Abstract
The present study was carried out to fabricate the food grade vitamin E acetate nanoemulsion using edible mustard oil and to evaluate its improved bioactivities. A food-grade vitamin E acetate nanoemulsion was fabricated using the edible mustard oil and surfactant Tween-80. Flocculation was not observed for 15 days. The nanoemulsion was characterized for droplet morphology and size distribution using atomic force microscope and zetasizer, respectively. We observe a stable nanoemulsion of spherical morphology and a size distribution of 86.45 ± 3.61 nm. Further, the high-performance liquid chromatography method was used to determine the vitamin E acetate concentration and encapsulation efficiency for the stable nanoemulsion. These nanoemulsions showed improved bioactivity, antioxidant, and antimicrobial activity and could be potentially used to increase the shelf life of fruit juice.
INTRODUCTION
Recently, the interest to use nanoemulsions (NEs) in the food, beverage, and pharmaceutical industries are growing due to their potential advantages over conventional emulsions. These NEs contain droplets size varied from 20–200 nm.[Citation1,Citation2] Additionally, NEs can act as a carrier system for delivering various functional lipophilic compounds, i.e., nutraceuticals, drugs, antioxidants, flavors, and anti-microbial agents, thus increases their bioavailability.[Citation3,Citation4] The food and beverage industries are interested in preparing such colloidal delivery systems where functional components can be delivered through an easy and efficient method. NEs are designed to possess high stability toward particle aggregation and gravitational separation, showing improved and efficient activity, mainly antimicrobial activity. Therefore, it can be used in the food industry to extend the shelf life of commercially available food products. Based on the need to design products with novel textural attributes, NEs can also be modified by using different types of emulsifiers and oil phase or by opting different fabricating protocols.[Citation5] Several bioactive compounds were nano-encapsulated to get higher bioactivity but vitamin E-based food-grade NE is least explored. α-tocopherol is the main component of vitamin E, which possesses antioxidant property and is fat-soluble. These properties are responsible for protecting cell membranes against peroxidation. The antioxidant property of vitamin E can be exploited to prevent chronic diseases such as cardiovascular diseases, arthrosclerosis, and cancer.[Citation6] It scavenges the free radicals and molecular oxygen, thus protecting the polyunsaturated fatty acids (PUFAs) and lipoproteins from peroxidation. Being lipophilic, it accumulates in lipoproteins, cell membranes, and fatty deposits.[Citation7] Vitamin-E (α-tocopherol) is prone to oxidation because of its polyunsaturated structure (Fig. 1) and thus it is also used in its esterified form: Vitamin-E acetate (α-tocopherol acetate).[Citation5]
NEs are thermodynamically stable system of two phases consisting of at least two immiscible liquids with droplet size in nano range. One phase is dispersed throughout the other in the form of small droplets with the help of an emulsifying agent. The dispersed liquid is known as the internal or discontinuous phase, whereas the dispersion medium is known as the external or continuous phase.[Citation4,Citation8] High-energy and low-energy approaches are the two major ways for the formulation of NE. Industries are preferably using high-energy equipment that is capable of generating intense mechanical forces which can disrupt and fuse the oil and water phases. Usually, homogenizers or ultrasonicators are used to impart external forces that help in formulation of NE.[Citation9] Main factors that influence the size of NE include energy intensity and duration, the surfactant type and concentration, and the physicochemical properties of the oil and water phases.[Citation8,Citation10] Moreover, low-energy approaches make use of spontaneous formation of emulsions either by altering the concentration of oil, water, or surfactant or physical parameters, such as temperature or pH. However, low-energy approaches are not fully exploited at industrial use, even though it is regarded as better than the high-energy approaches at laboratory scale.[Citation11]
NE based systems are valuable delivery vehicles as long as they are stable and have acceptable release property at target site. Thus, it is a necessary step to analyze the encapsulation efficiency (EE) and release property while emulsifying active compounds. In the present study, vitamin E acetate was nanoemulsified using food grade mustard oil and characterized by dynamic light scattering (DLS) and atomic force microscopy (AFM). EE and release property of vitamin E NE was further analyzed by high-performance liquid chromatography (HPLC) method to calculate free and bound vitamin E. Further, the antioxidant and antimicrobial properties were studied and the NE was used to increase the shelf life of fruit juice.
MATERIALS AND METHODS
NE was prepared using mustard oil, Tween-80 (surfactant), and vitamin-E acetate. Mangoes were purchased from the local market of Vellore. All the chemicals used in the experiment were as per the National Institute of Standards and Technology (NIST). The following solvents; methanol, iso-propanol, and tetrahydrofuran (THF) were purchased from Sigma Aldrich (Bangalore, India). All other chemicals, drugs, and solvents were of analytical grade and procured from Merck (India) and HiMedia (India).
Preparation of NE
NE was fabricated by the wash-out method.[Citation12] In this method, water phase was added continuously to the mixture of oil phase (mustard oil), surfactant (Tween-80), and the functional compound (vitamin E acetate). Various concentrations of surfactant and oil were used as described in the and further the conditions were optimized for stability. Initially, a mixture of surfactant Tween-80 (5.0%w/w) and vitamin E acetate (2.0% w/w) were dissolved in the mustard oil phase (3.0% w/w) such that vitamin E completely gets solubilized in the oil phase with the help of the surfactant. The prepared mixture and water were preheated separately at 75 ± 2°C. The water phase was slowly added drop by drop to the oil phase containing surfactant and functional compound using magnetic stirrer. To obtain homogeneity throughout the NE, the mixture was centrifuged at 400 rpm for 15 min at 25°C.
TABLE 1 Different trials to determine optimum concentration of surfactant and mustard oil to fabricate nanoemulsion based on the flocculation and phase separation
Stability Study
Intrinsic stability of NEs was investigated by storing them at room temperature. It was then observed visually for instability-phase separation or creaming or flocculation. The stable sample was further analyzed for physicochemical characterization.
Droplet Size Distribution and Zeta Potential
Particle size distribution was determined by dynamic laser light scattering method using Malvern ZetasizerNano ZS90 (Malvern Instruments, UK). Zeta potential, the electrical charge on the oil droplets in the emulsions was determined under holder temperature of 25°C and electrical voltage 3.9 V.
Atomic Force Microscope (AFM)
To examine the detailed morphology of the NE, a thin film of the samples were prepared on a glass slide by dropping 100 µL of the sample on the slide, and was allowed to dry. The slides were then scanned with the AFM (Nanosurf Easyscan 2, Switzerland).
pH Measurement and Absorption Spectrum
The pH of formulated NE was measured as per the protocol described by Mayes et al.[Citation5] The absorption spectrum of NEs was recorded between 200 and 400 nm using ultraviolet-visible–near infrared spectrophotometer (UV-VIS-NIR) Spectrophotometer (UV-3600, Shimadzu Scientific Instruments, Kyoto, Japan). UV scan testing was performed in order to verify the maximum absorption wavelength (λmax) for vitamin E acetate, mustard oil (oil phase), Tween-80 (surfactant) using methanol, iso-propanol, and THF as solubilizing media. The objectives of this were to: (1) determine the strongest λ maximum which would then be used for the HPLC method, since different values were reported in the literature[Citation13–Citation16] (2) quantify the vitamin E acetate by drawing the HPLC calibration curve; and (3) verify possible interfering peaks (overlapping) of the oil phase constituents (which contains e.g., α-tocopherol)[Citation1,Citation17] and of the surfactants on the drug detection peak. Samples were prepared by dissolving known amounts of vitamin E acetate, mustard oil, Tween-80 in methanol, iso-propanol, and THF. The results were reported as mean ± SD (n = 3).
Calibration Curve for Determining Vitamin E Acetate
Solvent was selected as suggested and verified by Diane and Burgess.[Citation1] Linearity of the detector response was verified using increasing amounts of vitamin E acetate (with three different initial standard solutions). Calibration solutions of formulated NE of different concentrations (0.5 to 100.0 mg/mL) were prepared. Peak areas and retention times were measured and a calibration curve was plotted. Linear regression analysis was carried out. The selectivity of the reverse phase–high-performance liquid chromatography (RP-HPLC) method for the determination of vitamin E acetate NEs was investigated at the retention times of the analyte.[Citation13] The data are presented as mean values ± SD (n = 3). The significance difference was evaluated using Student’s t-test and one way analysis of variance (ANOVA; single factor) with the level of significance at p-value ≤ 0.05.
Vitamin E Acetate Analysis by HPLC
Vitamin E acetate was analyzed using the RP-HPLC method. The optimal HPLC conditions determined were as follows: mobile phase iso-propanol: methanol: THF (47.6:47.6:4.8) with 1% of phosphoric acid solution (10% v/v); flow rate = 0.7 mL/min; λ = 294.1 nm, injection volume = 20 µL; running time: 10 min. A Ascentis® C18 column (25 cm × 4.6 mm) was used. Vitamin E acetate was detected using a UV/Vis detector. The mobile phase was filtered using a 0.45 mm filter (Type HVLP, Millipore) and sonicated for 30 min.[Citation1]
Determination of EE
The EE of vitamin E acetate in the NE was determined by ultrafiltration, the method established by Diane and Burgess.[Citation1] NE was subjected to centrifugal forces by submitting samples to 5000 × g for 30 min at 4°C (Remi Laboratory Instruments, India). Four milliliters was withdrawn from the pellet and diluted using iso-propanol. Subsequently, the samples were filtered using a microfilter of size (0.45 µm) and injected in the HPLC (as previously described) for the estimation of vitamin E acetate concentration.
Antioxidant Activity
Antioxidant activity test of the vitamin E acetate NE was undertaken to check whether it can act as an antioxidant if incorporated in food. To accomplish this, DPPH (α, α-diphenyl β-picrylhydrazyl) radical scavenging assay was performed. In this assay, 10 mL of DPPH (0.001 M) was prepared using methanol. Two milliliters of NE is mixed with 4 mL of DPPH solution. The control contained 2 mL of methanol instead of NE. Both the test and the control were incubated in dark place for 30 min to allow the reaction to occur. After this, absorbance was checked at 517 nm.[Citation18,Citation19] Free radical scavenging activity or antioxidant activity was calculated using formula 1:
where AD: absorbance of sample, AE: absorbance of control.
Shelf Life of Fruit Juice
The influence of the NE on the preservation of fruit juice against microbiological spoilage was evaluated. Fresh mango juice (control) was prepared in the laboratory, and its microbial load was observed by plating 1 µL of juice onto nutrient agar plates to mark the population of microbes in the small amount of the juice.[Citation20] The same was done for fortified nanoemusified mango juice (samples in 1:9, 2:8, 3:7, 4:6, 5:5 NE and control ratio). After every 6 h, streaking was done until microbial growth was observed. Microbial growth was observed after incubation for 24 h at 37°C. Control and samples were kept in programmable environmental test chamber (Remi Laboratory Instruments, India) at 37°C with 70% humidity.
RESULTS AND DISCUSSION
Preparation of NE and Its Stability
Vitamin E is a lipophilic molecule, consists of α, β, γ, and δ derivatives of tocopherol and tocotrienol. However, its application in the food and beverage industry as an active ingredient is limited due to its poor bioavailability. Vitamin E is highly sensitive to heat and oxygen and sparingly soluble in water. NE-based systems can be used as a relatively safe method to overcome this problem. Emulsion-based systems can be fabricated using relatively simple processing operations and commercially viable ingredients.[Citation21] The wash out method is an appreciable process for the preparation of NE. Vitamin-E acetate is only moderately soluble in water as it is a fat soluble vitamin. It has a high affinity for the internal oil phase due to its high partition coefficient.[Citation11] Therefore, to formulate o/w NEs, vitamin E is first completely dissolved in oil, surfactant Tween-80 aids in this process. Then addition of water is accompanied by magnetic stirring to obtain a homogeneous solution of NE. This method provides uniformity and also allows the NE to be stable for 15 days at room temperature (27°C). Vitamin E acetate o/w NEs were formulated and found to be stable when centrifugation was carried out at 400 rpm. While for higher speed separation of phases were observed. No instability phenomena, such as creaming, phase separation, or flocculation, were observed visually for a period of 15 days. This preparation was analyzed for further studies.
Droplet Size Distribution and Zeta Potential
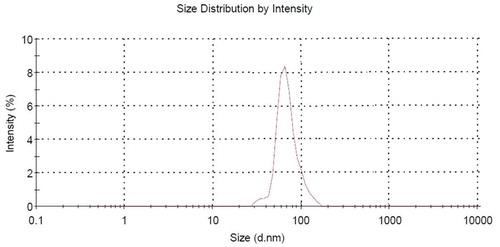
The droplet diameter greatly affects the in vitro release capacity from the dispersed to continuous phases.[Citation12] The average diameter for formulated NE (z-average) was found to be 86.45 ± 3.61 nm with the polydispersivity index (PDI) as 0.391 ± 0.43. The size distribution graph has been shown in Fig. 2. We observed that vitamin E acetate incorporated mustard oil NE maintained a mean droplet size of 86.45 ± 3.61 nm before phase separation.
Shape Analysis by AFM
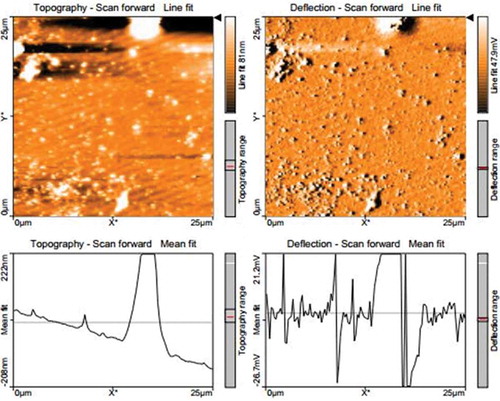
The NE formed was spherical in morphology as confirmed by AFM (Fig. 3). This shape is preferable for the use in food applications.
pH Measurement for Formulated NE
The pH of the formulated NE was 5.45 which suggest that it was slightly acidic in nature. The NE can be successfully employed in fruit juice and the beverage industry for the fortification of liquid foods having pH in the range 5–6. Further, the formulation properties of vitamin E acetate NE were determined using HPLC.
Determination of Absorption Spectrum
The λ maximum values were 286.0 ± 0.1, 294.1 ± 0.2, and 285.0 ± 0.1 nm for vitamin E acetate in methanol, iso-propanol, and THF solutions, respectively. Mustard oil and surfactants do not have absorption peaks (λ) in the spectrum range evaluated, demonstrating that mustard oil constituents (e.g., α-tocopherol) in their usual concentrations do not have detectable absorption peaks that could overlap and interfere with vitamin E acetate detection. Accordingly, 294.1 nm was chosen for further HPLC analysis of vitamin E acetate.
Calibration Curve for Determining Vitamin E Acetate
The calibration curve was drawn from the HPLC graphs got for the standards (data not shown). The variance among the standard solutions was not significantly different and linearity was indicated by R2-values close to unity. The selectivity of the method was indicated by vitamin E acetate retention times, 3.40 ± 0.02 min in iso-propanol solution.
Vitamin E Acetate Analysis by HPLC
The total concentration of vitamin E acetate in o/w NE was identified using calibration curve which was found to be 17.57 ± 1.54 mg/mL. Thus, it can be inferred that the formulated NE can be used for fortification in beverage industries as a health supplement.
Determination of EE
For the industrial application of NE as a drug or active component; it is necessary to check for the free and bound drug. For this property we have analyzed EE. Thus the matrix can be formulated accordingly to achieve desired release properties. Similarly, the free vitamin E acetate was determined by HPLC calibration curve and 0.06 ± 0.03 mg/mL of free vitamin E acetate was detected in o/w NE. Thus, the EE was calculated as 99.65%.
Antioxidant Activity
The antioxidant activity was 62.55% as obtained by the DPPH radical scavenging assay. These NEs can act as preservatives for the liquid foods because of their higher antioxidant property as compared with mustard oil and vitamin E acetate in their native form. Antioxidant activity is attributed to the presence of vitamin-E acetate in the NE and thus, can scavenge reactive oxygen species formed in the body during oxidation of the fats which can cause damage to the cells.
Shelf Life of Fruit Juice after Adding Formulated NE
Another important aspect of these NEs which make them fit for fortification of liquid foods is their ability to increase the shelf life of the fruit juices. Microbial growth was found at 6 h in control, as well as fortified sample with ratio 1:9. Similarly, 12 and 18 h were observed for fortified sample with 2:8 and 3:7 ratios, respectively. Interestingly, shelf life of fortified sample (4:6 ratios) was found as 30 h and the same for 5:5 fortifications. Therefore, it suggests that NEs possess anti-bacterial properties. The reduction in microbial growth can be attributed because of the use of mustard oil. The essential oil extracted from mustard comes under the generally recognized as safe (GRAS) category for food application based on 21 code of federal regulations (CFR) part 182.20. The main constituent of mustard oil is allylisothiocyanate. It is a non-phenolic volatile compound which significantly inhibits growth of variety of pathogenic microorganisms even when used at low concentrations.[Citation22–Citation24] Notably, the authors have detected more antioxidant and antimicrobial activities for vitamin E encapsulated NE than the mustard oil/water NE without vitamin E.
CONCLUSION
The present study identified a simple and convenient method to formulate spherical vitamin E NE using food-grade oil. The NE was stable at room temperature for 15 days. The average size diameter was found to be 86.45 ± 3.61 nm with the PDI as 0.391 ± 0.43. Maximum absorption spectrum (λ max) was found at 294.1 nm by using UV/Vis spectrophotometer which was considered for further HPLC analysis and calibration curve was drawn for standard. From calibration curve a concentration of 17.57 ± 1.54 mg/mL vitamin E acetate was determined. Accordingly, this analytical method might also be applied for the detection and quantification of vitamin E, its esters, and potentially other lipophilic vitamins in lipid formulations, and for quality control purposes. To use this NE at industrial level, it is necessary to analyze free and bound bioactive compounds; with this focus EE has been analyzed by using HPLC and was found as 99.65% (~100%). DPPH radical scavenging assay shows the higher antioxidant activity of 62.55% for mustard oil–vitamin E acetate based NE; also, it has increased anti-bacterial activity and thus, can be implemented for increasing shelf life of fruit juices. The formulated NE can be used in the beverage industry as a health supplement. Additionally, this method gives the formulation, stability analysis, concentration determination by HPLC, EE—which can also be studied for other lipophilic NEs. Further the results not only reveal how to formulate, characterize, and analyze the free/bound bioactive compounds but also provide information on the applications of NEs which can be carried out for other NEs. Additionally, these properties have added new information to the current food-grade mustard NE stability data, and will assist on the development of vitamin E (NEs) and in vitro release testing methods for these formulations along with the applications.
ACKNOWLEDGMENTS
Nandita Dasgupta, Shivendu Ranjan, and Shraddha Mundra would like to acknowledge VIT University in Vellore, India for providing the opportunity to carry out the research work. The authors are acknowledging VIT-TBI, VIT University, Vellore for providing access to the HPLC facility.
FUNDING
The authors would also like to thank the Department of Biotechnology (DBT, India) for the project grant (Grant Number: BT/PR10414/PFN/20/961/2014). The authors wish to acknowledge VIT University in Vellore, India for providing RGEMS-VC Fund for carrying out the research work for the nano-food research group. The authors would also like to thank the Veer Kunwar Singh Memorial Trust, Chapra, Bihar, India for partial support: VKSMT/SN/NFNA/2013/013.
Additional information
Funding
REFERENCES
- Diane, M.J.M.; Burgess, J. Vitamin E Nanoemulsions Characterization and Analysis. International Journal of Pharmaceutics 2014, 465(1–2), 455–463.
- Daniel, H.S.; Miguel, A.C.; António, A.V. Nanoemulsions for Food Applications: Development and Characterization. Food Bioprocess Technology 2011, 5, 854–867.
- McClements, D.J.; Rao, J. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity. Critical Reviews in Food Science and Nutrition 2011, 51, 285–330.
- Ranjan, S.; Dasgupta, N.; Chakraborty, A.R.; Samuel, S.M.; Ramalingam, C.; Shanker, R.; et al. Nanoscience and Nanotechnologies in Food Industries: Opportunities and Research Trends. Journal of Nanoparticle Research 2014, 16, 2464.
- Mayer, S.; Weiss, J.; McClements, D.J. Vitamin E-Enriched Nanoemulsions Formed by Emulsion Phase Inversion: Factors Influencing Droplet Size and Stability. Journal of Colloid and Interface Science 2013, 402, 122–130.
- Brigelius-Flohé, R.; Traber, M.G. Vitamin E: Function and Metabolism. Faseb Journal 1999, 13(10), 1145–1155.
- Debier, C.; Larondelle, Y. Vitamins A and E: Metabolism, Roles, and Transfer to Offspring. British Journal of Nutrition 2005, 93(2), 153–174.
- Dasgupta, N.; Ranjan, S.; Deepa, M.; Ramalingam, C.; Rishi, S.; Ashutosh, K. Nanotechnology in Agro-Food: From Field to Plate. Food Research International 2015, 69, 381–400.
- Abbas, S.; Hayat, K.; Karangwa, E.; Bashari, M.; Zhang, X. An Overview of Ultrasound-Assisted Food-Grade Nanoemulsions. Food Engineering Reviews 2013, 5, 139–157.
- Komaiko, J.; McClements, D.J. Optimization of Isothermal Low-Energy Nanoemulsion Formation: Hydrocarbon Oil, Non-Ionic Surfactant, and Water Systems. Journal of Colloid and Interface Science 2014, 425, 59–66.
- Saberi, A.H.; Fang, Y.; McClements, D.J. Fabrication of Vitamin E-Enriched Nanoemulsions: Factors Affecting Particle Size Using Spontaneous Emulsification. Journal of Colloid and Interface Science 2013, 391, 95–102.
- Morais, J.M.; Burgess, D.J. In Vitro Release Testing Methods for Vitamin E Nanoemulsions. International Journal of Pharmaceutics 2014, 475(1–2), 393–400.
- Alencastre, J.B.; Bentley, M.V.L.B.; Garcia, F.S.; Moragas, M.; Viladot, J.L.; Marchetti, J.M. A Study of the Characteristics and in Vitro Permeation Properties of CMC/Chitosan Microparticles As a Skin Delivery System for Vitamin E. Brazilian Journal of Pharmaceutical Sciences 2006, 42, 69–76.
- Dubbs, M.D.; Gupta, R.B. Solubility of Vitamin E (A-Tocopherol) and Vitamin K3(Menadione) in Ethanol-Water Mixture. Journal of Chemical and Engineering Data 1998, 43, 590–591.
- Guaratini, T.; Gianeti, M.D.; Campos, P.M.B.G.M. Stability of Cosmetic Formulations Containing Esters of Vitamins E and A: Chemical and Physical Aspects. International Journal of Pharmaceutics 2006, 327, 12–16.
- Scalia, S.; Renda, A.; Ruberto, G.; Bonina, T.F.; Menegatti, E. Assay of Vitamin Apalmitate and Vitamin E Acetate in Cosmetic Creams and Lotions by Supercritical Fluid Extraction and HPLC. Journal of Pharmaceutical and Biomedical Analysis 1995, 13, 273–277.
- Ying, C.F.; DeMan, J.M. Sulfur and Chlorophyll Content of Ontario Canola Oil. Canadian Institute of Food Science and Technology Journal 1989, 22, 222–226.
- Gülçin, I.; Huyut, Z.; Elmastas, M.; Hassan, Y.A.E.Radical Scavenging and Antioxidant Activity of Tannic Acid. Arabian Journal of Chemistry 2010, 3(1), 43–53.
- Gülçin, I.; Zübeyr, H.; Mahfuz, E.; Hassan, Y. A.-E. Radical Scavenging and Antioxidant Activity of Tannic Acid. Arabian Journal of Chemistry 2010, 3, 43–53.
- Ranjan, S.; Dasgupta, N.; Saha, P.; Rakshit, M.; Ramalingam, C. Comparative Study of Antibacterial Activity of Garlic and Cinnamon at Different Temperature and Its Application on Preservation of Fish. Advances in Applied Science Research 2012, 3(1), 495–501.
- Guttoff, M.; Saberi, A.H.; McClements, D.J. Formation of Vitamin D Nanoemulsion-Based Delivery Systems by Spontaneous Emulsification: Factors Affecting Particle Size and Stability. Food Chemistry 2014, 171, 117–122.
- Turgis, M.; Han, J.; Caillet, S.; Lacroix, M. Antimicrobial Activity of Mustard Essential Oil Against Escherichia Coli O157:H7 and Salmonella Typhi. Food Control 2009, 20(12), 1073–1079.
- Jayeeta, B.; Runu, C.; Utpal, R. Quality Enhancement of Mustard Oil by Tocotrienol Rich Fraction from Rice Bran Oil. International Journal of Food Properties 2014, 17(10), 2312–2321.
- Lee, S.T.; Radu, S.; Ariffin, A.; Ghazali, H.M. Physico-Chemical Characterization of Oils Extracted from Noni, Spinach, Lady’s Finger, Bitter Gourd, Mustard Seeds, and Copra. International Journal of Food Properties 2014, 18(11), 2508–2527.