?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
There is a traditional pickled tea fermented under anaerobic condition in many Asian countries, but its quality characteristics are still unclear. The dynamic qualities, chemical components, and volatile components of pickled tea processed by submerged fermentation were investigated. The results showed that the sensory qualities of the pickled tea gradually improved with increasing fermentation time. Sensory qualities decreased gradually after 7 d of fermentation, and the change was extremely significant (p < 0.01). Correspondingly, biochemical indicators (except for soluble extract) decreased continuously and significantly (p < 0.01) within 5–7 d of fermentation, but the decrease was much slower in the later stage. The proper control of fermentation time is a key step for obtaining the desired quality of pickled tea. By submerged fermentation for 7 d, the best sensory quality of pickled tea was obtained: the taste of pickled tea was less bitter and astringent, but certainly acidic; the flavor of pickled tea was sour with sweet and floral tastes; the tea had low caffeine content, high gamma-amino butyric acid content, and high contents of special volatile components, such as hotrienol and 2,6-bis (1,1-dimethylethyl)-4-methyl-phenol. Results indicated that submerged fermentation is potentially a new method of processing pickled tea to obtain the desired quality. Our results contribute to the understanding of the microbial transformation of tea components under anaerobic conditions.
Introduction
Tea is one of the most popular beverages in the world, and it has many unique health benefits, such as strengthening immunity, quenching thirst, and reducing cholesterol.[Citation1] Preliminary tea products processed from the leaves of Camellia sinensis are mainly classified into six types according to the processing technology and quality characteristics, as follows: green, oolong, black, yellow, white, and dark teas.[Citation1,Citation2] Among these types, only dark tea is processed by using microbes through microbial pile-fermentation technology.[Citation3,Citation4] Dark teas, such as Pu-erh and Fuzhuan brick teas, have a special flavor and unique health benefits, which are usually formed through a pile-fermentation; in this process, microbes can transform tea ingredients to other active components and simultaneously secret functional metabolites into tea.[Citation3–Citation5] Dark teas generally are fermented under aerobic conditions during the pile-fermentation process, and there are numerous reports on the quality of these teas.[Citation3–Citation7]
However, there is a traditional kind of pickled tea processed by anaerobic fermentation; this tea is produced and drank or eaten in many Asian countries and is given different names, such as Yancha (or Suancha) in China,[Citation8] Lahpet (or Leppet-so) in Myanmar,[Citation9] Miang tea in Thailand and Laos,[Citation10,Citation11] and Goishi cha and Awaban cha in Japan.[Citation11] In China, Yancha is a special and important food for DeAng, BuLang, and JingPo people in Yunnan province.[Citation8] In Thailand, chewing Miang tea is common in all age groups.[Citation10] In Myanmar and Singapore, Lahpet is a special and important side dish or is eaten as a salad, whereas its dried form was used for drinks.[Citation9] In Japan, Awaban cha and Goishi cha are dried after fermentation, and are only brewed as a drink.[Citation11] A prominent feature that is common in pickled tea is that a completely anaerobic fermentation occurs during their processing. Pickled tea is refreshing and thirst-quenching function when consumed and benefit the human digestive system by regulating bile and mucus; thus, people maintain the habit of consuming pickled tea.[Citation12–Citation15]
Some studies on pickled tea have been performed, but most of these studies focused on the isolation and identification of microbial strains in the tea, especially the lactic acid bacteria.[Citation16,Citation17] In addition to the antibacterial and antioxidant activities, the properties relevant to probiotic lactobacilli and the catechin composition of pickled tea have also been investigated.[Citation11,Citation14,Citation15,Citation18] Except for these studies, research on quality characteristics of pickled tea is lacking.[Citation8–Citation11] Known pickled tea is usually fermented via a solid-state fermentation process under anaerobic conditions. A previous study reported that a black tea was manufactured by submerged fermentation only with enzyme oxidization and its quality increased.[Citation19] However, reports on pickled tea or dark teas processed by submerged fermentation are lacking.[Citation3,Citation4] To develop pickled tea, the quality characteristics of a pickled tea processed by submerged fermentation were determined in this study.
Materials and Methods
Tea Leaves and Reagents
Camellia sinensis buds with three or four leaves were plucked during summer and autumn at the tea plantation of Huazhong Agricultural University, Wuhan City, Hubei Province, China. All the reagents, such as ferrous sulfate, potassium sodium tartrate, disodium hydrogen phosphate, stannous chloride, and concentrated sulfuric acid, were purchased from Tianyuan Reagent Co. Ltd., Wuhan City, China and were of analytical grade.
Preparation of Samples of Pickled Tea
After washing and draining, fresh tea leaves were blanched for 90 s and immediately cooled by using a fan. The blanched leaves were fanned continuously until about 30% reduction in weight was observed. Subsequently, these leaves were placed in a sterilized glass bottle. Saline solution (40 g/L) was boiled and cooled to room temperature and added to submerge the leaves. The amount of the added saline solution was 50 times the mass of leaves placed in the bottle. The bottle was then completely sealed and placed at room temperature (about 26–27°C) to ferment for several days. A parallel experiment was set up, and each treatment was performed in triplicate.
Samples of the submerged leaves were collected at 0, 1, 3, 5, 7, 9, and 11 d of fermentation time. To prevent potential contamination, the samples were collected from three different bottles each time. The sensory quality was evaluated immediately after the samples were collected, and then the samples were dried at 80°C for 4 h for the component analysis. Simultaneously, the pH of the fermented liquid was measured. The samples fermented for 7 d with the best sensory qualities, were chosen for analysis of various quality characteristics such as free amino acid (FAA) composition, catechin composition, tea pigments, volatile components, and soluble protein. The sample collected at 0 d was the control.
Evaluation of the Sensory Quality
In this study, 10 sensory panelists, who are all specialists in tea science at Huazhong Agricultural University and professionally trained in sensory evaluation, evaluated the sensory characteristics of the wet fermented samples. The sensory qualities of the samples were scored according to the criteria for color, odor, flavor, and texture features which were established in advance (Suppl. 1).
Determination of the pH Value
The pH value of the fermented liquid was precisely measured by with an electronic pH meter (FE20 type, made in Shanghai, China).
Analysis of the Components
The components of the samples, including tea polyphenols (TPs), FAAs, soluble sugar, caffeine, soluble protein, and soluble extract, were determined by using a ultra violet–visible (UV-Vis) spectrometer in accordance with China National Standard.[Citation20,Citation21]
Reversed Phase–High-Performance Liquid Chromatography (RP–HPLC) Analysis of the Catechin Composition
The samples fermented for 7 d had the best sensory qualities and were chosen for the analysis of catechin composition by RP–HPLC (Waters 600, USA; Column: Phenomenex Gemini C18, 250 mm×4.6 mm, 5 µm, USA). The sample at 1.5 g was extracted with 125 mL boiling ultrapure water in a boiling water bath for 45 min with shaking (3–5 times), and the extract was then filtered to 250 mL constant volume with ultrapure water. The detection wavelength was 278 nm. The mobile phase (A) was 0.1% acetic acid in water, and the mobile phase (B) was pure acetonitrile. The operating column temperature was maintained at 20°C. The injection volume was 5 µL, and flow speed was 1.0 mL/min. For the detection, pure (+) catechin C, (−) epicatechin (EC), (−) epigallocatechin (EGC), (−) epicatechin gallate (ECG), and (−) epigallocatechin gallate (EGCG) were used as standards. All of the operations and data acquisition were controlled by Waters Empower.[Citation21,Citation22]
Determination of the Theaflavins (TFs), Theabrownines (TBs), and Thearubigins (TRs)
The analysis for the main tea pigments including the TBs, TFs, and TRs, was performed by using a systematic approach.[Citation23] The sample at 3.0 g was extracted with 125 mL boiling distilled water in a boiling water bath for 10 min with shaking (1–2 times) and subsequently filtered. The tea filtrate was rapidly cooled to room temperature. The tea filtrate (30 mL) was shaken with 30 mL of ethyl acetate extract (EtOAc) for 5 min, the layers were separated after equilibration. A sample of the EtOAc layer (2 mL) was diluted to 25 mL with 95% ethanol (solution A). The 15 mL EtOAc layer was mixed with 25 mL of NaHCO3 solution (2.5 g/100 mL) for 30 s, and a 4 mL sample of the regenerated EtOAc layer was diluted to 25 mL with 95% ethanol (solution C). A 2 mL sample of the aqueous layer (first) was mixed with 6 mL distilled water and 2 mL saturated oxalic acid solution and then diluted to 25 mL with 95% ethanol (solution D). Tea filtrate (15 mL) was mixed with 15 mL of butyl alcohol for 3 min, and the layers were separated after equilibration. Then, a 2 mL sample of the aqueous layer (second) was mixed with 2 mL of saturated oxalic acid solution and 6 mL of distilled water and diluted to 25 mL with 95% ethanol (solution B).
The optical densities (EA, EB, EC, and ED) of solutions A, B, C, and D, respectively, were measured in a 1 cm cell at 380 nm using a spectrophotometer (Model-755B, Shanghai Optical Instrument Factory). Total concentrations of TFs, TRs, and TBs were calculated from the following equations:
where EA, EB, EC, and ED are the corresponding absorbance readings of the solutions.
RP–HPLC Analysis of the FAA Composition
The FAA composition was determined using RP–HPLC (Waters 600, USA; Column: Nova-PakTM C18, 150 × 4.6 mm, 4 µm, USA). Dried sample (1.5 g) was extracted with 125 mL boiling ultrapure water in a boiling water bath for 45 min with shaking (3–5 times), and the extract was filtered to 250 mL constant volume with ultrapure water. The detection excitation wavelength of fluorescence was 278 nm, and the emission wavelength was 395 nm. The mobile phase A (purchased from Waters China, Ltd.) was diluted 10-fold, the mobile phase B was pure acetonitrile. The mobile phase C was ultrapure water. The operating column temperature was maintained at 37°C. The injection volume was 5 µL, and the flow speed was 1.0 mL/min. All of the operations and the acquisition of data were controlled by Waters Empower.[Citation24]
GC–MS Analysis of the Volatile Components
The GC–MS analysis of the samples was performed using a gas chromatograph coupled with mass spectrometer (GC-2010 coupled with a GC–MS QP-2010) equipped with an auto-sampler (AOC-5000, Shimadzu, Japan) and a DB-5 fused silica capillary column (30 m × 0.25 mm, 0.25 μm). The extraction of volatile compounds was based on solid-phase microextraction (SPME) method using a 75 μm CAR/PDMS fiber (Sigma-Aldrich Co. LLC, USA). Each 1.00-g sample of tea, previously homogenized, was weighed into a 20 mL vial, and the SPME fiber head was rapidly inserted into the headspace of the vial. The extraction was kept in a water bath at 65°C for 45 min with constant stirring. The SPME fiber was pre-conditioned for 30 min at 250°C in the injection port of the GC prior to extraction. After extraction, the fiber was desorbed in the injection port of the GC at 230°C for 1.5 min. The oven temperature was held at 40°C for 5 min and then increased to 230°C at a rate of 5°C/min, and finally maintained at 230°C for 3 min. Ion-source temperature was at 230°C and spectra were produced in the electron impact (EI) mode at 70 eV. The mass spectrometer was operated in the full scan, and the peak area was determined using ChemStation software (Agilent Technologies).[Citation25]
Statistical Analysis
All statistical analysis was conducted using the statistical package of SAS software. Analysis of variance (ANOVA) followed by least significance difference (LSD) tests was performed to assess the statistical significance. The capitalized letter shows a highly significant difference (p < 0.01), whereas the lowercase letter shows a significant difference (p < 0.05). All data were expressed as mean ± standard deviation (SD) of three replicates.
Results
Change in the Sensory Quality
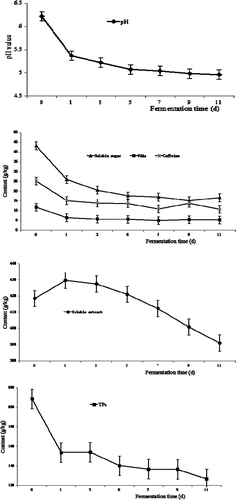
The sensory quality of the pickled tea collected every after 2 d during the 11 d submerged fermentation experiment was evaluated according to the scoring criteria established in advance (Suppl. 1). Depending on the results of the sensory evaluation, a change in the color associated with the formation of the characteristic aroma was observed (). During the submerged fermentation, the leaf color varied from green to dark yellow-green. Moreover, the pickled tea had a certain acidic taste and a sour smell (including ester aroma). However, the color of pickled tea deepened, and the acidity increased during prolonged fermentation (). During the fermentation, the scores increased in the first 7 d and showed an extremely significant change (p < 0.01). Then, the scores decreased from the 8th day to the 11th day. The score at the 7th day was the highest and showed an extremely significant difference (p < 0.01) compared with the scores in the latter 5 d. At the 7th day, the texture of pickled tea became soft, the bitterness and astringency decreased ().
TABLE 1 The sensory quality of pickled tea during submerged fermentation
Change in the pH Value
The pH value has a positive correlation with acidity and is usually chosen as the main quality attribute and fermentation index of pickles.[Citation26] During the submerged fermentation, the pH value of pickled tea decreased continuously from 6.2 at 0 d to 4.98 at 11th day. The pH value was reduced rapidly with a highly significant change (p < 0.01) during the previous 5 d, but gradually in the later stage (). The change in pH values was consistent with changes in terms of the smell and taste, i.e., the sample’s sourness increased ().
Change in the Soluble Sugar Content
Tea leaf contains 20–25% carbohydrates and 4–6% soluble sugars on a dry basis.[Citation2] Soluble sugar, which is a significant constituent of tea taste, presented a unique sweet aftertaste. Soluble sugar of pickled tea decreased continuously throughout the fermentation process (). The soluble sugar content decreased rapidly in the first 5 d, and then slowly in the next 6 d. Soluble sugar content stabilized at approximately 16 g/kg in the last 4 d (). Soluble sugar declined to about 61.58% throughout the 11 d fermentation process (about 37.75% at the first day and 59.22% at the 5th day). Variance analysis and multiple comparison indicated that the differences in the first 5 d were extremely significant (p < 0.01).
Change in the Caffeine Content
From , we can see that the caffeine content decreased during the fermentation process, which could also explain the reduction of the bitterness of the samples (). Similar to soluble sugar, caffeine decreased rapidly by 39.70% at the first day, and was reduced by almost 57% throughout the entire fermentation process. The variance analysis showed that the difference during the first 7 d reached extremely significant levels (p < 0.01).
Change in the Soluble Extract Content
The soluble extract of tea has traditionally been regarded as an important international standard for tea quality,[Citation2] and can be directly absorbed and utilized by human body. In the present study, the content of the soluble extract of pickled tea increased mildly during the first day, decreased slightly during the next 10 d, and finally stabilized at the 11th day. The final content was 6.5% lower than that at 0 d (). There were no significant differences (p > 0.05) in the soluble extract contents in the first 7 d according to variance analysis, but the final soluble extract content had an extremely significant difference (p < 0.01) when compared with those in the first 7 d ().
Change in the FAA, FAA Composition, and Soluble Protein
Generally, FAAs of tea contribute to the formation of taste and aroma quality, and their content is relative to the tenderness of fresh tea leaves.[Citation2] The FAA of pickled tea decreased sharply to 54.07% of the original content during the first day, and then the reduction became gradual in the next 10 d (). The final content of FAAs decreased to almost 55.85% throughout the entire fermentation process. Variance analysis showed that the difference in FAA content during the fermentation process reached an extremely significant level (p < 0.01; ).
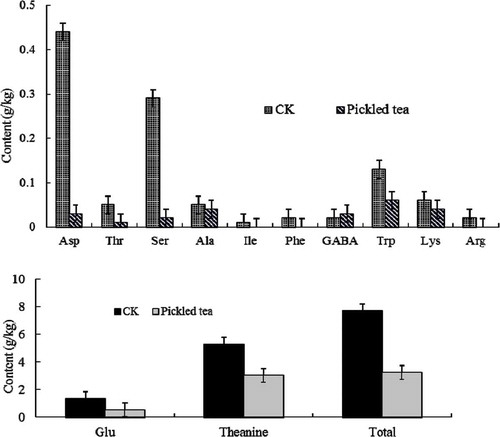
To further understand the quality characteristics of pickled tea during the fermentation process, the catechins, FAAs, pigments, and soluble protein content of pickled tea fermented for 7 d, which had the best sensory quality, were further analyzed and compared with those of the control sample. The total content of the FAAs and soluble protein decreased by 57.90% () and 41.86% (), respectively, during the first 7 d. Ile, Phe, and Arg were not detected, and Asp and Ser contents both decreased by 93% (). However, only gamma-amino butyric acid (GABA) content increased by 50% ().
TABLE 2 Tea pigments and soluble protein in pickled tea (g/kg)
Change of TPs and Tea Pigments
TPs are the most significant characteristic of tea.[Citation2] During the 11 d submerged fermentation, TPs of pickled tea decreased continuously (). The reduction was rapid on the first day (26.32%), but became gradual and fluctuating during the next 10 d (). The TPs of pickled tea dropped to a total of 39.27% throughout the entire submerged fermentation process. An extremely significant change in TPs (p < 0.01) was observed during the fermentation process as shown by variance analysis. The decrease in TPs positively correlated with the disappearance of the astringent taste (). Throughout the first 7 d, TPs of pickled tea decreased by 34.50% (). Interestingly, TFs and TBs also decreased by 15.80 and 31.91%, respectively. However, TRs increased by 175% in the first 7 d (). The decrease in content of TFs and TBs was much larger than the increase in content of TRs.
Change in the Catechin Composition
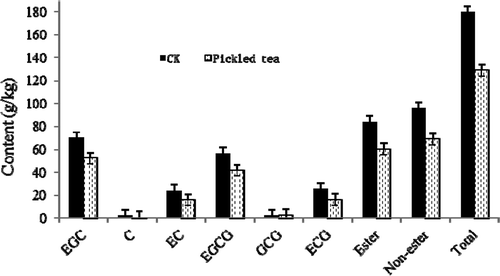
The catechin composition of pickled tea fermented for 7 d was analyzed using RP–HPLC. Interestingly, ester-, and non-ester-catechins of pickled tea were both reduced by 28%, and the total catechins were correspondingly reduced by 28% (, Suppl. 2). The reduction in content of C was maximum at 60%. The content of other catechins decreased by 25–35%, whereas only GCG increased by 9.09%. The decrease in the content of the ester-, and non-ester-catechins in pickled tea was consistent with the reduction of the bitterness and astringency of the sample (), which would contribute to the quality formation of pickled tea.
Change in the Volatile Compounds
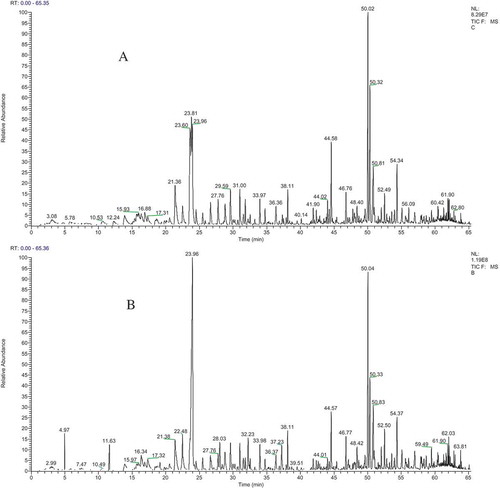
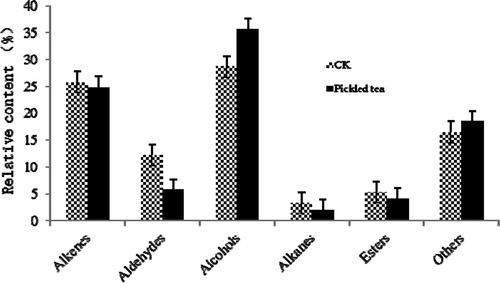
The volatile compounds of pickled tea fermented for 7 d were analyzed using GC–MS (). The total content of the identified compounds in pickled tea, with a slight reduction, was basically equal to that of the control. The identified 44 volatiles represented 92.57 and 91.34% of the essential oils extracted from the control sample and pickled tea, respectively (Suppl. 3). The volatile compounds of pickled tea could be classified into the following six types as listed in : alkenes (24.88%), aldehydes (5.77%), alcohols (35.57%), esters (4.11%), alkanes (2.01%), and others (18.50%). Among the six types of the volatile components, the alkene, aldehyde, ester, and alkane contents decreased during the fermentation. However, the contents of alcohol and other compounds increased compared with that of the control. The compounds showing an increase in percentage over 100% were hotrienol (144.57%), 4-ethylphenol (47040%), 2-bornene (401.58%), and cis-3-hexenyl caproate (156.55%), whereas those that showed a decrease in percentage over 50% were 1-methoxy-4-methyl-benzene (85.35%), octanal (54.97%), β-linalool (70.77%), nonanal (76.49%), and tetradecane (51.11%). Hotrienol (25.64%), 2,6-bis (1,1-dimethylethyl)-4-methyl-phenol (11.35%), α-farnesene (5.28%), δ-cadinene (5.00%), trans-linalool oxide (4.75%), and β-caryophyllene (3.33%) were the major components in pickled tea, and these compounds promote the formation of a unique odor from the major components of the sweet floral flavor constituents (). These observations also supported the production of ester aroma in the scent of pickled tea ().
Discussions
We already identified that Lactobacillus plantarum was the dominant strain during submerged fermentation.[Citation27] In this study, the changes in the main chemical components and sensory qualities of C. sinensis leaves processed by submerged fermentation were analyzed. In total, the sensory qualities of pickled tea gradually improved during fermentation, but started to degrade slowly beyond the optimum fermentation time (). This finding was similar to that observed during Kombucha fermentation,[Citation28] wherein the fermentation time played a key role in the sensory quality formation of the tea. The desired sensory quality can only be obtained through the proper control of fermentation time during the submerged fermentation process,[Citation28] which was also supported by the change in the chemical components of pickled tea ().
The chemical indicators (except for soluble extract) of pickled tea decreased continuously with an extremely high significance (p < 0.01) within 5–7 d. The reduction in the contents of various chemical indicators was generally rapid on the first day and then slowed down thereafter (). This result indicated that the changes in the chemical components in pickled tea were completely different from those in liquid-fermented black tea with enzyme oxidization alone.[Citation19] The change in pickled tea’s TPs content was the same as that in Pu-erh tea’s TPs, which decreased from 220 to 100 g/kg before 50 d.[Citation5] The appropriate TP reduction was attributed to the oxidative polymerization caused by the growth of bacteria or yeasts, which produced polyphenol oxidase and other oxidases.[Citation28,Citation29] Nevertheless, the decrease in pickled tea’s TPs did not directly increase the contents of tea pigments in pickled tea. TFs and TBs of pickled tea both decreased during submerged fermentation, and only the TRs increased by 175% (), and such changes were evidently different from those in common dark teas, such as Pu-erh and brick tea[Citation5] Thus, yeasts and bacteria in pickled tea could secrete some enzymes that could catalyze the biodegradation of tea catechins, TFs and TBs.[Citation28,Citation29] The change in pickled tea catechin after fermentation for 7 d is in accordance with the changes that occurred after fermentation of Miang,[Citation18] Kombucha,[Citation30] and green tea.[Citation31] EGCG in pickled tea was mostly reserved, i.e., the acidic condition may hinder the biodegradation of catechins.[Citation31] In pickled tea, the content of ester-catechins was essentially equal to that of the non-ester form (). The decrease in TPs, TFs, and TBs would be due to the polymerization of more insoluble TBs or other insoluble tea pigments.
C. sinensis is among several plants that contain caffeine. Caffeine, which is a powerful stimulant of the central nervous system and cardiac muscle, contributes to the unique quality of tea.[Citation32] However, high levels of caffeine might cause a noticeable irritation of the gastrointestinal tract, as well as other adverse effects.[Citation32] Hence, it may be desirable to reduce the content of caffeine in tea leaves. Pickled tea’s caffeine content was different from that of Pu-erh tea, which was higher than that of pickled tea by 23% ().[Citation33] Yeast fermentation can reportedly reduce caffeine content, whereas fermentation with Aspergillus niger can increase the content of caffeine in green tea, from an initial concentration of 3.47% to 9.63%.[Citation34] Hence, the reduction of caffeine in pickled tea may be caused by microbial transformation,[Citation34] and a tea product with much lower caffeine content could be processed through submerged fermentation. Moreover, only GABA and TRs increased through submerged fermentation ( and ), which would offer a new special function activity to pickled tea. More organic acids could be formed in pickled tea through submerged fermentation, which is indicated by the production of a pleasant acidic scent. Thus, further study is needed to identify the composition of organic acids or other functional ingredients in pickled tea.
Conclusions
Based on the analysis of the changes of the chemical components and sensory quality of pickled tea, this process is conducive to understanding its quality formation under anaerobic conditions. The proper control of fermentation time is a key step in obtaining the desired quality of pickled tea. After 7 d of submerged fermentation, pickled tea achieved better sensory quality, and its taste was less bitter and astringent, but was certainly acidic. Pickled tea’s flavor was sour with sweet and floral notes. Pickled tea had low caffeine content, high GABA content, and high hotrienol and 2,6-bis (1,1-dimethylethyl)-4-methyl-phenol contents. The quality characteristics of pickled tea were clearly different from those of other dark teas, and submerged fermentation is a new potential method of processing pickled tea products for its desired quality.
Nomenclature
| Free amino acid: | = | FAA |
| Tea polyphenol: | = | TP |
| Theaflavins: | = | TFs |
| Theabrownines: | = | TBs |
| Thearubigins: | = | TRs |
| Gamma-amino butyric acid: | = | GABA |
Funding
The authors are very grateful for the financial support from Projects in the National Science & Technology Pillar Program during the 12th 5-year Plan Period, China (Grant No. 2013BAD20B06).
Supplemental Material
Supplemental data for this article can be accessed on the publisher’s website.
LJFP_S_1075217.docx
Download MS Word (33.7 KB)Additional information
Funding
References
- Yang, C.S.; Lambert, J.D. Research on Tea and Health. Pharmacological Research 2011, 64(2), 85–86.
- Harbowy, M.E.; Balentine, D.A. Tea Chemistry. Critical Reviews in Plant Sciences 1997, 16(5), 415–480.
- Mo, H.Z.; Zhu, Y.; Chen, Z.M. Microbial Fermented Tea—A Potential Source of Natural Food Preservatives. Trends in Food Science & Technology 2008, 19(3), 124–130.
- Luo, Z.-M.; Du, H.X.; Li, L.X.; An, M.Q.; Zhang, Z.Z.; Wan, X.C.; Bao, G.H.; Zhang, L.; Li, T.J. et al. Fuzhuanins A and B: The B-Ring Fission Lactones of Flavan-3-ols from Fuzhuan Brick-Tea. Journal of Agricultural and Food Chemistry 2013, 61(28), 6982–6990.
- Abe, M.; Takaoka, N.; Idemoto, Y.; Takagi, C.; Imai, T.; Nakasaki, K. Characteristic Fungi Observed in the Fermentation Process for Puer Tea. International Journal of Food Microbiology 2008, 124(2), 199–203.
- Zhang, L.; Deng, W.-W.; Wan, X.-C. Advantage of LC-MS Metabolomics to Identify Marker Compounds in Two Types of Chinese Dark Tea After Different Post-Fermentation Processes. Food Science and Biotechnology 2014, 23(2), 355–360.
- Wang, W.N.; Zhang, L.; Wang, S.; Shi, S.P.; Jiang, Y.; Li, N.; Tu, P.F. 8-C N-Ethyl-2-Pyrrolidinone Substituted Flavan-3-Ols As the Marker Compounds of Chinese Dark Teas Formed in the Post-Fermentation Process Provide Significant Antioxidative Activity. Food Chemistry 2014, 152, 539–545.
- Nanba, A.; Miyagawa, K.; Omori, M.; Kato, M.; Tamura, A.; Saito, H. Non-Salted Pickled Tea (Sour Tea) in South-East Yunnan in China. Japan Society of Home Economics 1998, 49(8), 907–915.
- Nanba, A.; Nyein, M.M.; Win, S.Y.; Miyagawa, K. Post-Heated and Fermented Edible Teas and Their Dried Forms Used for Drinking in Myanmar. Journal of Home Economics of Japan 1999, 50(6), 639–646.
- Reichart, P.A.; Philipsen, H.P.; Mohr, U.; Geerlings, H.; Srisuwan, S. Miang Chewing in Northern Thai Villagers. Tropical and Geographical Medicine 1988, 40(1), 39–44.
- Kato, M.; Tamura, A.; Omori, M.; Nanba, A.; Miyagaw, K.; Nishimura, O.; Kameda, W. Changes of Flavor During Manufacturing Process of Japanese Fermented Tea (Goishi-Cha) and Its Characteristic. Japan Society of Home Econmics 1994, 45(6), 527–532.
- Yokota, J.; Jobu, K.; Yoshioka, S.; Moriyama, H.; Murata, S.; Oishi, M.; Ukeda, H.; Miyamura, M. Effect of Goishi-Tea on Adipocytokine Changes. Journal of the Japanese Society for Food Science and Technology-Nippon Shokuhin Kagaku Kogaku Kaishi 2011, 58(8), 398–402.
- Hirota, R.; Ngatu, N.R.; Miyamura, M.; Nakamura, H.; Suganuma, N. Goishi Tea Consumption Inhibits Airway Hyperresponsiveness in BALB/C Mice. BMC Immunology 2011, 12, 1–8.
- Klayraung, S.; Viernstein, H.; Sirithunyalug, J.; Okonogi, S. Probiotic Properties of Lactobacilli Isolated from Thai Traditional Food. Scientia Pharmaceutica 2008, 76, 485–503.
- Klayraung, S.; Okonogi, S. Antibacterial and Antioxidant Activities of Acid and Bile Resistant Strains of Lactobacillus Fermentum Isolated from Miang. Brazilian Journal of Microbiology 2009, 40(4), 757–766.
- Okada, S.; Daengsubha, W.; Uchimura, T.; Ohara, N.; Ki, A.I.K. Flora of Lactic Acid Bacteria in Miang Produced in Northern Thailand. Journal of General and Applied Microbiology 1986, 32(1), 57–65.
- Tanasupawat, S.; Pakdeeto, A.; Thawai, C.; Yukphan, P.; Okada, S. Identification of Lactic Acid Bacteria from Fermented Tea Leaves (Miang) in Thailand and Proposals of Lactobacillus Thailandensis sp. Nov., Lactobacillus Camelliae sp. Nov., and Pediococcus Siamensis sp. Nov. Journal of General and Applied Microbiology 2007, 53(1), 7–15.
- Phromrukachat, S.; Tiengburanatum, N.; Meechui, J. Assessment of Active Ingredients in Pickled Tea. Asian Journal of Food and Agro-Industry 2010, 3(3), 312–318.
- Tao, X.; Qi-qing, T.; Wei-xiang, X. Comparative Study on the Quality of Suspension-Fermented Black Tea and Orthodox Black Tea. Journal of Tea Science 2000, 20(2), 105–109.
- The First Research Institute of China Standards Publisher, The First Research Institute of China Standards Publisher, Standards Collection for Tea. China Standards Publisher, PR China, Beijing, 2003.
- Zhou, D.; Chen, Y.; Ni, D. Effect of Water Quality on the Nutritional Components and Antioxidant Activity of Green Tea Extracts. Food Chemistry 2009, 113(1), 110–114.
- Ahmad, R.S.; Butt, M.S.; Huma, N.; Sultan, M.T.; Okada, S.; Arshad, M.U.; Mushtaq, Z.; Saeed, F. Quantitative and Qualitative Portrait of Green Tea Catechins (GTC) Through HPLC. International Journal of Food Properties 2014, 17(7), 1626–1636.
- Yao, L.H.; Jiang, Y.M.; Caffin, N.; D’Arcy, B.; Datta, N.; Liu, X.; Singanusong, R.; Xu, Y. Phenolic Compounds in Tea from Australian Supermarkets. Food Chemistry 2006, 96(4), 614–620.
- Tan, F.; Tan, C.; Zhao, A.P.; Li, M.L. Simultaneous Determination of Free Amino Acid Content in Tea Infusions by Using High-Performance Liquid Chromatography with Fluorescence Detection Coupled with Alternating Penalty Trilinear Decomposition Algorithm. Journal of Agricultural and Food Chemistry 2011, 59(20), 10839–10847.
- Liang, Y.R.; Ye, Q.; Jin, J.; Liang, H.; Lu, J.L.; Du, Y.Y.; Dong, J.J. Chemical and Instrumental Assessment of Green Tea Sensory Preference. International Journal of Food Properties 2008, 11(2), 258–272.
- Panda, S.H.; Panda, S.; Sivakumar, P.S.; Ray, R.C. Anthocyanin-Rich Sweet Potato Lacto-Pickle: Production, Nutritional and Proximate Composition. International Journal of Food Science and Technology 2009, 44(3), 445–455.
- Xiao, P.; Huang, Y.Y.; Yang, W.P.; Zhang, B.W.; Quan, X.X. Screening Lactic Acid Bacteria with High Yielding-Acid Capacity from Pickled Tea for Their Potential Uses of Inoculating to Ferment Tea Products. Journal of Food Science and Technology 2015, 52(10), 6727–6734.
- Chen, C.; Liu, B.Y. Changes in Major Components of Tea Fungus Metabolites During Prolonged Fermentation. Journal of Applied Microbiology 2000, 89(5), 834–839.
- Jayabalan, R.; Subathradevi, P.; Marimuthu, S.; Sathishkumar, M.; Swaminathan, K. Changes in Free-Radical Scavenging Ability of Kombucha Tea During Fermentation. Food Chemistry 2008, 109(1), 227–234.
- Jayabalan, R.; Marimuthu, S.; Swaminathan, K. Changes in Content of Organic Acids and Tea Polyphenols During Kombucha Tea Fermentation. Food Chemistry 2007, 102, 392–398.
- Tu, Y.Y.; Xia, H.L.; Watanabe, N. Changes in Catechins During the Fermentation of Green Tea. Applied Biochemistry and Microbiology 2005, 41(6), 574–577.
- Gramza-Michalowska, A. Caffeine in Tea Camellia Sinensis—Content, Absorption, Benefits, and Risks of Consumption. Journal of Nutrition Health & Aging 2014, 18(2), 143–149.
- Yang, T.; Rong, X.; Kunlong, X.; Bo, J.; Congyin, S. Changes and Correlations of Main Chemical Components During Puer Tea Processing. Food Science (China) 2010, 31(11), 20–24.
- Wang, X.G.; Wan, X.C.; Hu, S.X.; Pan, C.Y. Study on the Increase Mechanism of the Caffeine Content During the Fermentation of Tea with Microorganisms. Food Chemistry 2008, 107(3), 1086–1091.