Abstract
The aim of this study was to characterize and classify 10 honey samples collected from different regions of Iran based on their physicochemical properties. Moisture, ash and hydroxymethylfurfural content, pH, electrical conductivity, diastase activity, color, and fructose to glucose ratio were determined. Phenolic and flavonoid compounds, antioxidant activity, and the ability to inhibit enzymatic browning were also evaluated in the samples. Based on diastase activity and hydroxymethylfurfural content, Tamarisk honey showed the best quality. The highest ratio of fructose to glucose (1.5) belonged to Ziziphus. Coriander honey with the lowest L* (18.9) was considered darker than other samples. High correlation coefficients between phenolic, flavonoid compounds, and antioxidant activity indicated that these compounds are mainly responsible for the antioxidant capacity of honey. Based on the first principal component, honey samples were classified into four main groups. The first group included coriander and ziziphus, the second group was dill, the third group contained thyme, parsley, and qanqal, and the fourth group included astragal, alfalfa, tamarisk, and orange blossom.
INTRODUCTION
Honey cannot be considered a complete food by human nutritional standards, but has the potential to be a dietary supplement.[Citation1] It has been used as an important part of traditional medicines, and its function in the treatment of burns, asthma, infections, gastrointestinal disorders, skin ulcers, various inflammatory processes, as well as cataracts and other eye diseases have been widely reported.[Citation2,Citation3] In addition, the antioxidants naturally present in honey help its antioxidant capacity.[Citation4] These components include flavonoids, phenolic acids, enzymes like glucose oxidase and catalase, ascorbic acid, carotenoid-like substances, organic acids, Maillard reaction products, amino acids, and proteins.[Citation4,Citation5] Phenolic acids and flavonoids are two main compounds responsible for the antioxidant activity of honey that prevent auto-oxidation reactions and have a scavenging effect on free radicals by different mechanisms.[Citation6] Their amount varies greatly depending on the honey floral source, season, and environmental factors.[Citation4,Citation7] The botanical origin of honey has the most influence on its antioxidant activity, while processing, handling, and storage are effective only to a minor degree.[Citation2]
For quality control of honey, several physical and chemical features, which mostly include water content, enzyme activity of invertase, hydroxymethylfurfural (HMF), electrical conductivity, and sugar composition, have to be determined.[Citation8] There are several reports on the use of physical and chemical parameters to characterize honeys from different countries. Bertoncelj et al.[Citation9] studied sensory and physicochemical characteristics of seven main types of Slovenian honeys such as electrical conductivity, pH, free acidity, proline content, protein content, optical rotation, phenolic content, and antioxidant activity. The results showed that there was a wide variation among the honey types and lower values generally belonged to light honeys such as Acacia, Linden, and multifloral honeys. Socha et al.[Citation7] examined the antioxidant activity and phenolic acids profile of seven types of Polish honeys from different regions. The results showed that the total phenolic acids content of Polish honeys varied from 4.46 to 15.04 mg of gallic acid equivalents per 100 g of product. There was a positive linear correlation between the amount of total phenolic and antioxidant activity of honey extracts. Rodriguez et al.[Citation10] reported antioxidant properties of 14 Mexican honeys and their methanolic extracts by 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and ferric-reducing antioxidant power (FRAP) assays. Eucalyptus flower and orange blossom honeys were good sources of antioxidant compounds. The ability of 19 Spanish honeys to inhibit enzymatic browning in apple homogenate and juice was investigated. By increment in honey concentration, its anti-browning activity was increased but the effects did not follow a standard pattern.[Citation11]
Since there is no comprehensive study on the physicochemical properties of Iranian honeys, in this work, the physicochemical characteristics of 10 honey samples collected from different regions of Iran were determined. Moreover, total phenolic and flavonoid content, antioxidant activity, as well as the ability of honeys to inhibit enzymatic browning were investigated. Finally, the correlation between analyzed parameters was also evaluated and various honeys were classified based on their properties.
MATERIALS AND METHODS
Honey Samples
Monofloral honey samples and their collection regions are shown in . Honey samples were stored at 4°C in the dark until analyzed. All tests were performed in triplicate.
TABLE 1 Monofloral honey samples and their collection regions
Chemicals
Folin-Ciocalteu reagent, sodium carbonate, potassium ferrocyanide, zinc acetate, metabisulfite, sodium choloride, acetate, acetic acid glacial, starch, iodine, potassium iodide, copper (II) sulfate, hydrochloric acid, sodium hydroxide, methanol, glucose, fructose, and sucrose (as standard of sugars present in honey) were of analytical grade and purchased from Merck (Darmstadt, Germany). Gallic acid, quercetin, and DPPH were prepared from Sigma-Aldrich (St. Louis, USA).
Physicochemical Analyses
The moisture and ash content, pH, diastase activity, and HMF were determined according to Association of Official Analytical Chemists (AOAC) methods.[Citation12] The electrical conductivity was measured by a conductivity meter at 20°C (Elmetron, type CC-401) for a 20% (w/v) solution of honey (dry matter basis) in deionized water.[Citation13] Glucose and fructose contents were determined using high-performance liquid chromatography (HPLC; Shimadzu, Japan) fitting with a refractive index detector at 60°C. The HPLC column was SCR-101N (30 cm × 9.7 mm i.d.) fitted a guard column SCR (N; 5 cm × 4 mm i.d.). The mobile phase was deionized water at a flow rate of 0.7 mL/min.
The honey color was measured by a colorimeter (Texflash colorimeter, Data color, Swiss). Color parameters of L* (Lightness: L* = 100 for white and 0 for black), a* (redness/greenness axis: positive a* is red and negative a* is green) and b* (yellowness/blueness axis: positive b* is yellow and negative b* is blue) were used to express the colors of honey samples. Color intensity was determined by reading the absorbance at 450 and 720 nm using a spectrophotometer (Camspec M350 UV-Vis Double Beam, England). The difference in absorbance is expressed as color intensity.[Citation14] The total phenolic content (TPC) was determined with Folin-Ciocalteu reagent by absorbance measurement at 670 nm using gallic acid as standard.[Citation5,Citation15] Total flavonoid content (TFC) was measured by the absorbance at 415 nm using quercetin as the standard.[Citation5,Citation16,Citation17]
Determination of Antioxidant Activity
The antioxidant activity of honey samples in the presence of the stable free radical DPPH was measured spectrophotometracally. Briefly, 1.25 mL of honey solution was dissolved in distilled water (0.025 g/mL) and was mixed with 1.5 mL of a 90 μg/mL solution of DPPH in methanol. After 5 min, the absorbance was read at 517 nm against water/methanol (1:1 v/v) blank. For the control sample, 1.25 mL of methanol was mixed with 1.5 mL DPPH. The antioxidant activity of honey was expressed as a percentage of inhibition and was calculated using the following formula:[Citation5,Citation11]
The Effect of Honey on Minced Apple Browning
Fresh homogenates of Golden Delicious apple (10 g) were mixed with 0.5 g of honey (5% w/w per homogenate) and were incubated for 1 h. Then, 20 mL of a solution of aqueous methanol (1:1) was added and mixed for 10 min; and it was filtered and centrifuged at 18,500 × g for 10 min. The absorbance of the extract was read at 420 nm against an aqueous methanol blank and was expressed as browning inhibition (%) of each honey solution. In order to avoid the interference of honey color, 0.5 g of honey was mixed with 20 mL of a solution of aqueous methanol (1:1) and after performing the above steps; absorbance was read at 420 nm and was subtracted from the sample absorbance values. Two control samples were needed to determine the highest and lowest brownings: The homogenate apple without honey (highest browning) and the homogenate apple after thermal treatment in a boiling water bath for 5 min, followed by a refrigeration step, in order to inactivate polyphenol oxidase (PPO; lowest browning).[Citation11]
The Effect of Honey on Apple Juice Browning
Apple juice was prepared by a conventional blender, and was instantly filtered under vacuum. The filtered fraction was placed in an ice bath to minimize juice browning before the experiment. Then, the apple juice (10 g) was mixed with 0.5 g of honey (5% w/w). After 4 h of incubation, the effect of honey was read spectrophotometrically at 420 nm against a blank (mixture of 10 g of water and 0.5 g honey to avoid the interference of honey color) and the results were expressed as the percentage of ascorbic acid equivalent using two reference values: The absorbance after the incubation of a mixture of juice and ascorbic acid (1%), i.e., the highest juice clarification value (100% inhibition), and the absorbance of the juice without the addition of honey, i.e., the lowest juice clarification value.[Citation11]
Statistical Analysis
All of the physicochemical analyses were performed in three replications. Data were processed using Statistix 8. The means evaluation was done using the least significant difference (LSD) test at a confidence level of 95%. The dendograms based on data were constructed using SPSS version 17. Principal component analysis (PCA) was carried out using Statgraphics statistical 2007 software.
RESULTS AND DISCUSSION
Physicochemical Properties
Moisture content
Water content is a good criterion to evaluate honey quality[Citation6] and to determine honey shelf life during storage.[Citation18,Citation19] The moisture contents of honey samples are shown in . These values ranged from 14.3 to 16%. All honey samples had the moisture content below 20%, which is the maximum allowed limit for honey in the Codex standard.[Citation20] The results were indicative of good storage ability of the honey sample,[Citation21] since, during storage, a high moisture content could lead to fermentation caused by the action of osmotolerant yeast.[Citation1,Citation15] Moreover, high moisture content could also accelerate crystallization in some types of honey.[Citation21] Alfalfa honey had the highest moisture (16%) but not significantly different from thyme, orange blossom, and coriander honeys. High moisture in honey can be an indicator of premature extraction or extraction under high-humidity conditions.[Citation22] The moisture content of honey depends on several factors such as the harvest season, climatic conditions,[Citation23] level of maturity,[Citation19] floral origin, geographical location,[Citation24] and the moisture content of the original plant.[Citation18]
TABLE 2 Physicochemical properties of Iranian monofloral honeys
Ash content
The ash content is an index of honey quality indicating its botanical origin.[Citation14] The ash content in the analyzed samples ranged from 0.01 to 0.23% (). The Codex standard has proposed an ash content of not more than 0.6% for normal honey.[Citation20] The highest and lowest contents were related to the ziziphus and orange blossom honeys, respectively. The low ash content is probably due to several factors such as differences in soil and atmospheric conditions, the type, and physiology of each plant.[Citation1] It is reported that the ash content has a positive relation with pH so that high mineral content results in high buffering capacity and pH.[Citation9] This might be the reason ziziphus with high ash showed low acidity among samples.
Electrical conductivity
The electrical conductivity values of honey samples were in the range of 0.12–0.47 mS/cm (). None of the samples exceeded the maximum allowed by the Codex Alimentarius of 0.8 mS/cm. The electrical conductivity depends on the minerals, organic acids, proteins, some complex sugars, and polyols contents.[Citation18] Therefore, the highest and lowest electrical conductivity was related to the ziziphus (with high ash) and orange blossom (with low ash) honeys, respectively. Alvarez-Suarez et al.[Citation15] reported the electrical conductivity values of five samples of Cuban honey in the range of 0.1–0.6 mS/cm. This parameter is a good criterion of botanical origin of honey[Citation19] and thus is often used for differentiating honeys with various floral origins.[Citation1] Moreover, it can be considered as a tool to differentiate between nectar and honeydew honeys[Citation9] because the latter have a high electrical conductivity and ash.[Citation18]
pH
The pH values of different samples of honey are shown in . All of the honey samples were acidic in nature with the pH ranging between 4.1 and 5.5. Ziziphus honey possessed the highest pH and was significantly different from other samples. The lowest pH belonged to alfalfa, which had no significant difference with thyme, tamarisk, and astragal honeys. The pH of honey depends on the amount of minerals, especially iron.[Citation16] Therefore, the ziziphus honey, which had more ash content compared to other samples, possessed a high pH. Gulfraz et al.[Citation19] reported the pH of four samples of Pakistan honey in the range of 3.32–6.56. Honey is generally acidic in nature regardless of its variable geographical origin.[Citation6,Citation14] Geographic origins and floral can cause large variations in honey pH, because the nectar pH and soil conditions can largely affect honey physicochemical properties.[Citation16] The acidic pH in honeys depends on the amount of gluconic acid produced mainly by glucose oxidase during glucose oxidation. In addition, other non-aromatic (formic, acetic acids) and aromatic acids (hydroxybenzoic and hydroxycinnamic group of acids) could affect the final pH of honeys.[Citation25]
Fructose to glucose ratio
The glucose, fructose, and their ratio in honey samples are presented in . Glucose and fructose values of the honey samples ranged from 19.2 to 31.8% and 25.4 to 39.2%, respectively, while the ratio of fructose to glucose was 1.1–1.5. Escuredo et al.[Citation17] reported glucose, fructose, and the ratio of fructose to glucose values of 34 samples of honey from the north-west of Spain in the range of 24.4–35.2, 33.1–42.1, and 0.9–1.7%, respectively. Dill honey possessed the highest amount of fructose and glucose showing higher content of sugars (70.7%) compared to other samples. The lowest content of sugars (44.6%) belonged to Astragal honey (p < 0.05). The highest and the lowest ratio of fructose to glucose belonged to ziziphus (1.5) and coriander (1.1) honeys, respectively. Fructose to glucose ratio gives information about the crystallization state of honey. When fructose to glucose ratio is 1.1 or less, the crystallization would occur while in values more than 1.5, no such tendency[Citation1] would be observed due to the modification of the saturated level of glucose by the attendance of the larger level of fructose. In addition, the fructose/glucose ratio affects honey taste because fructose is much sweeter than glucose. This ratio can be largely dependent upon the nectar source.[Citation22]
Diastase activity
Diastase activity is the amount of enzyme that hydrolyzes 0.01 g of starch in 1 h at 40°C under test conditions.[Citation22] Diastase (α-amylase and β-amylase mixture) is a natural enzyme catalyzing the degradation of starch and viscosity loss in honey.[Citation8] Diastase activity in honey depends on the amount of nectar the bee processes at each period,[Citation17] geographic and floral origins of the product.[Citation21] Diastase activity can be used as an index of aging and temperature abuse, but with precaution, because its variability is high.[Citation21] According to , diastase activity of honey samples ranged from 5.8–21.3 gothe which was higher than that reported by Codex Alimentarius standard (at least 3 gothe). Ziziphus and coriander honeys had the highest and lowest diastase activity, respectively. Very low and/or very high diastase activity, both in honey are undesirable. Very high diastase activity may pertain to the formation of acid resulting from fermentation since the acid helps the enzyme to break down starch.[Citation8]
HMF
The HMF content of honey samples varied from 2.2–39.4 mg/kg (). Isla et al.[Citation6] reported the HMF values of 13 samples of honey from the Northwestern Argentina in the range of 4–28.2 mg/kg. HMF levels in all samples were lower than the value proposed by the Codex Alimentarius standards (lower than 40 mg/kg). Dill honey had the highest level (39.4), which was very close to the Codex limit, while the lowest HMF belonged to tamarisk. HMF is widely known as one of the freshness parameters of honey because it is present in trace amounts in fresh honeys.[Citation26] This factor would increase during processing and/or aging of the product.[Citation15] A high value of HMF in dill honey showed that probably the sample had been heat treated and/or stored for a long time before study. This can lead to compositional changes due to caramelization of carbohydrates, Maillard reaction, and the decomposition of fructose in the acidic environment of honey.[Citation27] Decomposition of fructose occurs at high temperatures while Maillard reaction is accelerated at low temperatures.[Citation8]
Color
The colors of honey samples are shown with three parameters of L* (Lightness), a* (redness), and b* (yellowness) in . The amount of parameters L*, a*, and b* were in the range of 19–45.6, 2.3–23, and 21.8–60, respectively. Parsley honey (L = 45.6) possessed the highest lightness and coriander honey with the lowest L* (18.9) was darker than other honeys. The highest redness and yellowness were related to coriander and ziziphus honeys, respectively, while tamarisk honey had the lowest redness and yellowness. Gonzalez-Miret et al.[Citation28] classified honey samples into two groups according to their lightness values: light honeys with L* > 50; and dark honeys with L* < 50. According to this classification, all samples studied here can be considered dark honeys. Positive values of a* and b* indicated that the honey samples showed a large proportion of red and yellow color. Rodriguez et al.[Citation10] reported three parameter values L*, a*, and b* of 14 samples of Mexican honey in the range of 14.4–31.6, 0.1–15.6, and 17.2–38.1, respectively, which is slightly similar to the results obtained in this research. The honey color is one of the factors determining its price as well as its acceptance in the world market.[Citation1] This parameter depends on several factors such as botanical origin, minerals, as well as phenolics and the HMF content of the honey.[Citation24] Light-colored honeys usually have low ash content, while dark-colored types contain higher ash content.[Citation15] Ziziphus honey with high ash content showed high a* and b*, whereas the lowest a* and b* values belonged to orange blossom honey with low ash.
TABLE 3 Color characteristics of honey samples
The color intensity of honey samples were in the range of 0.244–1.917 AU (). The highest and lowest color intensities were related to coriander and orange blossom honeys, respectively. Qanqal, astragal, and alfalfa honeys were not significantly different from each other. Saxena et al.[Citation14] reported the color intensity of seven samples of Indian honey in the range of 0.524–1.678 AU. Honeys from different botanical sources consist of different compositions and concentrations of pigments mainly polyphenols and carotenoids.[Citation25] The conjugated systems of double bonds, like those existing in phenolics (flavonoids and long-chain phenolics), as well as other components like terpene and isoprene units are chromophores, which can absorb visible light leading to a wide range of colors.[Citation29] Color intensity in honey might also be related to the products resulting from the Maillard reaction.[Citation30]
TPC
TPC of honey samples ranged from 19.0 to 55.7 mg of GAE/100 g of honey (). Ziziphus and orange blossom honeys had the highest and lowest TPC, respectively, and were significantly different from other samples. Dong et al.[Citation2] reported the phenolic content of 33 samples of Chinese honey in the range of 10.4 mg/100 g in acacia honey to 149.6 mg/100 g in red date honey. Di Marco et al.[Citation31] reported the phenolic content of 38 samples from Rom in the range of 8.6–34.1 mg/100 g. Phenolic acids are an important group of compounds effective in the appearance and functional properties of honey.[Citation15] The concentration and type of polyphenolic substances are variable[Citation14] in honey and strongly influenced by the floral and geographical origin and climatic characteristics of the production location.[Citation32] The determination of the TPC of honey can be a good parameter for the evaluation of its quality and therapeutic potential.[Citation18] Therefore, it could be suggested that ziziphus honey can have more nutritional value regarding to TPC.
TABLE 4 Total phenolic content, total flavonoid content, and antioxidant activity of honey samples
TFC
TFC of honey samples ranged from 1.7 to 4.5 mg of QE/100 g of honey, which was determined using quercetin as standard (). Ziziphus honey exhibited the highest TFC but had no significant difference with coriander sample. Orange blossom had the lowest TFC, which was not significantly different from alfalfa honey. Escuredo et al.[Citation17] reported TFC of 34 honey samples from north-west Spain in the range of 1.4–10.3 mg/100 g, while Di Marco et al.[Citation31] reported the TFC of 38 honey samples from Rom in the range of 2.7–22.6 mg/100 g.The flavonoids make up a great family of plant phenolic pigments[Citation32] and are effective in aroma and antioxidant properties of honey.[Citation26] The concentration and type of these compounds depend on the floral origin of honey.[Citation33]
Antioxidant Activity Using DPPH
A stable nitrogen-centered free radical called DPPH, is often used to determine antioxidant activity. The color change of DPPH occurs from purple to yellow when the unpaired electron of DPPH forms a pair with hydrogen donated by a free radical scavenging antioxidant. Thus, the free radical DPPH is reduced to the corresponding hydrazine, and its absorbance at 517 nm decreases.[Citation14] The antioxidant activity of honey samples ranged from 45.6 to 54.2% (). The results showed that all samples had antioxidant activity, and ziziphus honey possessed the highest potential. Astragal had the lowest antioxidant activity, which was not significantly different from parsley, qanqal, alfalfa, tamarisk, and orange blossom honeys. The antioxidant activity of natural honeys can be attributed to the presence of various substances such as enzymes, Maillard reaction products, organic acids, phenolic acids, flavonoids, amino acids, peptides, ascorbic acid, and carotenoid-like compounds. The difference in the antioxidant activity between various types of honey results from antioxidant content of honey, especially phenolic compounds.[Citation7] Thus, high levels of antioxidant activity of ziziphus and coriander honeys result probably from high amounts of phenolic compounds.
Inhibition of Enzymatic Browning
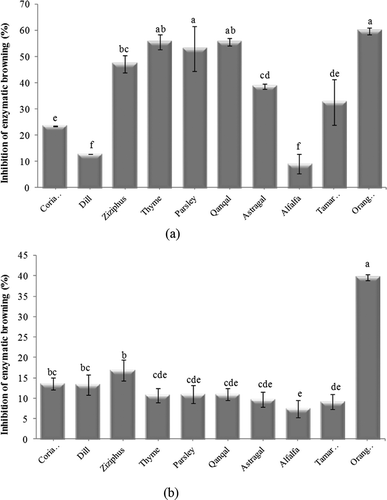
Enzymatic browning is one of the limiting factors in the shelf life of fresh-cut fruits and vegetables. Control of enzymatic browning is a high priority for food processing industries due to its harmful effects on the health in most commercially important food products. Honey is one of the potential inhibitors of enzymatic browning.[Citation34] The antibrowning activity of honeys was assayed on fresh apple homogenates after 1 h of incubation and on apple juice after 4 h with a concentration of 5% w/w. The inhibiting activity of the samples was 7.4–39.6% and 9.2–59.8% for apple homogenates and apple juice, respectively (). Orange blossom showed the highest antibrowning activity in apple homogenates and apple juice. This honey was significantly different from other samples in apple homogenates but had no significant difference with thyme, qanqal, and parsley honeys in apple juice. The lowest antibrowning activity was related to alfalfa honey in apple homogenates and apple juice. This honey was not significantly different from tamarisk, astragal, thyme, qanqal, and parsley honeys in apple homogenates and had no significant difference with coriander honey in apple juice. The difference in the results of apple homogenates and apple juice is probably due to the presence of fiber and other compounds in apple homogenates, which prevent better mixing of honey with substrate leading to lower activity of honey in apple homogenates compared to apple juice. Vela et al.[Citation11] assessed the ability of 19 samples of Spanish honey to inhibit enzymatic browning in apple homogenates and apple juice with five honey concentrations ranging from 0.5 to 4%. The results showed that the average ability of inhibition enzymatic browning of 19 samples was 33.7 and 36.3% in apple homogenates and apple juice, respectively. They also reported that antibrowning activity increases by increasing the concentration of honey, but the effects did not follow a standard pattern. Different types of honey contain substances like flavonoids, which are known for their metal-chelating activity. The chelation of copper ion present in the enzyme PPO may be one of the factors affecting the reduction in the activity of this enzyme. Inhibition of enzymatic browning reaction may also be partly due to the presence of reducing substances such as ascorbic acid and riboflavin in honey. Ascorbic acid possesses a lower redox potential than the PPO-generated quinones; thus, it is oxidized and the quinones are reduced to dihydroxyphenols before they can undergo further reaction to form melanin pigments.[Citation34]
Correlation Between Physicochemical Parameters
Correlation between the physicochemical parameters of honey samples is shown in . Electrical conductivity and ash had a positive relationship together and also with pH, phenolic compounds, flavonoid, antioxidant activity, and color (parameters a* and b*). Electrical conductivity and pH increased with an increase in minerals since the electrical conductivity depends on the mineral content of the honey.[Citation9] On the other hand, ash content can largely increase color intensity in honey. In this research, a* and b* parameters showed a positive correlation with the amount of ash (r = 0.743 and r = 0.787, respectively). Minerals being complex with phenolic compounds can show a significant enhancement in antioxidant capacity because many metals can act as electron donors, and their charge is easily stabilized by the polyphenolic structure. High correlation was found between pH and antioxidant capacity (r = 0.703) as well as pH and phenolic and flavonoid compounds (r = 0.797 and r = 0.673), which might be related to the complex formation of metallic ions with phenolic compounds.[Citation16] Generally, light-colored honeys showed more alkaline pH values while darker ones were more acidic. In this study, color intensity showed a moderate correlation with pH (r = 0.424). Moreover, strong correlations between antioxidant activity, phenolic and flavonoid compounds and color intensity were found. High correlation coefficient between phenolic compounds and antioxidant activity (r = 0.955) and also flavonoid compounds and antioxidant activity (r = 0.812) indicated that polyphenolic compounds are one of the main compounds responsible for the antioxidant behavior of honey. Dong et al.[Citation2] reported that there is a high correlation between DPPH radical scavenging activity and phenolic compounds (r = 0.89) and flavonoids (r = 0.71). They stated that variations in antioxidant activities of honeys are due to the quantitative and qualitative nature of their phenolic substances. High correlations were observed between antioxidant activity and color intensity (r = 0.822), the phenolic compounds and color intensity (r = 0.772) as well as flavonoid compounds and color intensity (r = 0.746). As mentioned above, honey color intensity is related to pigments such as flavonoids, carotenoids, etc., which are involved in the antioxidant activity of honey samples. Therefore, in general, dark honeys had a higher antioxidant activity than light types.[Citation15] Saxena et al.[Citation14] showed that color intensity had a high correlation with DPPH radical scavenging activity (r = 0.72) and phenolic compounds (r = 0.9). They reported that this correlation shows the probable involvement of pigments in antioxidant properties.
TABLE 5 Correlation between physicochemical parameters
Classification of Honey Samples Based on the Physicochemical Properties
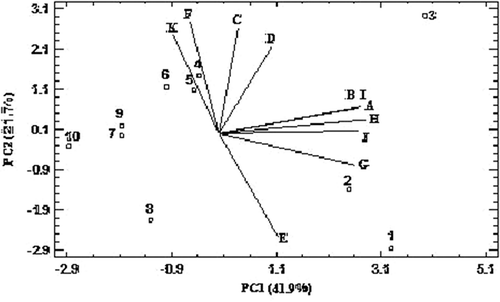
Dendrograms of honey samples according to cluster analysis on the basis of physicochemical properties are shown in . Accordingly, honey samples were enerally placed in three groups. The first group included ziziphus, thyme, parsley, and qanqal honeys; the second group contained astragal, tamarisk, and orange blossom honeys; and the third group included coriander, dill, and alfalfa honeys. PCA was carried out to determine the best parameters to classify honey samples. The results of PCA are shown in . Accordingly, the two principal components (PC1 and PC2) explained the 63.6% of total variation. The first component included ash, electrical conductivity, a*, TPC, TFC, and antioxidant activity parameters. The second component contained the ratio of fructose/glucose, diastase activity, HMF, L*, and the inhibition of enzymatic browning in apple juice parameters. It was determined that these parameters were more effective than other factors (such as moisture, pH, fructose, glucose, b*, color intensity, and inhibition of enzymatic browning in minced apple) and were used for the classification of honey samples. Based on the first principal component (PC1), honey samples were placed into four main groups. The first group included coriander and ziziphus honeys; the second group contained dill honey; the third group included thyme, parsley, and qanqal honeys; and the fourth group contained astragal, alfalfa, tamarisk, and orange blossom honeys. Based on the second principal component (PC2), honey samples were also placed into four main groups. The first group included coriander, dill, and alfalfa honeys; the second group contained ziziphus honey; the third group included thyme, parsley, and qanqal honeys; the fourth group contained astragal, tamarisk, and orange blossom honeys.
FIGURE 2 Dendrogram of honey samples according to cluster analysis on the basis of physicochemical properties using the Ward Method (1: coriander honey; 2: dill honey; 3: ziziphus honey; 4: thyme honey; 5: parsley honey; 6: qanqal honey; 7: astragal honey; 8: alfalfa honey; 9: tamarisk honey; 10: orange blossom honey).
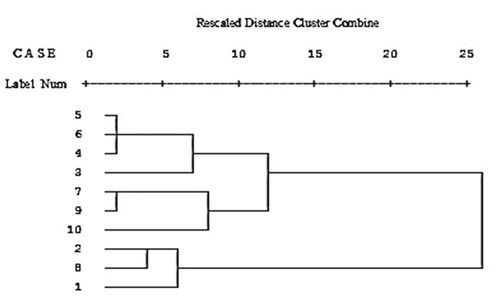
FIGURE 3 Principal component analysis of honey samples (1: coriander honey; 2: dill honey; 3: ziziphus honey; 4: thyme honey; 5: parsley honey; 6: qanqal honey; 7: astragal honey; 8: alfalfa honey; 9: tamarisk honey; 10: orange blossom honey; A: ash; B: electrical conductivity; C: ratio of fructose/glucose; D: diastase activity; E: HMF; F: L*; G: a*; H: total phenolic content; I: total flavonoid content; J: antioxidant activity; K: inhibition of enzymatic browning in apple juice).
CONCLUSION
The results showed that the physicochemical parameters analyzed in this study can provide enough information for classification and distinction between honey samples. Dill honey was the most viscous type because of the lowest moisture content and can have longer shelf life than other samples during storage. Based on diastase activity and HMF content, tamarisk honey had the best quality. The lightest and darkest honeys were coriander and parsley honey, respectively. Due to the high ratio of fructose to glucose, ziziphus honey was determined as the sweetest. In addition, this honey had the highest phenolic and flavonoid compounds and antioxidant activity. High correlation coefficients between the phenolic compounds, flavonoids and antioxidant activity indicated that these compounds are among the main components responsible for the antioxidant behavior of honeys.
REFERENCES
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Zaldivar-Cruz, J.M.; Kuri, V.; Fernández-López, J.; Carbonell-Barrachina, Á.A.; Pérez-Álvarez, J. Aroma Profile and Physico‐Chemical Properties of Artisanal Honey from Tabasco, Mexico. International Journal of Food Science and Technology 2010, 45(6), 1111–1118.
- Dong, R.; Zheng, Y.; Xu, B. Phenolic Profiles and Antioxidant Capacities of Chinese Unifloral Honeys from Different Botanical and Geographical Sources. Food and Bioprocess Technology 2013, 6(3), 762–770.
- Dong, R.; Zheng, Y.; Xu, B. Floral Origin Identification and Amino Acid Profiles of Chinese Unifloral Honeys. International Journal of Food Properties 2013, 16(8), 1860–1870.
- Alvarez-Suarez, J.M.; Tulipani, S.; Romandini, S.; Bertoli, E.; Battino, M. Contribution of Honey in Nutrition and Human Health: A Review. Mediterranean Journal of Nutrition and Metabolism 2010, 3(1), 15–23.
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the Total Phenolic, Flavonoid, and Proline Contents in Burkina Fasan Honey, As Well As Their Radical Scavenging Activity. Food Chemistry 2005, 91(3), 571–577.
- Isla, M.I.; Craig, A.; Ordoñez, R.; Zampini, C.; Sayago, J.; Bedascarrasbure, E.; Alvarez, A.; Salomón, V.; Maldonado, L. Physico Chemical and Bioactive Properties of Honeys from Northwestern Argentina. LWT–Food Science and Technology 2011, 44(9), 1922–1930.
- Socha, R.; Juszczak, L.; Pietrzyk, S.; Gałkowska, D.; Fortuna, T.; Witczak, T. Phenolic Profile and Antioxidant Properties of Polish Honeys. International Journal of Food Science and Technology 2011, 46(3), 528–534.
- Furkan Yardibi, M.; Gumus, T. Some Physico-Chemical Characteristics of Honeys Produced from Sunflower Plant (Helianthus Annuus L.). International Journal of Food Science and Technology 2010, 45(4), 707–712.
- Bertoncelj, J.; Golob, T.; Kropf, U.; Korošec, M. Characterisation of Slovenian Honeys on the Basis of Sensory and Physicochemical Analysis with a Chemometric Approach. International Journal of Food Science and Technology 2011, 46(8), 1661–1671.
- Rodriguez, B.A.; Mendoza, S.; Iturriga, M.H.; Castaño-Tostado, E. Quality Parameters and Antioxidant and Antibacterial Properties of Some Mexican Honeys. Journal of Food Science 2012, 77(1), C121–C127.
- Vela, L.; de Lorenzo, C.; Perez, R.A. Antioxidant Capacity of Spanish Honeys and Its Correlation with Polyphenol Content and Other Physicochemical Properties. Journal of the Science of Food and Agriculture 2007, 87(6), 1069–1075.
- AOAC. Official Methods of Analysis, 15th Ed.; Association of Official Analytical Chemists: Arlington, TX, 1990.
- Gómez-Díaz, D.; Navaza, J.M.; Quintáns-Riveiro, L.C. Physicochemical Characterization of Galician Honeys. International Journal of Food Properties 2012, 15(2), 292–300.
- Saxena, S.; Gautam, S.; Sharma, A. Physical, Biochemical, and Antioxidant Properties of Some Indian Honeys. Food Chemistry 2010, 118(2), 391–397.
- Alvarez-Suarez, J.M.; Tulipani, S.; Díaz, D.; Estevez, Y.; Romandini, S.; Giampieri, F.; Damiani, E.; Astolfi, P.; Bompadre, S.; Battino, M. Antioxidant and Antimicrobial Capacity of Several Monofloral Cuban Honeys and Their Correlation with Color, Polyphenol Content and Other Chemical Compounds. Food and Chemical Toxicology 2010, 48(8), 2490–2499.
- Sant’Ana, L.D.O.; Sousa, J.P.; Salgueiro, F.B.; Lorenzon, M.C.A.; Castro, R.N. Characterization of Monofloral Honeys with Multivariate Analysis of Their Chemical Profile and Antioxidant Activity. Journal of Food Science 2012, 77(1), C135–C140.
- Escuredo, O.; Seijo, M.C.; Fernández‐González, M. Descriptive Analysis of Rubus Honey from the North-West of Spain. International Journal of Food Science and Technology 2011, 46(11), 2329–2336.
- Ouchemoukh, S.; Louaileche, H.; Schweitzer, P. Physicochemical Characteristics and Pollen Spectrum of Some Algerian Honeys. Food Control 2007, 18(1), 52–58.
- Gulfraz, M.; Iftikhar, F.; Imran, M.; Zeenat, A.; Asif, S.; Shah, I. Compositional Analysis and Antimicrobial Activity of Various Honey Types of Pakistan. International Journal of Food Science and Technology 2011, 46(2), 263–267.
- Codex Alimentarius. Codex Standard for Honey; FAO: Rome, Italy, 2001.
- Gomes, S.; Dias, L.G.; Moreira, L.L.; Rodrigues, P.; Estevinho, L. Physicochemical, Microbiological, and Antimicrobial Properties of Commercial Honeys from Portugal. Food and Chemical Toxicology 2010, 48(2), 544–548.
- Ajlouni, S.; Sujirapinyokul, P. Hydroxymethylfurfuraldehyde and Amylase Contents in Australian Honey. Food Chemistry 2010, 119(3), 1000–1005.
- Kukurova, K.; Karovičová, J.; Greif, G.; Kohajdová, Z.; Lehkoživová, J. Determination of 5-Hydroxymethylfurfural after Winkler and by the HPLC Method for Authentication of Honey. Chemical Papers 2006, 60(3), 186–191.
- Ram, A.K. Production of Spray-Dried Honey Powder and Its Application in Bread, Agricultural and Mechanical College; Louisiana State University: Baton Rouge, Louisiana, 2011.
- Alvarez, L.M. Honey Proteins and Their Interaction with Polyphenols; Faculty of Mathematic and Sciences, Brock University: St. Catharines, Ontario, 2011.
- Khalil, M.I.; Moniruzzaman, M.; Boukraâ, L.; Benhanifia, M.; Islam, M.A.; Islam, M.N.; Sulaiman, S.A.; Gan, S.H. Physicochemical and Antioxidant Properties of Algerian Honey. Molecules 2012, 17(9), 11199–11215.
- Saxena, S.; Gautam, S.; Sharma, A. Microbial Decontamination of Honey of Indian Origin Using Gamma Radiation and Its Biochemical and Organoleptic Properties. Journal of Food Science 2010, 75(1), M19–M27.
- González-Miret, M.L.; Terrab, A.; Hernanz, D.; Fernandez-Recamales, M.A.; Heredia, F.J. Multivariate Correlation Between Color and Mineral Composition of Honeys and By Their Botanical Origin. Journal of Agricultural and Food Chemistry 2005, 53(7), 2574–2580.
- Brudzynski, K.; Miotto, D. The Relationship Between the Content of Maillard Reaction-Like Products and Bioactivity of Canadian Honeys. Food Chemistry 2011, 124(3), 869–874.
- Miotto, D. Elucidation of the Components Involved in the Antioxidant Activity of Honey; Faculty of Biological Sciences, Brock University: St. Catharines, Ontario, 2011.
- Di Marco, G.; Canuti, L.; Impei, S.; Leonardi, D.; Canini, A. Nutraceutical Properties of Honey and Pollen Produced in a Natural Park. Agricultural Sciences 2012, 3(2), 187.
- Escuredo, O.; Silva, L.R.; Valentão, P.; Seijo, M.C.; Andrade, P.B. Assessing Rubus Honey Value: Pollen and Phenolic Compounds Content and Antibacterial Capacity. Food Chemistry 2012, 130(3), 671–678.
- Ulusoy, E.; Kolayli, S.; Sarikaya, A.O. Antioxidant and Antimicrobial Activity of Different Floral Origin Honeys from Turkiye. Journal of Food Biochemistry 2010, 34(s1), 321–335.
- Gacche, R.; Shinde, B.; Dhole, N.; Pund, M.; Jadhav, A. Evaluation of Floral Honey for Inhibition of Polyphenol Oxidase-Mediated Browning, Antioxidant, and Antimicrobial Activities. Journal of Food Biochemistry 2009, 33(5), 693–706.