also found that bioactive compounds of loquat fruit skin are very sensitive to ultrasound waves as they cause compounds to degrade.
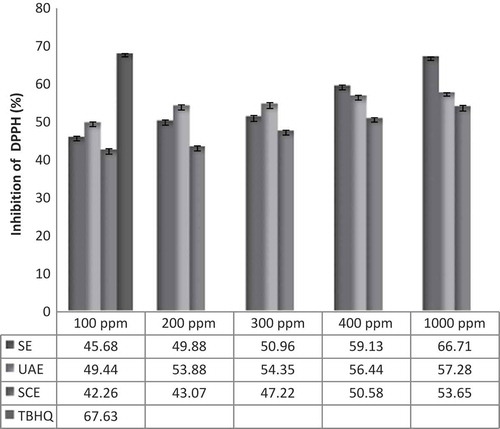
DPPH• Radical Scavenging Activity
In the reaction of the free radical DPPH• with antioxidant species, antioxidant inhibits, and stabilizes the free radical which cause the color to change from purple to yellow, and decreases the absorbance which was monitored using a spectrophotometer at 517 nm.[Citation29] shows the ability of loquat fruit skin extracts to scavenge DPPH• radical as inhibition percentage at concentrations of 100 to 1000 ppm. In this assay, scavenging free radicals increased as concentrations of the extracts increased which due to increasing amount of phenolic and tocopherol compounds at higher concentrations of the extracts. Similar to previously published results of Chaillou and Nazareno[Citation34] and Zhang et al.[Citation35] With increasing concentrations of phenolic compounds the number of hydroxyl groups available in the reaction medium increased. So, the possibility of hydrogen donation to free radicals is increased.[Citation27] The result showed UAE of skin at concentrations of 100 to 300 ppm had the highest DPPH• radical-scavenging capacity, but SE of skin had a better performance at 400 and 1000 ppm compared to other extracts. Our result also demonstrated that skin extracts have good antioxidant activity, but the highest inhibitory effect was observed in TBHQ. These results concurred with previously published results of Silva et al.[Citation36] in which the antioxidant effect of quince fruit skin and pulp extracts compared to TBHQ.
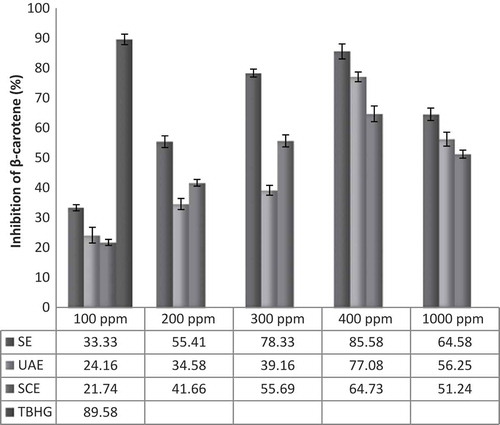
β-Carotene Bleaching Assay
In this assay β-carotene is oxidized by free radicals derived from the oxidation of linoleic acid, which can better simulate the food system compared to DPPH assay.[Citation37] The auto-oxidation products of linoleic acid attack double bonds of β-carotene. In the absence of an antioxidant, the β-carotene molecule loses its chromosphere and undergoes rapid discoloration, which can be monitored spectrophotometrically.[Citation38] The antioxidant activities of the loquat skin extracts were measured by bleaching of β-carotene which is illustrated in . The SCE indicated the lowest significant antioxidant activity in levels of 100, 400, and 1000 ppm, while the SE showed better performance to prevent of β-carotene oxidation in all the tested concentrations. The SE at 400 ppm exhibited antioxidant activity which was comparable to that of standard synthetic antioxidant TBHQ. The results of antioxidant activity were in agreement with previously published results of Koba et al.[Citation39] that examined the antioxidant effect of loquat fruit skin by β-carotene bleaching assay.
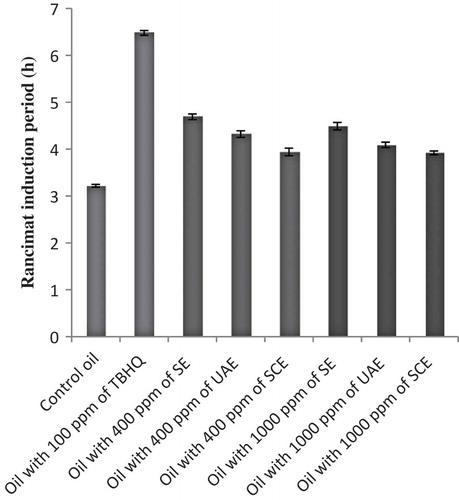
Rancimat Analysis
The Rancimat analysis was performed at 110°C and the induction period (h) was evaluated for soybean oils with or without loquat fruit skin extracts at 400 and 1000 ppm. Our results showed both skin extracts and TBHQ had a strong antioxidant activity in soybean oil. As shown in , the presence of extracts retarded the oxidation of soybean oil. Generally, the lowest thermal stability in oils treated with extracts was for SCE sample (3.92 h), which was higher compared to control oil (3.32 h). On the other hand the highest thermal stability in oils containing loquat fruit skin extracts was for SE at 400 and 1000 ppm (4.69 and 4.49 h), but the best protection effect was observed in soybean oil containing 100 ppm of TBHQ (6.48 h). These results concurred with the results of Sun and Ho[Citation40] and Aladedunye and Matthäus[Citation41] that reported the induction period of oils containing buckwheat (Fagopyrum esculentum Möench), rowanberry (Sorbus aucuparia), and crabapple (Malus baccata) extracts was higher than control oil, but the most antioxidant effect was in TBHQ. Moreover, from the findings of Rancimat assay it is understood that the samples extracted by solvent extraction method exhibited strong antioxidant activity under the Rancimat conditions compared to other techniques.
Change in PV
The PV is a measurement of the concentrations of peroxides and hydroperoxides produced in the first stage of oxidation of oils and fats. Change in PV of the oil samples during deep frying is shown in . The PV of fresh oil should be less than 2 meqO2/kg.[Citation42] The initial PV of oil samples was 0.65 (meq O2/kg). It was generally observed that the presence of extracts significantly (p < 0.05) reduced the PV compared to control oil. The PV of soybean oil with 400 ppm of solvent extract (SOEA), soybean oil with 1000 ppm of supercritical CO2 extract (SOSB), and soybean oil with 100 ppm of TBHQ (SO-TBHQ) treatments has no significant differences during 4 h of frying. The peroxide levels during frying after 8 h in soybean oil with 1000 ppm of ultrasound-assisted extract (SOUB), after 12 h in SOEA, after 16 h in SOUB, after 20 h in SOEA, after 24 h in SOEA and soybean oil with 400 ppm of ultrasound-assisted extract (SOUA) were lower than the other oil samples. The SOEA showed greater ability to prevent augmentation in PV compared to other oil samples. There was an initial increase in PV for SOUB from 0 to 12 h, for SOSB, SO-TBHQ, and soybean oil with no antioxidant added (SBO) from 0 to 16 h, for SOEA, soybean oil with 1000 ppm of solvent extract (SOEB), soybean oil with 400 ppm of supercritical CO2 extract (SOSA), and SOUA from 0 to 20 h and after which the rate decelerated. Peak values for PV were obtained as follows: SBO (6.58 meq O2/kg) after 16 h, SOEA (4.55 meq O2/kg), SOEB (5.43 meq O2/kg), SOSA (5.84 meq O2/kg), SOSB (5.66 meq O2/kg), SOUA (4.68 meq O2/kg), and SOUB (5.42 meq O2/kg) after 20 h and SO-TBHQ (5.11 meq O2/kg) after 24 h. The PV decreased in oil samples after the reached to peak point. These results were agreement with previously studies results by Abdulkarim et al.[Citation2] and Casal et al.[Citation43] The PV test is not a valid parameter to assess oils’ oxidative changes during deep frying process, because peroxidase under frying conditions are unstable and are converted into other compounds, such as carbonyl and aldehyde that cause PV abatement.[Citation44]
TABLE 2 Changes in peroxide value (PV) and total polar compounds (TPC) of the oil samples
Change in TPC
In many European countries the TPC value considered as a major oil degradation indicator and it is acceptable at maximum 25–27% for used frying oil.[Citation45] The increase in the amount of TPC in the oils shows the formation of compounds of high polarity such as triacylglycerols and secondary oxidation products. Changes in TPC contents of oil samples are shown in . The TPC of fresh frying oil (5.84%) also reflected the good quality of the oil, as the TPC of unused oils normally range from 0.4 to 6.4%.[Citation45] There were significant differences between the TPC values of all frying times for each oil sample. The results showed that the contents of TPC increased almost linearly with the frying time similar to those reported by Bansal et al.[Citation46] and Marmesat et al.[Citation47] During frying time it is observed that the rate increase in the amount of TPC in SOEA was relatively slower than other oil samples and later reached to the critical limit (25%). There was no significant difference between the TPC values of SOEA and SO-TBHQ after 8 h of frying, while values during 12 to 24 h in SOEA was significantly lower than the other oil samples. Generally, the rate of oxidation of oil samples (25% of TPC) was as follows:
which indicated that the highest oxidative stability were obtained by addition of SOEA. The result was in agreement with previously published results by Casarotti and Jorge[Citation48] and Urbančič et al.[Citation49]that indicated that rosemary extract was more effective in reduced production of polar compounds in frying oil compared to TBHQ.
Change in CV
The CV is an appropriate method for evaluation of frying oil quality because carbonyl compounds are cause undesirable flavors and reduce nutritional value of fried foods. CV measures secondary products of oxidation such as aldehydes and ketones.[Citation23] On the basis of the National Standards of Japan the frying oils containing more than 50 µmol/g of CV should be discarded, because undesirable changes have occurred in their flavor.[Citation50] As it is shown in the CV of a set of oil samples increased and reached to maximum value during the heating process, and then decreased. The result were concurred with previously published results by Farhoosh and Kenari[Citation51] and Farhoosh and Tavassoli-Kafrani[Citation19] During deep frying process carbonyl compounds may convert to new compounds which are not detectable by the CV assay.[Citation51] The greatest increases in CV were seen in control frying oil, which confirms that the unprotected frying oil is more susceptible to degradation during deep-frying than the frying oils with the addition of skin extracts. The CV of the SOEA, SOEB, and SOUB didn’t reach 50 µ mol/g (critical limit). Generally, the CV of the SOSA, SOUA, SO-TBHQ, SOSB after 24 h and control oil sample (SBO) after 16 h reached to the critical limit (50.66, 52.06, 52.46, 53.09, and 54.47 µ mol/g, respectively). Therefore, SOEA showed higher antioxidant activity compared to other oil samples to prevent the increase of carbonyl compounds during deep frying process.
TABLE 3 Changes in carbonyl value (CV) and free fatty acids content (FFA) of the oil samples
Change in FFA Content
shows change in FFA content of oil samples during deep frying process at 180°C. The FFA is used as an indicator for assessment of oil deterioration during frying.[Citation49] The steady rise in FFA can be attributed partly to hydrolysis of triacylglycerols and partly to carboxyl groups of compounds that present in oxidative and/or polymeric products of heating.[Citation52] There was no significant difference between the initial FFA (0 h) of oil samples. Similar to results reported by Kim and Choe[Citation53] and Aladedunye and Przybylski[Citation54] it was observed that the FFA content increased with the frying time. At the end of the deep frying process, the FFA contents of the SOEA, SO-TBHQ, SOSA, SOUA, SOEB, SOSB, SOUB, and SBO were 3.55, 3.85, 4.22, 4.22, 4.35, 4.44, 4.52, and 7.44%, respectively. The FFA contents of the SOSB, SOUB, and SO-TBHQ after 4 h, SOEA, SOUB, and SO-TBHQ after 8 h with no significant difference were lower than other oil samples. The low level of FFA for SOEA during frying time (at 8, 12, 16, 20, 24 h) indicated high ability to reduce oxidation of unsaturated fatty acids. The results also showed that the natural antioxidants in the loquat skin extracts better protected the oils from hydrolysis compare to TBHQ and control oil sample. These results concurred with the results of Casarotti and Jorge[Citation48] and Urbančič et al.[Citation49] that examined the antioxidant effect of rosemary extract in soybean and sunflower oil.
Change in CDV and CTV
The CDV and CTV are measured the primary and secondary oxidation products, respectively. An increase in CDV and CTV showed a decrease in oxidative stability of oils.[Citation55] The changes in CDV and CTV during 24 h of frying are shown in . The CDV and CTV were initially present in the fresh oil in small amounts (2.85 and 0.64 mmol/L), which increase in both with increased deep-frying time, as it is reported in other studies such as the works by Abdulkarim et al.[Citation2] and Bou et al.[Citation52] The CDV and CTV of soybean oil treated with skin extracts were significantly different from the control oil and from the oils with the addition of the TBHQ. The CDV of the SOEA, SO-TBHQ, SOUA, SOEB, SOUB, SOSA, SOSB, and SBO after 24 h of frying were 16.58, 16.66, 17.44, 17.61, 17.82, 18.05, 18.43, and 23.5 mmol/L, respectively. The CDV for SO-TBHQ after 4 h, for SOEA, SOUB, and SO-TBHQ (with no significant difference) after 8 h and for SOEA after 12 to 24 h, were lower than other oil samples.
FIGURE 4 Effect of deep frying conditions on conjugated dienes and trienes of oil samples. Soybean oil with 400 (SOEA) and 1000 ppm (SOEB) of solvent extract of skin, soybean oil with 400 (SOSA) and 1000 ppm (SOSB) of supercritical CO2 extract of skin, soybean oil with 400 (SOUA) and 1000 ppm (SOUB) of ultrasound-assisted extract of skin, soybean oil with 100 ppm of TBHQ (SO-TBHQ), soybean oil with no antioxidant added (SBO).
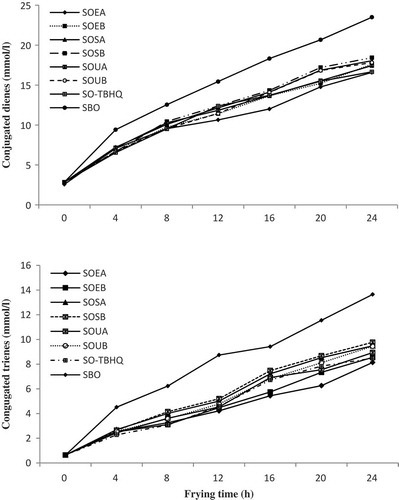
The CTV for the control oil at the end of 24 h of frying was greater than that for the oils treated with skin extracts and TBHQ. For the all treatments of the SOEA, SOEB, SOSA, SOSB, SO-TBHQ, SOUA, SOUB, and SBO, the levels of the CTV at the end of the frying time were 8.14, 8.55, 8.94, 9.45, 9.48, 9.48, 9.77, and 13.65 (mmol/L), respectively. The CTV of the SO-TBHQ after 8 h and SOEA after 12 to 20 h were lowest. Therefore, the low levels of both CDV and CTV in SOEA are indications of good oxidative stability of the oil. The CDV and CTV results are approved by other studies such as Casarotti and Jorge[Citation48] and Urbančič et al.[Citation49] that showed the antioxidant effect of rosemary extract in prevention of conjugated compounds augmentation in vegetable oil was higher than TBHQ.
CONCLUSION
In this study, the antioxidant mixture extracted from loquat fruit skin using solvent extraction with ethanol was found to be the most active in terms of TPC, CV, PV, FFA, CDV, and CTV. Our results also confirmed that the protection offered by SOEA is comparable with, or in some cases better than that of widely used synthetic antioxidant (TBHQ). The loquat fruit skin extracts showed acceptable oxidation prevention activity in soybean oil and could be used as a substitute for synthetic antioxidants to increase the shelf life of vegetable oils.
REFERENCES
- Xua, T. Lia, J. Fana, Y.V. Zhenga, T. Deng, Z.Y. Comparison of Oxidative Stability Among Edible Oils under Continuous Frying Conditions. International Journal of Food Properties 2014, 18(7), 1478–1490.
- Abdulkarim, S. Long, K. Lai, O. Muhammad, S. Ghazali, H. Frying Quality and Stability of High-Oleic Moringa Oleifera Seed Oil in Comparison with Other Vegetable Oils. Food Chemistry 2007, 105(4), 1382–1389.
- Katalinica, V. Mozinab, S.S. Generalica, I. Skrozaa, D. Ljubenkovc, I. Klancnik, A. Phenolic Profile, Antioxidant Capacity, and Antimicrobial Activity of Leaf Extracts from Six Vitis Vinifera L. Varieties. International Journal of Food Properties 2013, 16(1), 45–60.
- Omena, C.M.B. Valentim, I.B. Guedes, G.d.S.; Rabelo, L.A. Mano, C.M. Bechara, E.J.H. Sawaya, A.C. Trevisan, M.T.S. da Costa, J.G.; Ferreira, R.C.S. Antioxidant, Anti-Acetylcholinesterase and Cytotoxic Activities of Ethanol Extracts of Peel, Pulp, and Seeds of Exotic Brazilian Fruits: Antioxidant, Anti-Acetylcholinesterase, and Cytotoxic Activities in Fruits. Food Research International 2012, 49(1), 334–344.
- Arabshahi-D, S. Vishalakshi Devi, D. Urooj, A. Evaluation of Antioxidant Activity of Some Plant Extracts and Their Heat, pH, and Storage Stability. Food Chemistry 2007, 100(3), 1100–1105.
- Wells, J. Raju, B. Huang, H. Weisburg, W. Mandelco-Paul, L. Evaluation of Loquats (Eriobotrya japonica (thunb.) Lindl.) at the Tropical Research and Education Center, Homestead. Proc. Ha. State Hort. Soc 1999, 112, 187–190.
- Ercisli, S. Gozlekci, S. Sengul, M. Hegedus, A. Tepe, S. Some Physicochemical Characteristics, Bioactive Content, and Antioxidant Capacity of Loquat (Eriobotrya Japonica (Thunb.) Lindl.) Fruits from Turkey. Scientia Horticulturae 2012, 148, 185–189.
- Lin, S. Sharpe, R.H. Janick, J. Loquat: Botany and Horticulture. Horticultural Reviews 1999, 23, 233–276.
- Tosun, M. Ercisli, S. Karlidag, H. Sengul, M. Characterization of Red Raspberry (Rubus Idaeus L.) Genotypes for Their Physicochemical Properties. Journal of Food Science 2009, 74(7), 575–579.
- Yasoubi, P. Barzegar, M. Sahari, M. Azizi, M. Total Phenolic Contents and Antioxidant Activity of Pomegranate (Punica Granatum L.) Peel Extracts. Journal of Agricultural Science and Technology 2010, 9, 35–42.
- Tachakittirungrod, S. Okonogi, S. Chowwanapoonpohn, S. Study on Antioxidant Activity of Certain Plants in Thailand: Mechanism of Antioxidant Action of Guava Leaf Extract. Food Chemistry 2007, 103(2), 381–388.
- Luengthanaphol, S. Mongkholkhajornsilp, D. Douglas, S. Douglas, P.L. Pengsopa, L.-I. Pongamphai, S. Extraction of Antioxidants from Sweet Thai Tamarind Seed Coat––Preliminary Experiments. Journal of Food Engineering 2004, 63(3), 247–252.
- Albu, S. Joyce, E. Paniwnyk, L. Lorimer, J. Mason, T. Potential for the Use of Ultrasound in the Extraction of Antioxidants from Rosmarinus Officinalis for the Food and Pharmaceutical Industry. Ultrasonics Sonochemistry 2004, 11(3), 261–265.
- Tian, Y. Zeng, H. Xu, Z. Zheng, B. Lin, Y. Gan, C. Lo, Y.M. Ultrasonic-Assisted Extraction and Antioxidant Activity of Polysaccharides Recovered from White Button Mushroom (Agaricus Bisporus). Carbohydrate Polymers 2012, 88(2), 522–529.
- McDonald, S. Prenzler, P.D. Antolovich, M. Robards, K. Phenolic Content and Antioxidant Activity of Olive Extracts. Food Chemistry 2001, 73(1), 73–84.
- Wong, M. Timms, R. Goh, E. Colorimetric Determination of Total Tocopherols in Palm Oil, Olein, and Stearin. Journal of the American Oil Chemists Society 1988, 65(2), 258–261.
- Burits, M. Bucar, F. Antioxidant Activity of Nigella Sativa Essential Oil. Phytotherapy Research 2000, 14(5), 323–328.
- Amarowicz, R. Estrella, I. Hernández, T. Robredo, S. Troszyńska, A. Kosińska, A. Pegg, R.B. Free Radical-Scavenging Capacity, Antioxidant Activity, and Phenolic Composition of Green Lentil (Lens Culinaris). Food Chemistry 2010, 121(3), 705–711.
- Farhoosh, R. Tavassoli-Kafrani, M.H. Simultaneous Monitoring of the Conventional Qualitative Indicators During Frying of Sunflower Oil. Food Chemistry 2011, 125(1), 209–213.
- Farhoosh, R. Tavassoli-Kafrani, M.H. Polar Compounds Distribution of Sunflower Oil As Affected by Unsaponifiable Matters of Bene Hull Oil (BHO) and Tertiary-Butylhydroquinone (TBHQ) During Deep-Frying. Food Chemistry 2010, 122(1), 381–385.
- Shantha, N.C. Decker, E.A. Rapid, Sensitive, Iron-Based Spectrophotometric Methods for Determination of Peroxide Values of Food Lipids. Journal of AOAC International 1993, 77(2), 421–424.
- Schulte, E. Economical Micromethod for Determination of Polar Components in Frying Fats. European Journal of Lipid Science and Technology 2004, 106(11), 772–776.
- Endo, Y. Li, C.M. Tagiri-Endo, M. Fujimoto, K. A Modified Method for the Estimation of Total Carbonyl Compounds in Heated and Frying Oils Using 2-Propanol As a Solvent. Journal of the American Oil Chemists’ Society 2001, 78(10), 1021–1024.
- AOCS. (1998). Official Methods and Recommended Practices of the American Oil Chemists’ Society, 5th Ed; Vol. 1; AOCS Press: Chicago, IL.
- Fathi, A. Sahari, M. Barzegar, M. Badi, H.N. Antioxidant Activity of Satureja Hortensis L. Essential Oil and its Application in Safflower Oil. Journal of Medicinal Plants 2013, 12(45), 51–67.
- Plánder, S. Gontaru, L. Blazics, B. Veres, K. Kéry, Á. Kareth, S. Simándi, B. Major Antioxidant Constituents from Satureja Hortensis L. Extracts Obtained with Different Solvents. European Journal of Lipid Science and Technology 2012, 114(7), 772–779.
- Sánchez-Vioque, R. Polissiou, M. Astraka, K. Mozos-Pascual, M. Tarantilis, P. Herraiz-Peñalver, D.; Santana-Méridas, O. Polyphenol Composition and Antioxidant and Metal Chelating Activities of the Solid Residues from the Essential Oil Industry. Industrial Crops and Products 2013, 49, 150–159.
- Wang, Y. Chen, X. Zhang, Y. Chen, X. Antioxidant Activities and Major Anthocyanins of Myrobalan Plum (Prunus Cerasifera Ehrh.). Journal of Food Science 2012, 77(4), C388–C393.
- Li, Y. Guo, C. Yang, J. Wei, J. Xu, J. Cheng, S. Evaluation of Antioxidant Properties of Pomegranate Peel Extract in Comparison with Pomegranate Pulp Extract. Food Chemistry 2006, 96(2), 254–260.
- Ferreres, F. Gomes, D. Valentão, P. Gonçalves, R. Pio, R. Chagas, E.A. Seabra, R.M. Andrade, P.B. Improved Loquat (Eriobotrya Japonica Lindl.) Cultivars: Variation of Phenolics and Antioxidative Potential. Food Chemistry 2009, 114(3), 1019–1027.
- Rop, O. Juríková, T. Sochor, J. Mlcek, J.; Kramarova, D. Antioxidant Capacity, Scavenging Radical Activity and Selected Chemical Composition of Native Apple Cultivars from Central Europe. Journal of Food Quality 2011, 34(3), 187–194.
- Milivojevic, J. Slatnar, A. Mikulic-Petkovsek, M. Stampar, F. Nikolic, M. Veberic, R. The Influence of Early Yield on the Accumulation of Major Taste and Health-Related Compounds in Black and Red Currant Cultivars (Ribes spp.). Journal of Agricultural and Food Chemistry 2012, 60(10), 2682–2691.
- Mussatto, S.I. Ballesteros, L.F. Martins, S. Teixeira, J.A. Extraction of Antioxidant Phenolic Compounds from Spent Coffee Grounds. Separation and Purification Technology 2011, 83, 173–179.
- Chaillou, L.L. Nazareno, M.A. New Method to Determine Antioxidant Activity of Polyphenols. Journal of Agricultural and Food Chemistry 2006, 54(22), 8397–8402.
- Zhang, Y. Yang, L. Zu, Y. Chen, X. Wang, F. Liu, F. Oxidative Stability of Sunflower Oil Supplemented with Carnosic Acid Compared with Synthetic Antioxidants During Accelerated Storage. Food Chemistry 2010, 118(3), 656–662.
- Silva, B.M. Andrade, P.B. Valentão, P. Ferreres, F. Seabra, R.M. Ferreira, M.A. Quince (Cydonia Oblonga Miller) Fruit (Pulp, Peel, and Seed) and Jam: Antioxidant Activity. Journal of Agricultural and Food Chemistry 2004, 52(15), 4705–4712.
- Razali, N. Mat-Junit, S. Abdul-Muthalib, A.F. Subramaniam, S. Abdul-Aziz, A. Effects of Various Solvents on the Extraction of Antioxidant Phenolics from the Leaves, Seeds, Veins, and Skins of Tamarindus Indica L. Food Chemistry 2012, 131(2), 441–448.
- Martinez-Correa, H.A. Magalhaes, P.M. Queiroga, C.L. Peixoto, C.A. Oliveira, A.L. Cabral, F.A. Extracts from Pitanga (Eugenia Uniflora L.) Leaves: Influence of Extraction Process on Antioxidant Properties and Yield of Phenolic Compounds. The Journal of Supercritical Fluids 2011, 55(3), 998–1006.
- Koba, K. Matsuoka, A. Osada, K. Huang, Y.-S. Effect of Loquat (Eriobotrya Japonica) Extracts on LDL Oxidation. Food Chemistry 2007, 104(1), 308–316.
- Sun, T. Ho, C.-T. Antioxidant Activities of Buckwheat Extracts. Food Chemistry 2005, 90(4), 743–749.
- Aladedunye, F. Matthäus, B. Phenolic Extracts from Sorbus Aucuparia (L.) and Malus Baccata (L.) Berries: Antioxidant Activity and Performance in Rapeseed Oil During Frying and Storage. Food Chemistry 2014, 159, 273–281.
- Man, Y.C. Hussin, W.W. Comparison of the Frying Performance of Refined, Bleached, and Deodorized Palm Olein and Coconut Oil. Journal of Food Lipids 1998, 5(3), 197–210.
- Casal, S. Malheiro, R. Sendas, A. Oliveira, B.P. Pereira, J.A. Olive Oil Stability Under Deep-Frying Conditions. Food and Chemical Toxicology 2010, 48(10), 2972–2979.
- Ramadan, M.F. Amer, M.M.A. Sulieman, A.E.R.M. Correlation Between Physicochemical Analysis and Radical‐Scavenging Activity of Vegetable Oil Blends As Affected by Frying of French Fries. European Journal of Lipid Science and Technology 2006, 108(8), 670–678.
- Lumley, I. Polar Compounds in Heated Oils. Frying of Food. Principles, Changes, New Approaches. 1988; 166–173 pp.
- Bansal, G. Zhou, W. Barlow, P.J. Lo, H.L. Neo, F.-L. Performance of Palm Olein in Repeated Deep Frying and Controlled Heating Processes. Food Chemistry 2010, 121(2), 338–347.
- Marmesat, S. Morales, A. Velasco, J. Carmen Dobarganes, M. Influence of Fatty Acid Composition on Chemical Changes in Blends of Sunflower Oils During Thermoxidation and Frying. Food Chemistry 2012, 135(4), 2333–2339.
- Casarotti, S.N. Jorge, N. Antioxidant Activity of Rosemary Extract in Soybean Oil Under Thermoxidation. Journal of Food Processing and Preservation 2014, 38(1), 136–145.
- Urbančič, S. Kolar, M.H. Dimitrijević, D. Demšar, L. Vidrih, R. Stabilisation of Sunflower Oil and Reduction of Acrylamide Formation of Potato with Rosemary Extract During Deep-Fat Frying. LWT–Food Science and Technology 2014, 57(2), 671–678.
- Hara, S. Ogawa, E. Totani, Y. Evaluation of Heat-Deteriorated Oils. I. TLC-FID Method for Determining Polar Compounds Content. Journal of Oleo Science 2006, 55(4), 167–172.
- Farhoosh, R. Kenari, R.E. Anti-Rancidity Effects of Sesame and Rice Bran Oils on Canola Oil During Deep Frying. Journal of the American Oil Chemists’ Society 2009, 86(6), 539–544.
- Bou, R. Navas, J. Tres, A. Codony, R. Guardiola, F. Quality Assessment of Frying Fats and Fried Snacks During Continuous Deep-Fat Frying at Different Large-Scale Producers. Food Control 2012, 27(1), 254–267.
- Kim, H. Choe, E. Effects of Egg Yolk Powder Addition to the Flour Dough on the Lipid Oxidation Development During Frying. LWT–Food Science and Technology 2008, 41(5), 845–853.
- Aladedunye, F. Przybylski, R. Frying Stability of High Oleic Sunflower Oils As Affected By Composition of Tocopherol Isomers and Linoleic Acid Content. Food Chemistry 2013, 141(3), 2373–2378.
- Karoui, I.J. Dhifi, W. Ben Jemia, M. Marzouk, B. Thermal Stability of Corn Oil Flavoured with Thymus Capitatus under Heating and Deep‐Frying Conditions. Journal of the Science of Food and Agriculture 2011, 91(5), 927–933.