Abstract
Daikon (Japanese white radish, Raphanus sativus L.) is widely consumed across Japan. The characteristic pungent flavor of daikon is derived from 4-methylthio-3-butenyl isothiocyanate. Herein, we propose an easy method for the approximate quantitation of this compound. This method does not require an expensive analytical apparatus such as a high-performance liquid chromatograph but only an ultra violet-visible spectrophotometer to measure the absorbance of a daikon n-hexane extract, at 231 nm, against a standard. The estimation of the concentration of 4-methylthio-3-butenyl isothiocyanate by this method can sort the pungency of daikon, because this isothiocyanate is a principal isothiocyanate of daikon.
INTRODUCTION
Radish (Raphanus sativus L.) is widely consumed in various forms (raw, cooked, brined, fermented, and dried) in Japan, China, India, Korea, Europe, and the Americas.[Citation1] In China, red radish cultivars are grown all over the country.[Citation2] In Korea, radish root is a main ingredient for kimchi (Korean fermented vegetables).[Citation3] Radish sprouts contain beneficial glucosinolates,[Citation4] which breakdown products are reported to have chemopreventive activity against cancer.[Citation5] Epidemiological investigations have revealed that a high intake of plant-originated foods strongly correlates with a reduced risk of cancer.[Citation6]
Among the kinds of radish available, daikon (Japanese white radish, Raphanus sativus L.) is the most popular in Japan. Daikon oroshi (grated raw daikon) is often used as a condiment with grilled fish, tempura (a Japanese dish of fried fish and vegetables), udon (thick white noodles), soba (buckwheat noodles), etc. 4-Methylthio-3-butenyl isothiocyanate is the main isothiocyanate generated from 4-methylthio-3-butenyl glucosinolate by the action of myrosinase when raw daikon is grated.[Citation7] This isothiocyanate imparts the characteristic pungent odor and flavor to daikon oroshi and is reported to be present in very high proportions (78% of total isothiocyanates) in an n-hexane extract of Aokubi, a major daikon strain in Japan.[Citation8] Moreover, the yellow pigment in takuan (Japanese salted-fermented daikon pickle) is produced from the major degradation product of this isothiocyanate.[Citation9,Citation10] Because of the previously mentioned reasons, 4-methylthio-3-butenyl isothiocyanate is considered as a significant odor, taste, and color component of daikon.
Breeding of daikon varieties includes the evaluation of the pungent taste of daikon, i.e., selection between pungent and non-pungent daikon varieties. These investigations do not require accurate quantitation for each type of isothiocyanate that imparts a pungent flavor to the daikon. A sensory evaluation is thought to be an easy method for a crude evaluation of the pungent flavor of daikon. However, the strongly pungent flavor of daikon restricts the assessors to a limited number of samples, and consequently, fewer evaluations.
Since 4-methylthio-3-butenyl isothiocyanate is a principal component contributing to the characteristic pungent flavor of daikon,[Citation8] a method for easy quantification of this isothiocyanate is thought to be a substitute for the aforesaid sensory evaluation. In this study, we present method that can approximately quantify 4-methylthio-3-butenyl isothiocyanate of daikon, without the need for the tedious and unpleasant sensory evaluation process. The proposed method does not require expensive analytical techniques, such as gas chromatography (GC) or high-performance liquid chromatography (HPLC), but requires only a spectrophotometer; further, it is simple and easy to perform. An ultra violet-visible (UV-VIS) spectrophotometer is expected to be available in many laboratories, including daikon breeding facilities; hence, this method can be used in simple facilities as well. Although this analytical method is not precise, it allows for easier and faster quantitative analysis than do conventional methods, such as GC and HPLC.
MATERIALS AND METHODS
Materials
Daikon cultivars “Akimine”, “Kenkaaokubi”, “Tenankoushin”, and “Tenpou” (Sakata Seed, Kanagawa, Japan), “Fukuhomare” and “Fukutenka” (Mikado Kyowa Seed, Tokyo, Japan), “Gensuke” (Ishikawa Sand Dune Agricultural Experimental Station, Ishikawa, Japan), “Haruhime” (Musashino seed, Tokyo, Japan), “Harumeki”, “Kanpaku”, “Natsuminowasesangou”, “Oshin”, and “Taibyousoubutori” (Takii, Kyoto, Japan), and “Takamiya” (Watanabe Seed, Miyagi, Japan) were cultivated at the experimental field of the Ishikawa Sand Dune Agricultural Experimental Station and were harvested for this research in August 2007. 4-Methylthio-3-butenyl isothiocyanate standard was prepared from fresh daikon roots purchased from a local supermarket.[Citation11] HPLC-grade organic solvents were used for preparing the HPLC eluent.
HPLC-UV and UV Analyses of n-Hexane Extract from Daikon
The extraction of 4-methylthio-3-butenyl isothiocyanate with n-hexane from the harvested daikon cultivars was carried out as follows. A non-peeled daikon root was symmetrically cut into eight pieces along the longitudinal axis. One of the pieces was pulped using a ForceMill (Osaka Chemical, Osaka, Japan) to promote the release of 4-methylthio-3-butenyl isothiocyanate from 4-methylthio-3-butenyl glucosinolate. The pulp of the sample was incubated for 10 min at 25°C and centrifuged at 12,000 × g for 5 min at 4°C. The daikon juice was obtained by filtration from the supernatant. The juice (4 mL) and n-hexane (4 mL) were placed in a stoppered conical tube and then shaken vigorously for 30 s on a vortex mixer. The tube was centrifuged at 1400 × g for 5 min at 25°C, and the n-hexane fraction was collected for use in HPLC-UV and UV analyses.
HPLC-UV analysis of 4-methylthio-3-butenyl isothiocyanate was performed according to our previously reported method.[Citation11] The silica column (Develosil 30-3, 250 mm × 4.6 mm; Nomura Chemical, Aichi, Japan) was isocratically eluted with an n-hexane/2-propanol (99.5/0.5) mixture. The wavelength for detecting 4-methylthio-3-butenyl isothiocyanate was 232 nm.
The n-hexane extract from the daikon cultivar was diluted five times with n-hexane, and its UV spectrum was measured using a U-2810 spectrophotometer (Hitachi, Tokyo, Japan). The concentration of 4-methylthio-3-butenyl isothiocyanate was estimated by comparing the absorbance at 231 nm with that of the n-hexane solution of 4-methylthio-3-butenyl isothiocyanate.
RESULTS AND DISCUSSION
HPLC Analysis of Daikon Cultivars
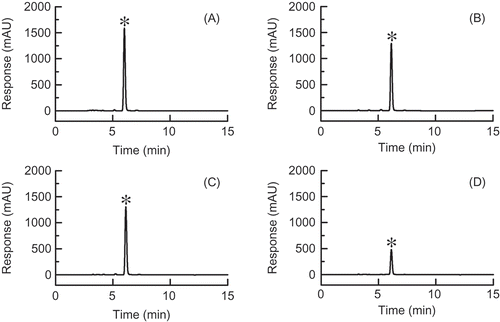
The HPLC chromatograms of the n-hexane extracts of “Kanpaku” (), “Kenkaaokubi” (), “Taibyousoubutori” (), and “Tenankoushin” () are presented as representative examples of HPLC-UV analyses for the other daikon cultivars used in this study. In the example chromatograms, 4-methylthio-3-butenyl isothiocyanate clearly exhibited the largest peak. The peak corresponding to 4-methylthio-3-butenyl isothiocyanate was largest in the chromatograms (data not shown here) of other cultivars as well.
UV Analysis of Daikon Cultivars
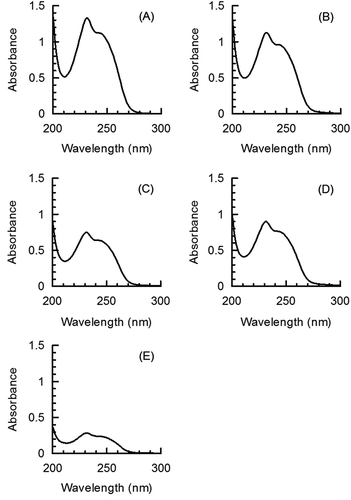
shows the UV spectra of 4-methylthio-3-butenyl isothiocyanate (130 μM) in n-hexane (A) and the extracts in n-hexane of “Kanpaku” (B), “Kenkaaokubi” (C), “Taibyousoubutori” (D), and “Tenankoushin” (E) as representative examples of the UV analysis of daikon cultivars. The maximum absorption wavelength of 4-methylthio-3-butenyl isothiocyanate in n-hexane is 231 nm (). The isothiocyanate concentrations in the extracts were estimated by comparison of the absorbance of n-hexane extracts from daikon cultivars at 231 nm with that of the 4-methylthio-3-butenyl isothiocyanate standard.
Correlation between UV and HPLC Analyses of Daikon Cultivars
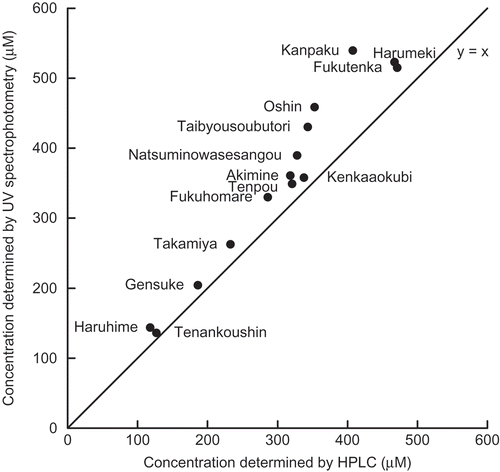
The relationship between the concentration of 4-methylthio-3-butenyl isothiocyanate (Ca) estimated by spectrophotometry at 231 nm and the concentration (Ch) determined by HPLC analysis is shown in . The deviations from the value determined by HPLC (Ca/Ch-100) were 6% (“Kenkaaokubi”) to 32% (“Kanpaku”). The deviations of the daikon cultivars other than “Haruhime”, “Kanpaku”, “Oshin”, and “Taibyousoubutori” were less than 20%. Thus, approximate quantitation of the 4-methylthio-3-butenyl isothiocyanate concentration in a root extract is possible by comparing the UV absorbance at 231 nm with that of a standard, assuming that the absorbance of the daikon n-hexane extract at 231 nm is principally accounted for by that of this isothiocyanate. This is supported by the fact that the peak of 4-methylthio-3-butenyl isothiocyanate is largest in the chromatograms of the daikon cultivars, regardless of the differences in the detection wavelength (232 nm) and the solvent (n-hexane/2-propanol, 99.5/0.5) used for HPLC. Because 4-methylthio-3-butenyl isothiocyanate is the principal isothiocyanate responsible for the characteristic pungent flavor of daikon,[Citation8] estimation of its concentration by absorbance measurements of a daikon n-hexane extract at 231 nm might be a suitable alternative to the sensory evaluation of the pungent taste.
FIGURE 3 Relationship between the 4-methylthio-3-butenyl isothiocyanate concentrations determined by UV spectrophotometry and HPLC analysis.
4-Methylthio-3-butenyl isothiocyanate is very unstable in fresh daikon juice, and approximately 25% of this isothiocyanate disappears within 60 min after grating.[Citation12] Sensory evaluation cannot be applied for estimation of the pungency of multiple daikon samples, because it requires a long time resulting in the disappearance of 4-methylthio-3-butenyl isothiocyanate. This isothiocyanate is, however, stable once it is extracted into n-hexane.[Citation13] For this reason, our newly developed spectrophotometric method is suitable for the evaluation of numerous daikon samples without the stability concerns that may impact a sensory evaluation. As opposed to the use of only sensory evaluation to choose between pungent and non-pungent daikon varieties, the combination of sensory evaluation with our proposed analytical method can make the evaluation easier for the assessors.
CONCLUSION
The developed assay, which involves absorbance measurement of an n-hexane extract of daikon at 231 nm, can be used in laboratories evaluating the pungency of daikon, without any GC or HPLC apparatus. Our method is believed to be a substitute for sensory evaluation when accurate determination of 4-methylthio-3-butenyl isothiocyanate is not required, i.e., when only a choice between pungent and non-pungent daikon varieties is to be made.
ACKNOWLEDGMENTS
We would like to thank Ms. Takako Hayashi and Ms. Yukari Kokawa for their excellent technical assistance.
FUNDING
This work was supported in part by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan as part of its Research Project “Breeding and Integrated Research toward Enhancing Consumption of Domestic Farm Products in the Food Service Industry”.
Additional information
Funding
REFERENCES
- Carlson, D.G.; Daxenbichler, M.E.; VanEtten, C.H.; Hill, C.B.; Williams, P.H. Glucosinolates in Radish Cultivars. Journal of the American Society for Horticultural Science 1985, 110, 634–638.
- Jing, P.; Zhao, S.J.; Ruan, S.Y.; Xie, Z.H.; Dong, Y.; Yu, L.L. Anthocyanin and Glucosinolate Occurrences in the Roots of Chinese Red Radish (Raphanus sativus L.), and Their Stability to Heat and pH. Food Chemistry 2012, 133, 1569–1576.
- Lim, S.; Lee, E.J.; Kim, J. Decreased Sulforaphene Concentration and Reduced Myrosinase Activity of Radish (Raphanus sativus L.) Root during Cold Storage. Postharvest Biology and Technology 2015, 100, 219–225.
- Chen, J.; Li, L.; Wang, S.; Tao, X.; Wang, Y.; Sun, A.; He, H. Assessment of Glucosinolates in Chinese Kale by Near-Infrared Spectroscopy. International Journal of Food Properties 2014, 17, 1668–1679.
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Biotic Elicitors Effectively Increase the Glucosinolates Content in Brassicaceae Sprouts. Journal of Agricultural and Food Chemistry 2014, 62, 1881–1889.
- Şengül, M.; Yildiz, H.; Kavaz, A. The Effect of Cooking on Total Polyphenolic Content and Antioxidant Activity of Selected Vegetables. International Journal of Food Properties 2014, 17, 481–490.
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The Chemical Diversity and Distribution of Glucosinolates and Isothiocyanates among Plants. Phytochemistry 2001, 56, 5–51.
- Nakamura, Y.; Iwahashi, T; Tanaka, A.; Koutani, J.; Matsuo, T.; Okamoto, S.; Sato, K.; Ohtsuki, K. 4-(Methylthio)-3-Butenyl Isothiocyanate, a Principal Antimutagen in Daikon (Raphanus sativus; Japanese White Radish). Journal of Agricultural and Food Chemistry 2001, 49, 5755–5760.
- Ozawa, Y.; Kawakishi, S.; Uda, Y.; Maeda, Y. Isolation and Identification of a Novel β-Carboline Derivative in Salted Radish Roots, Raphanus sativus L. Agricultural and Biological Chemistry 1990, 54, 1241–1245.
- Ozawa, Y.; Uda, Y.; Matsuoka, H.; Abe, M.; Kawakishi, S.; Osawa, T. Occurrence of Stereoisomers of 1-(2’-Pyrrolidinethione-3’-Yl)-1,2,3,4-Tetrahydro-β-Carboline-3-Carboxylic Acid in Fermented Radish Roots and Their Different Mutagenic Properties. Bioscience, Biotechnology, and Biochemistry 1999, 63, 216–219.
- Ippoushi, K.; Ishida, M.; Takeuchi, A.; Azuma, K. Normal-Phase High-Performance Liquid Chromatography in the Study of 4-Methylthio-3-Butenyl Isothiocyanate from Daikon (Raphanus sativus). Bulletin of the National Institute of Vegetable and Tea Science 2013, 12, 61–66.
- Okano, K.; Asano, J.; Ishii, G. A Rapid Method for Determining the Pungent Principle in Root of Japanese Radish (Raphanus sativus L.). Journal of the Japanese Society for Horticultural Science 1990, 59, 545–550.
- Kosemura, S.; Yamamura, S.; Hasegawa, K. Chemical Studies on 4-Methylthio-3-Butenyl Isothiocyanate from Roots of Japanese Radish (Raphanus sativus L.) in Connection with Raphanusanins, Phototropism-Regulating Substances of Radish Hypocotyls. Tetrahedron Letters 1993, 34, 481–484.