Abstract
Sea cucumber is easy to undergo autolysis, causing serious economic loss in the aquaculture, fishery, and food processing. However, research on the autolysis of sea cucumber is limited. In the present study, the autoenzyme that could hydrolyze the structural protein of body wall was identified and characterized in sea cucumber Stichopus monotuberculatus. Although the main edible part of sea cucumber was the body wall, its microstructure was shown to be almost composed of collagen fibers belonging to extracellular matrix, indicating that the body wall of S. monotuberculatus belonged to dermis tissue. Starting from this perspective, crude collagen fibrils from dermis of S. monotuberculatus was isolated, and pepsin solubilized collagen was also obtained by hydrolyzing collagen telopeptides from crude collagen fibrils. Crude collagen fibrils and pepsin solubilized collagen were then acting as substrates, respectively, to identify dermis autoenzyme which had hydrolytic activity on dermis collagen. After that, a collagenase of 45 kDa was identified with the method of collagen zymograph, and the content of soluble protein was suppressed by collagenase inhibitor significantly in the autolysis of dermis. Finally, the effect of environmental conditions on collagenase activity was studied, results showed its best activity was at 40°C and pH 8, and divalent metal ion Mn2+ was essential for its activity. As a whole, our results showed that the dermis of sea cucumber was composed of collagen fibers, and collagenase was the main enzyme resulting in autolysis of sea cucumber.
INTRODUCTION
Sea cucumbers belong to phylum Echinodermata. There have been more than 1400 species of sea cucumbers found around the world, of which 50 species have higher edible and medical value.[Citation1] Many biological and pharmacological activities have been described in various species of sea cucumbers.[Citation2] Asia-Pacific Sea is the main output area of sea cucumber with an annual catches at 30,000 t, while the aquaculture production of sea cucumber could achieve to 140,000 t per year in China.[Citation3] However, in changing of environmental conditions, sea cucumber is easy to undergo autolysis, which brings serious economic losses to their food processing.[Citation4–Citation7]
Autolysis of tissue is a self-digestion process of cells and extracellular matrix (ECM), in which the digestive enzymes are secreted by cells. Autolysis usually occurs in non-viable tissues with their cell structure and ECM digested by autoenzyme.[Citation8,Citation9] Autoenzyme is the mixture of proteolytic enzymes participating in autolysis. Autoenzyme can be divided into enzymes, respectively, for cell digestion and ECM digestion according to the different substrates. The enzymes for cell digestion, such as calpain[Citation10,Citation11] and cathepsin,[Citation12Citation13] are usually activated in the cytoplasm of non-viable cell to degrade the organelles and cell structure.
The enzymes for ECM digestion can degrade the structure of ECM which is composed of collagen, elastin, glycosaminoglycan, and other matrix compounds.[Citation14] As the main structure in ECM, collagen fibers can endow tissue with their specific mechanical and biochemical properties.[Citation15] Usually, collagen fibers are hardly soluble, due to the covalent crosslinks formed by non-helical telopeptides of adjacent collagens.[Citation16] As a result, the enzymes for ECM digestion can digest collagen fibers into soluble protein, destroy ECM and lead to autolysis. Previous studies shown that soluble collagen would get away from the fibers with its telopeptides hydrolyzed.[Citation17Citation18] On the other hand, collagenase could break down collagen triple-helix directly and disintegrate the fibers.[Citation19Citation20] Collagenase has been demonstrated as the main enzyme for spoilage in fish. The process of muscle softening during iced storage is mainly caused by collagenase activity than by proteolysis of myofibrils, and this softening could be explained as a result of specific collagenases acting on the insoluble fraction of the collagen fibers.[Citation21,Citation22]
As the main edible part, dermis (body wall) of sea cucumber is rich in protein and mucopolysaccharide, and the crude protein can reach 83% of its dry weight in dried sea cucumber. The insoluble collagen fibers account for 70% of the protein in sea cucumber.[Citation16] The dermis of sea cucumber Stichipus japonicas was composed of collagen fibers with few muscle fibers by Van Gieson staining.[Citation23Citation24] So far, the studies on autolysis of sea cucumber were focused on the enzymes for cell digestion, such as alkaline protease,[Citation25] cysteine-like protease,[Citation4] cathepsin L-like enzyme,[Citation7] and cathepsin B.[Citation5] Some researches on collagenase of S. japonicas have been published recently, including: Sun et al.[Citation26] found that soluble protein could be controlled with the addition of collagenase inhibitor in the process of autolysis, and Wu et al.[Citation27] isolated and characterized the collagenase in the same species. However, degradation of collagen fibers was found to be not only performed by collagenase, but also other enzymes that could hydrolyze telopeptides.
As a tropical sea cucumber species with high economic value, sea cucumber (Stichopus monotuberculatus), belonging to family Stichopodidae, is distributed at reef and sandy bottoms along the Pacific Southwest coast.[Citation28] Researches on spawning, fertilization, embryo development, larval development, and juvenile growth of S. monotuberculatus have been explored recently in our group, which would promote the development of aquaculture in this species.[Citation29] As mentioned above, researches on the autoenzyme of sea cucumber have been carried out, and there was a wide variety of protease been reported. However, there was no agreement which protease played a main role in autolysis. Therefore, the objective of this study was to find the structural feature of dermis in S. monotuberculatus, identify the enzymes participating in the hydrolysis of its structural protein, and characterize them, for the purpose of developing its food processing.
MATERIAL AND METHODS
Materials
Sea cucumber, S. monotuberculatus, with an average weight of 100 g were provided by Beihai Zhengyu Biotechnology Limited Company. S. monotuberculatus were placed in plastic bags filling with 1/3 volume of sea water, shipped to the laboratory in Guangzhou at 4ºC and reared temporarily in cyclic aquarium at water temperature of 26–28ºC. Optimum cutting temperature compound (O.C.T. Compound) was from Sakura (Sakura Finetek, Torrance, CA, USA). Pepsin (EC 3.4.23.1), Ethylenediamine tetraacetate (EDTA), 1,10-phenanthroline, phenylmethanesulfonyl fluoride (PMSF), trypsin inhibitor from soybean (TI), benzamidine, DTNB (5,5-dithiobis-2-nitrobenzoic acid), iodoacetic acid, E-64 (cysteine peptidases inhibitor), leupeptin, and pepstatin A were purchased from Sigma-Aldrich Co. (Sigma-Aldrich Inc., St. Louis, MO, USA). Prestained protein marker ranging from 10 to 260 kDa was from Thermo Fisher Co. (Thermo Fisher Scientific Inc., MA, USA). Five times protein loading dye and 7.5% precast gels were obtained from Sangon Biotechnology Co. (Sangon Biotech. Inc., Shanghai, China). Triton X-100 was from MPBio Co. (MP Biomedicals, Santa Ana, CA, USA). Other reagents used in this study were all of analytical grade.
Preparation of Dermis for Light Microscope
The microstructure of dermis fibers was observed by optical microscope after frozen sectioning and Van Gieson staining.[Citation23,Citation24] Raw dermis of S. monotuberculatus were cut into 10 × 5 × 5 mm3 cubes, embedded in O.C.T. compound at –20°C. Samples were sliced into sections of 15 μm thickness using Leica CM 1900 cryostat (Leica Microsystems GmbH, Wetzlar, Germany), mounted on glass slides, and stained with Van Gieson staining. At last, light micrographs were taken with a Leica DM IRB optical microscopic.
Preparation of Crude Collagen Fibrils (CCF)
The water-EDTA-water method was used to extract sea cucumber CCF with a slight modification, and all operations were performed at 4ºC.[Citation30] The dermis of S. monotuberculatus was cut into small pieces, homogenized in 1 volume (v/w) of deionized water for 10 min by tissue disintegrator, and centrifuged at 10,000 × g for 10 min. Precipitate was stirred with new deionized water again for 1 h with magnetic stirrer, and centrifuged. Subsequently, 10 volumes of EDTA (4 mM, dissolved in 0.1 M Tris-HCl, pH 8.0) was added to the precipitate. After 48 h stirring, the precipitate was washed with deionized water for several times. Ten volumes of 0.1 M NaOH was then added and stirred for 72 h. After alkali extraction, the sample was centrifuged at 20,000 × g for 30 min, and precipitate was thoroughly washed with deionized water for several times to neutral. Finally, the CCF of upper precipitate was dialyzed against deionized water, lyophilized using Crist Freeze Dryer Alpha 1-2 LD (Martin Christ, Osterode am Harz, Germany) and stored at –20ºC.
Isolation of Pepsin Solubilized Collagen (PSC)
PSC of S. monotuberculatus was isolated and purified as previously described[Citation16] and all operations were performed at 4ºC. Briefly, lyophilized CCF was added to 500 volumes (v/w) of 0.5 M acetic acid (pH 3.0), mixed with pepsin at an enzyme/substrate ratio of 1:10, and stirred for 48 h. The supernatant containing PSC was collected by centrifugation at 25,000 × g for 60 min, and PSC was salted out by adding NaCl to a final concentration of 2% with slight stirring for 24 h. Precipitate containing PSC was collected by centrifugation at 7500 × g for 10 min and dissolved in 0.5 M acetic acid again. The sample was dialyzed against 0.02 M Na2HPO4 (pH 8.0), 0.1 M acetic acid and deionized water successively. Each dialysate was stirred slightly for 48 h and changed every 6 h. The purified PSC was finally lyophilized and stored at –20ºC.
Preparation of Crude Enzyme Extract (CEE)
The CEE from dermis of S. monotuberculatus was obtained as Fu et al.[Citation25] described, and all operations were performed at 4ºC. Ten S. monotuberculatus were dissected, and internal organs were removed. The remaining dermis were homogenized in 1 volume (v/w) 50 mM Tris-HCl buffer (pH 7.5), and centrifuged at 10,000 × g for 10 min. The supernatant containing enzymes was collected and stored at –80ºC. The soluble protein content of CEE was measured with bicinchoninic acid (BCA) method.[Citation31]
Collagen Zymograph
Collagen zymograph was performed according to Quiñones et al.[Citation9] with a slight modification. CEE of 20 μg was mixed with 5 × sample loading buffer (non-reducing condition), and applied to discontinuous Tris-glycine buffer system electrophoresis with 10% resolving gel (with 1 mg/mL PSC), along with 10-260 kDa prestained protein marker. Electrophoresis was conducted at temperature of 4ºC and voltage of 100 V for 2 h. After electrophoresis, the gels were shaken gently in 2.5% (v/v) Triton X-100 for 30 min to remove SDS and renature enzymes, followed by rinsing with deionized water. The gels were then incubated in 50 mM Tris-HCl buffer (pH 7.8, with 0.15 M NaCl and 1 mM CaCl2) at 37ºC for 18 h to catalyze the reaction between CEE and CCF or PSC. Finally, the gels were stained with 0.1% Coomassie Brilliant Blue, and then destained. The enzymes with activity were presented as lighter bands of gels.
Identification of Autoenzyme with Different Proteinase Inhibitors
The autoenzyme was identified with collagen zymograph method using CCF and PSC as substrate, respectively. After removing of SDS with 2.5% Triton X-100, the gels were then separated and incubated in 50 mM Tris-HCl buffer (pH 7.8, with 0.15 M NaCl and 1 mM CaCl2) at 37ºC for 18 h with different protease inhibitors, respectively. The protease inhibitors include metalloproteinase inhibitors (10 mM EDTA and 10 mM 1,10-phenanthroline), serine proteinase inhibitors (1 mM PMSF, 0.1 mg/ml TI and 10 mM benzamidine), thiol protease inhibitors (10 mM DTNB, 10 mM iodoacetic acid, 0.01 mM E-64 and 0.1 mM leupeptin) and aspartic protease inhibitors (0.1 mM pepstatin A).[Citation25Citation27]
Hydrolysis of CCF and PSC by Autoenzyme
The CEE (1 mg/mL) and different protease inhibitors were mixed, respectively, and incubated in 50 mM Tris-HCl buffer (pH 7.8, with 0.15 M NaCl and 1 mM CaCl2) at 25ºC for 1 h. CCF (or PSC) was then mixed with the CEE treated by protease inhibitors to final concentrations of 1 and 0.2 mg/mL, respectively, and incubated at 37ºC for 8 h. After reaction, the mixtures were centrifuged at 10,000 × g for 1 h, 10 μL of supernatant was applied to SDS-PAGE with 7.5% resolving gel.[Citation32]
Content of Soluble Protein in Autolysis of Dermis
The soluble protein in autolysis of S. monotuberculatus dermis was performed according to Sun et al.[Citation26] with modification, and all operations were performed at 4ºC. The fresh dermis was cut into small pieces, homogenized in 1 volume (v/w) 50 mM Tris-HCl buffer (pH 7.5) for 5 min, and centrifuged at 15,000 × g for 15 min. Afterward, the supernatant and precipitate were collected respectively. Then 0.5 g precipitate and 2 mL supernatant with the presence or absence of 10 mM 1,10-phenanthroline were mixed and vibrated at 30°C for 0, 2, 4, 6, and 8 h, respectively. Subsequently, the mixture was centrifuged at 15,000 × g for 15 min, and the soluble protein in supernatant was measured with BCA method.[Citation31]
Measurement of Collagenase Activity Against PSC
The activity of collagenase was measured with colorimetric method using PSC as a substrate.[Citation33] PSC and CEE were mixed in 50 mM Tris-HCl buffer (pH 7.8, with 0.15 M NaCl and 1 mM CaCl2) to final concentrations of 1 and 0.2 mg/mL, respectively, and incubated at 37ºC for 8 h. After reaction, 10 μL of each mixture was applied to SDS-PAGE with 7.5% resolving gel, along with PSC of 1, 2.5, 5, 7.5 and 10 μg, respectively, as controls for calculation of relationship between PSC concentration and band density. The activity of collagenase was reflected as digestibility of PSC α subunit with semi-quantitative analysis in Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA).[Citation32]
Effect of Temperature and pH on Collagenase Activity
The effect of temperature on collagenase activity was studied by incubation of PSC (1 mg/mL) and CEE (0.2 mg/mL) mixed at 4, 10, 20, 30, 40, 50, and 60ºC for 8 h, respectively. The effect of pH on collagenase activity was determined by incubation of PSC (1 mg/mL) and CEE (0.2 mg/mL) together in the following buffers with different pHs: 0.2 M HAc-NaAc (pH 6.0), 0.1 M Tris-HCl (pH 7.0–9.0), and 0.1 mM Gly-NaOH (pH 10.0–11.0)[Citation25Citation27] at 37ºC for 8 h, respectively. Activity of collagenase was then measured as described in section “Measurement of Collagenase Activity against PSC.”
Influence of Metal Ions on Collagenase Activity
The effect of metal ions on collagenase activity was studied as follows: the enzymes with different metal salts including 1 mM CaCl2, CuCl2, MgSO4, ZnCl2, HgCl2, and MnCl2 in 50 mM Tris-HCl buffer (pH 7.5), respectively, were incubated at 25ºC for 1 h. After that, PSC (1 mg/mL) and CEE (0.2 mg/mL) were incubated in 0.1 M Tris-HCl buffer (pH 8.0) at 40ºC for 8 h. Activity of collagenase was then measured as described in section “Measurement of Collagenase Activity against PSC.” The effect of metal ions on EDTA-inhibited collagenase activity was determined as follows: The CEE was incubated with 5 mM EDTA at 25ºC for 1 h.[Citation25] Then the mixture was incubated with PSC by adding 10 mM metal salts including: CaCl2, MgSO4, ZnCl2, and MnCl2 at 40ºC for 8 h. Activity of collagenase was then measured as described above.
Statistical Analysis
For all above experiments, triplicate experimental data were measured, while each experiment was conducted with three individuals. The data were presented as mean ± standard deviation and analyzed by one-way analysis of variance (ANOVA). Comparison of means was subjected to Fisher’s least significant difference (LSD) test. Significant differences between means were established at the level of p < 0.05. Analysis was performed using SPSS 19.0 package (SPSS Inc., Chicago, IL, USA).
RESULTS
The Fibrillar Structure in the Dermis of S. monotuberculatus
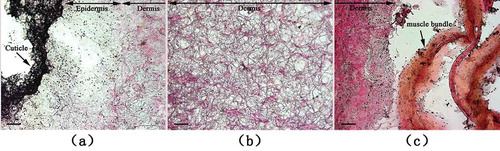
The raw dermis microstructure of S. monotuberculatus was observed by optical microscope after frozen sectioning and Van Gieson staining (). The muscle fibers, collagen fibers, and nucleus were stained yellow, red, and black, respectively, with Van Gieson staining. The result showed that dermis fibers were mainly composed of muscle and collagen fibers. The dermis of S. monotuberculatus could be divided into different layers including cuticle, epidermis, and dermis tissue from external to coelom, of which dermis tissue accounted for the major part. As shown in , the distribution of nucleus in epidermis and dermis tissue on the edge of coelom was more concentrated than in dermis tissue, indicating that there was fewer cellular structure in dermis tissue. The dermis was almost stained red because of collagen fibers, and the structure of fibers was sparse with large space, which endowed dermis of sea cucumber with good flexibility. Meanwhile, longitudinal muscle bundle closing to the edge of interior dermis was stained yellow, implying muscle fibers were the major structure in longitudinal muscle bundle that carried out the stretching function of dermis.
Isolation of Collagen from S. monotuberculatus
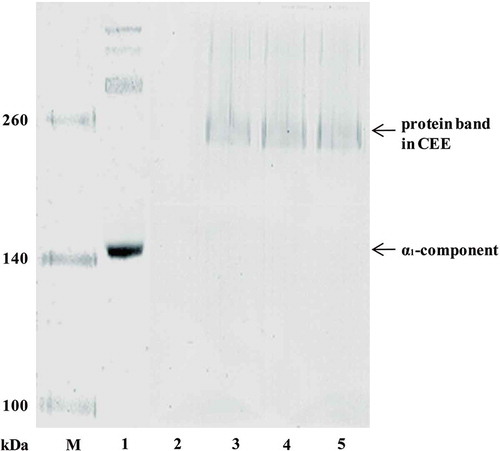
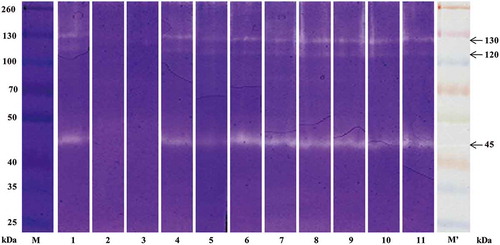
The isolated CCF and PSC of S. monotuberculatus were examined by SDS-PAGE (). There were three main subunit bands including α1, β, and γ subunits in the SDS-PAGE. In this study, the molecular weights of α1 was determined as about 137 by SDS-PAGE separation (). Contents of CCF and PSC were converted by measuring of hydroxyproline content with the method of Bergman and Loxley.[Citation34] Purified CCF and PSC were examined at the concentration of 1 mg/mL with SDS-PAGE (). In this case, however, the subunit bands of CCF were nearly invisible, suggesting CCF (collagen fibers) was insoluble protein with high molecular weight and soluble collagen couldn’t escape from CCF because of covalent crosslinks.
FIGURE 2 SDS-PAGE pattern of CCF and PSC from S. monotuberculatu.
Lane M: protein marker; Lane 1: CCF; Lane 2: PSC.
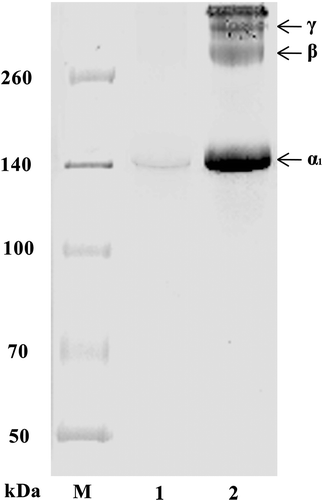
FIGURE 3 Zymograph for the analyses of autoenzyme activity on PSC.
Lane M, protein marker; lane 1: control (without inhibitor); lane 2: 1,10-phenathroline (10 mM); lane 3: EDTA (10 mM); lane 4: PMSF (1 mM); lane 5: TI (0.1 g/l); lane 6: Benzamidine (10 mM); lane 7: DTNB (10 mM); lane 8: Iodoacetic acid (10 mM); lane 9: E-64 (0.01 mM); lane 10: Leupeptin (0.1 mM); lane 11: Pepstatin A (0.1 mM); Lane M’: protein marker before staining.
Identification of Autoenzyme
Since collagen fibers were the main structural protein in the dermis of S. monotuberculatus, the hydrolytic activity of CEE on structural protein could be simulated by collagen zymograph with collagen as substrate. Therefore, our performed collagen zymograph couldn’t only detect the enzymes that hydrolyzed substrate qualitatively, but also obtain the molecular weight of these enzymes quantitatively.
A sample buffer on non-reducing condition was adopted in collagen zymograph of PSC. Results showed that PSC was hydrolyzed by enzymes with molecular weight of 45, 120, and 130 kDa, of which the 45 kDa enzyme had strongest hydrolyzing ability on PSC (). The protease inhibitors, including metalloproteinase, serine proteinase, thiol protease, and aspartic protease inhibitors, were added, respectively, to determine the enzymes with hydrolytic activity.[Citation25,Citation27] The hydrolyzation was inhibited only with metalloproteinase inhibitors, including EDTA and 1,10-phenanthroline, indicating that the hydrolytic enzyme in CEE might be metalloproteinase. In order to further verify, another experiment was conducted as described in section “The Hydrolysis of CCF and PSC by Autoenzyme,” in which α1 subunit of PSC was hydrolyzed by CEE, while α1 subunit of PSC could be prevented from hydrolyzing by adding metalloproteinase inhibitors (). Therefore, with the results above, a kind of collagenase with molecular weight of 45, 120, and 130 kDa in the CEE existed, which could hydrolyze triple-helix of collagen.
FIGURE 4 SDS-PAGE of PSC digested by autoenzyme.
Lane M, protein marker; lane 1: control (without inhibitor); lane 2: 1,10-phenathroline (10 mM); lane 3: EDTA (10 mM); lane 4: PMSF (1 mM); lane 5: TI (0.1 g/l); lane 6: Benzamidine (10 mM); lane 7: DTNB (10 mM); lane 8: Iodoacetic acid (10 mM); lane 9: E-64 (0.01 mM); lane 10: Leupeptin (0.1 mM); lane 11: Pepstatin A (0.1 mM).
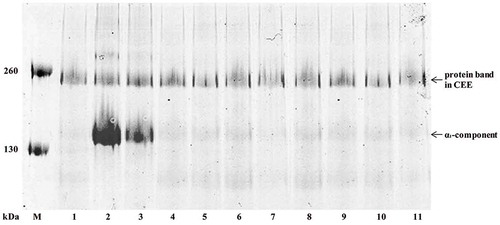
The collagenase could degrade collagen fiber by hydrolyzing triple-helix of collagen, while the enzymes having the ability in hydrolyzing collagen telopeptides could make PSC out from collagen fiber and degrade collagen fiber. In order to identify whether another enzyme was existed in CEE which participated in autolysis of dermis by hydrolyzing collagen telopeptides, an experiment was conducted by section “The Hydrolysis of CCF and PSC by Autoenzyme” with CCF as substrate (). There was no collagen subunit band in SDS-PAGE of CCF because of its insolubility (lane 2 in ), while there was no collagen subunit band in the hydrolyzing product of CCF and CEE yet (lane 4 in ), excepting the protein band in CEE. Since the collagenase in CEE could hydrolyze PSC, 1,10-phenanthroline was added in the reaction of CCF and CEE to prevent the hydrolysis of PSC. However, no collagen subunit band appeared (lane 5 in ), indicating there wasn’t any enzyme existed in CEE which could hydrolyze collagen telopeptides. Therefore, the main ECM autoenzyme in the dermis of S. monotuberculatus was collagenase.
Change of Soluble Protein Content in Autolysis of Dermis
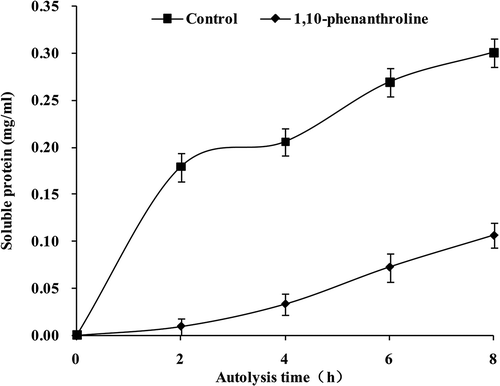
The change of soluble protein content in autolysis of dermis was monitored in this study (). The content of soluble protein was increased gradually to 0.30 mg/mL by incubation of 8 h with absence of 1,10-phenanthroline, while the content was just 0.10 mg/mL with present of 1,10-phenanthroline. As a result, the autolysis of dermis was inhibited by collagenase inhibitor significantly (p < 0.05).
Effect of Temperature and pH on Collagenase Activity
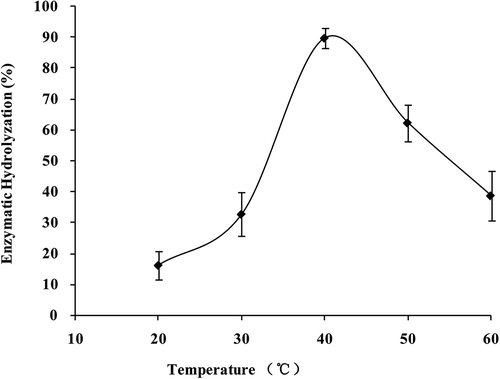
The activity of collagenase was affected by temperature significantly (p < 0.05; ). With temperature increasing, the reaction was accelerated, and on the contrary, the enzyme was denatured. Activity of collagenase was highest at 40ºC with its digestibility of α1 subunit up to 89.74 ± 3.26%. The activity reduced since temperature increased or decreased. Therefore, a parabolic curve was presented in collagenase activity, of which the highest point appeared at 40ºC.
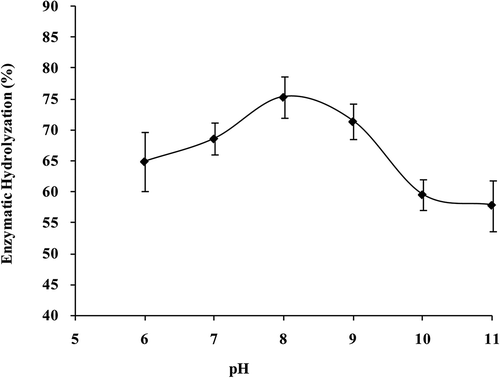
Effect of pH on activity of collagenase was shown in . As the enzyme activity could be greatly influenced by the structure of the enzyme, changing in pH would alter the shape of the enzyme and the active site as well the point where the substrate molecule could no longer fit to, and reaction activated by enzyme would be slowed down subsequently. Collagenase activity was highest at pH 8, with its digestibility of α1 subunit up to 75.36 ± 6.56%. A parabolic curve was also presented in collagenase activity along with the raising of pH from 6 to 11, of which the highest point arised at pH 8.
Influence of Metal Ions on Collagenase Activity
Influence of metal ions on collagenase activity was shown in . Cu2+ and Hg2+ inhibited collagenase activity significantly (p < 0.05), while Ca2+, Mg2+, Zn2+, and Mn2+ induced its activity. Then these four divalent metal ions were added to EDTA-inhibited collagenase (). In this case, Mg2+ and Mn2+ could improve the activity, with significant improvement by Mn2+ (p < 0.05).
TABLE 1 Effect of metal ions on collagenase activity
TABLE 2 Effect of metal ions on activity of EDTA-inhibited collagenase
DISCUSSION
Structure of the Body Wall
The phenomenon of autolysis was ubiquitous in food storage and processing of aquatic animal. Autoenzyme usually damaged the quality of stored fish, shrimp, and shellfish muscle foods, leading to mushiness or autolysis.[Citation35–Citation37] However, the speed of autolysis in sea cucumber was faster than other aquatic animals. The difference in speed of autolysis might be attributed to the structural differences of animals’ edible parts. The edible parts of fish, shrimp, and shellfish were mainly composed of muscle fibers, while the main fibers in sea cucumber were collagen fibers.[Citation14,Citation23Citation24] As autolysis happened, the degree of autolysis between these two fibers existed significant differences. The dermis of S. monotuberculatus included cuticle, epidermis, and dermis tissue, of which the major part was dermis tissue that consisted of collagen fibers (). Besides, since the structure of collagen fibers was sparse with large space, the fluidity of autoenzyme would increase, that accelerated the hydrolyzation of the structure. Therefore, on the basis of structural characteristics, it could be inferred that enzymes degrading collagen fibers of ECM played a key role in autolysis of sea cucumber.
Collagen from the Dermis
It was reported that the collagen of sea cucumber belonged to type I collagen, with the triple helix formed by three homologous α1 chains as (α1)3, while β and γ components of higher molecular weight were dimers and trimers of α1 chain, respectively.[Citation17Citation30] The collagen of S. monotuberculatus was demonstrated as type I collagen by SDS-PAGE, amino acid composition, and complete cleavage by collagenase (data not shown). The triple-helix collagen presented as microfibrillar structure, with the length and diameter of 300 and 1.5 nm, respectively. Many collagens would then pack together side-by-side, forming fibrils with a diameter of 50–400 nm. In fibers, adjacent collagens were displaced from one another by 67 nm, about one-quarter of their length, and a striated effect of fibers could be seen in electron microscope, with a periodicity of about 67 nm called the D-period.[Citation15Citation38] Therefore, collagen fibers could endow tissue with their specific mechanical and biochemical properties, and the covalent crosslinks formed by non-helical telopeptides of adjacent collagens had better stability when fibers were stretched.
As covalent crosslinks formed by non-helical telopeptides of adjacent collagens in fibers, the collagen fibers were hardly soluble. However, with the effect of pepsin on hydrolyzing non-helical telopeptides participating in crosslink, PSC was out from CCF and CCF was gradually degraded.[Citation17Citation30] In this study, SDS-PAGE of CCF and PSC demonstrated the insolubility of CCF, the solubility of PSC, and the solubility of collagen fibers could be improved with the hydrolyzation of telopeptides ().
Identification of Autoenzyme
The autoenzyme in dermis of sea cucumber can hydrolyze insoluble collagen fibers into soluble protein, leading to the phenomenon of autolysis. In this study, CCF and PSC isolated from S. monotuberculatus dermis were served as substrates to investigate the autoenzyme. The results showed that CEE from the dermis could not hydrolyze the telopeptides, but the triple-helix of collagen. The collagenase had hydrolytic activity on type I collagen and could be divided into catalytic domain (about 170 amino acids) and C-terminal hemopexin-like domain (about 210 amino acids) with molecular weight about 45 kDa.[Citation39] In this study, three enzymes of 45, 120, and 130 kDa could hydrolyze PSC, in according with S. japonicas[Citation27] and Holothuria glaberrima[Citation9] that there wasn’t only one enzyme existed to degrade collagen, but the enzyme of 45 kDa had the greatest activity. Besides, the fact that metalloproteinases (MMPs) inhibitor 1,10-phenanthroline prevented the autolysis of dermis significantly (p < 0.05) could confirm the importance of collagenase in autolysis ().
Metalloproteinases (MMPs) can be divided into several sub-types, including collagenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs, and other MMPs, of which only collagenases can hydrolyze the triple-helix of natural collagen. Most MMPs are secreted from the cell as inactive zymogens. The proteolytic activities of MMPs are precisely controlled during activation from their precursors and inhibition by endogenous inhibitors.[Citation39Citation40] As have the function of degrading ECM, MMPs play important roles in embryonic development, morphogenesis, reproduction, tissue resorption, and remodeling.[Citation15Citation39Citation40] In the present study PSC and CCF was performed as substrates to identify dermis autoenzyme, and the result showed that there wasn’t enzyme with hydrolytic activity on collagen telopeptides. Therefore, we considered that collagenase might be the main protease participating in ECM autolysis of S. monotuberculatus dermis.
Activities of Collagenase
Since collagenase was the major protease in autolysis of sea cucumber, it’s valuable to investigate the influence of environmental conditions on collagenase activity. The optimum temperature for collagenase activity in S. monotuberculatus was 40ºC, which was consistent with previous reported in S. japonicas (40ºC),[Citation27] Cyprinus carpio (40ºC),[Citation41] and Pagrus major (40ºC).[Citation42] S. monotuberculatus had its best collagenase activity at pH 8, which was in accord with C. carpio (pH 8),[Citation41] and P. major (pH 8),[Citation42] but lower than S. japonicas (pH 9).[Citation27] Therefore, it could be speculated that collagenase belonged to alkaline protease as previous known, and there was little relativity between its activity and animals’ living environment.
The Zn2+ and Ca2+ are indispensable in catalytic domains of MMPs to maintain the stability and catalytic activity, of which Ca2+ maintains the spatial structure of the enzyme and Zn2+ is necessary for the formation of catalytic domains.[Citation39] In this study, Ca2+, Mg2+, Zn2+ and Mn2+ were found to improve collagenase activity, of which significant improvement was performed by Mn2+ (p < 0.05), indicating that Mn2+ could maintain activity instead of Ca2+ and Zn2+. Researches shown that the gelatinase activity in sea urchin embryo was inhibited by Mg2+ and Zn2+, but activated by Ca2+,[Citation43] while collagenase activity in S. japonicus, could only be activated by Ca2+ and Ba2+.[Citation27] Since collagenase from different animals wasn’t activated uniformly by Zn2+ and Ca2+, therefore, influence of divalent cations on collagenase activity differed from the enzymatic origins.
CONCLUSION
Comparing with the main edible part of other aquatic animals, such as fish, shrimp, or shellfish, the body wall of sea cucumber S. monotuberculatus was mainly formed by collagen fibers, which belonged to dermis tissue indeed. Because of the difference in microstructure of edible part, sea cucumber was much easier to undergo autolysis than other aquatic animals. In this study, CCF and PSC were acting as substrates to identify enzymes from S. monotuberculatus dermis. The results showed that the CEE from S. monotuberculatus dermis didn’t contain the enzyme for hydrolyzing of collagen telopeptides, but the collagenase that could hydrolyze collagen triple-helix. Therefore, collagenase was the main ECM autoenzyme in the dermis of S. monotuberculatus. Then the best environmental conditions for collagenase activity were studied, in which its best activity was at 40°C and pH 8, indicating it was an alkaline metalloprotease. Divalent metal ion Mn2+ was essential for the activity, implying it was a manganese-dependent metalloproteinase. Through this study, the enzyme participating in autolysis of dermis ECM was found, which would be helpful for autolysis research and aquaculture of sea cucumber.
REFERENCES
- Toral-Granda, V.; Lovatelli, A.; Vasconcellos, M. Sea Cucumbers: A Global Review of Fisheries and Trade. SPC Bêche-de-mer Information Bulletin 2008, 28, 4–6.
- Bordbar, S.; Anwar, F.; Saari, N. High-Value Components and Bioactives from Sea Cucumbers for Functional Foods—A Review. Marine Drugs 2011, 9(10), 1761–1805.
- FAO. Fisheries and Aquaculture Information and Statistics Service. Capture Production & Aquaculture production 1950–2011. FISHSTAT Plus–Universal Software for Fishery Statistical Time Series. Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. Retrieved from http://www.fao.org/fishery/statistics/software/fishstat/en
- Qi, H.; Dong, X.; Cong, L.; Gao, Y.; Liu, L.; Mikiro, T.; Zhu, B. Purification and Characterization of a Cysteine-like Protease from the Body Wall of the Sea Cucumber Stichopus Japonicus. Fish Physiology and Biochemistry 2007, 33(2), 181–188.
- Sun, L.; Zhu, B.; Wu, H.; Yu, L.; Zhou, D.; Dong, X.; Yang, J.; Li, D.; Ye, W.; Murata, Y. Purification and Characterization of Cathepsin B from the Gut of the Sea Cucumber (Stichopus Japonicas). Food Science and Biotechnology 2011, 20(4), 919–925.
- Zhu, B.; Yu, J.; Zhang, Z.; Zhou, D.; Yang, J.; Li, D.; Murata, Y. Purification and Partial Characterization of an Acid Phosphatase from the Body Wall of Sea Cucumber Stichopus Japonicus. Process Biochemistry 2009, 44(8), 875–879.
- Zhu, B.; Zhao, L.; Sun, L.; Li, D.; Murata, Y.; Yu, L.; Zhang, L. Purification and Characterization of a Cathepsin L-Like Enzyme from the Body Wall of the Sea Cucumber Stichopus Japonicus. Bioscience Biotechnology and Biochemistry 2008, 72(6), 1430–1437.
- Foegeding, E.; Lanier, T.; Hultin, H. Characteristics of Edible Muscle Tissues. Food Chemistry 1996, 3(15), 879–942.
- Quiñones, J.L.; Rosa, R.; Ruiz, D.L.; Garcı́a-Arrarás, J.E. Extracellular Matrix Remodeling and Metalloproteinase Involvement during Intestine Regeneration in the Sea Cucumber Holothuria Glaberrima. Developmental Biology 2002, 250(1), 181–197.
- Geesink, G.; Morton, J.; Kent, M.; Bickerstaffe, R. Partial Purification and Characterization of Chinook Salmon (Oncorhynchus tshawytscha) Calpains and An Evaluation of Their Role in Postmortem Proteolysis. Journal of Food Science 2000, 65(8), 1318–1324.
- Ladrat, C.; Chaplet, M.; Verrez-Bagnis, V.; Noël, J.; Fleurence, J. Neutral Calcium-Activated Proteases from European Sea Bass (Dicentrarchus Labrax L.) Muscle: Polymorphism and Biochemical Studies. Comparative Biochemistry and Physiology B 2000, 125(1), 83–95.
- Ashie, I.; Smith, J.; Simpson, B.; Haard, N.F. Spoilage and Shelf‐Life Extension of Fresh Fish and Shellfish. Critical Reviews in Food Science and Nutrition 1996, 36(1–2), 87–121.
- Kolodziejska, I.; Sikorski, Z.E. Neutral and Alkaline Muscle Proteases of Marine Fish and Invertebrates—A Review. Journal of Food Biochemistry 1996, 20(3), 349–364.
- Thurmond, F.; Trotter, J. Morphology and Biomechanics of the Microfibrillar Network of Sea Cucumber Dermis. Journal of Experimental Biology 1996, 199(8), 1817–1828.
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, Function, and Biosynthesis. Advanced Drug Delivery Reviews 2003, 55(12), 1531–1546.
- Saito, M.; Kunisaki, N.; Urano, N.; Kimura, S. Collagen as the Major Edible Component of Sea Cucumber (Stichopus Japonicus). Journal of Food Science 2002, 67(4), 1319–1322.
- Liu, Z.; Oliveira, A.C.; Su, Y.-C. Purification and Characterization of Pepsin-Solubilized Collagen from Skin and Connective Tissue of Giant Red Sea Cucumber (Parastichopus Californicus). Journal of Agricultural and Food Chemistry 2010, 58(2), 1270–1274.
- Zhang, Y.; Liu, W.; Li, G.; Shi, B.; Miao, Y.; Wu, X. Isolation and Partial Characterization of Pepsin-Soluble Collagen from the Skin of Grass Carp (Ctenopharyngodon Idella). Food Chemistry 2007, 103(3), 906–912.
- Ando, M.; Yoshimoto, Y.; Inabu, K.; Nakagawa, T.; Makinodan, Y. Post-Mortem Change of Three-Dimensional Structure of Collagen Fibrillar Network in Fish Muscle Pericellular Connective Tissues Corresponding to Post-Mortem Tenderization. Fish Science 1995, 61(2), 327–330.
- Bracho, G.; Haard, N.F. Identification of Two Matrix Metalloproteinases in the Skeletal Muscle of Pacific Rockfish (Sebastes sp.). Journal of Food Biochemistry 1995, 19(4), 299–319.
- Hernández-Herrero, M.M.; Duflos, G.; Malle, P.; Bouquelet, S. Collagenase Activity and Protein Hydrolysis as Related to Spoilage of Iced Cod (Gadus Morhua). Food Research International 2003, 36(2), 141–147.
- Suarez, M.; Abad, M.; Ruiz-Cara, T.; Estrada, J.; García-Gallego, M. Changes in Muscle Collagen Content During Post Mortem Storage of Farmed Sea Bream (Sparus Aurata): Influence on Textural Properties. Aquaculture International 2005, 13(4), 315–325.
- Gao, X.; Zhang, Z.; Liu, L.; Liu, Q. Rheological Changes of Sea Cucumber Stichipus Japonicus During Different Heated Times. International Journal of Fisheries and Aquaculture 2011, 2(14), 258–262.
- Liu, L.; Zhang, Z.; Liu, Q.; Yang, B.; Huang, J.; Gao, X. Rheological and Structural Properties of Sea Cucumber Stichopus Japonicus During Different Heating Temperature. International Journal of Fisheries and Aquaculture 2012, 4(10), 209–216.
- Fu, X.; Xue, C.; Miao, B.; Li, Z.; Gao, X.; Yang, W. Characterization of Proteases from the Digestive Tract of Sea Cucumber (Stichopus Japonicus): High Alkaline Protease Activity. Aquaculture 2005, 246(1), 321–329.
- Sun, L.; Wang, T.; Zhu, B.; Niu, H.; Zhang, R.; Hou, H.; Zhang, G.; Murata, Y. Effect of Matrix Metalloproteinase on Autolysis of Sea Cucumber Stichopus Japonicus. Food Science and Biotechnology 2013, 22(5), 1–3.
- Wu, H.; Hu, Y.; Shen, J.; Cai, Q.; Liu, G.; Su, W.; Cao, M. Identification of a Novel Gelatinolytic Metalloproteinase (GMP) in the Body Wall of Sea Cucumber (Stichopus Japonicus) and Its Involvement in Collagen Degradation. Process Biochemistry 2013, 48(5), 871–877.
- Bruckner, A.; Johnson, K.; Field, J. Conservation Strategies for Sea Cucumbers: Can a CITES Appendix II listing Promote Sustainable International Trade. SPC Bêche-de-mer information Bulletin 2003, 18, 24–33.
- Hu, C.; Xu, Y.; Wen, J.; Zhang, L.; Fan, S.; Su, T. Larval Development and Juvenile Growth of the Sea Cucumber Stichopus sp. (Curry Fish). Aquaculture 2010, 300(1), 73–79.
- Cui, F.; Xue, C.; Li, Z.; Zhang, Y.; Dong, P.; Fu, X.; Gao, X. Characterization and Subunit Composition of Collagen from the Body Wall of Sea Cucumber Stichopus Japonicus. Food Chemistry 2007, 100(3), 1120–1125.
- Krieg, R.C.; Dong, Y.; Schwamborn, K.; Knuechel, R. Protein Quantification and Its Tolerance for Different Interfering Reagents using the BCA-Method with Regard to 2D SDS PAGE. Journal of Biochemical and Biophysical Methods 2005, 65(1), 13–19.
- Laemmli, U.K. Cleavage of Structural Proteins During the Assembly of the Head of Bacteriophage T4. Nature 1970, 227(5259), 680–685.
- Sulkala, M.; Tervahartiala, T.; Sorsa, T.; Larmas, M.; Salo, T.; Tjäderhane, L. Matrix Metalloproteinase-8 (MMP-8) is the Major Collagenase in Human Dentin. Archives of Oral Biology 2007, 52(2), 121–127.
- Bergman, I.; Loxley, R. Two Improved and Simplified Methods for the Spectrophotometric Determination of Hydroxyproline. Analytical Chemistry 1963, 35(12), 1961–1965.
- Pornrat, S.; Sumate, T.; Rommanee, S.; Sumolaya, K.; Kerr, W. Changes in the Ultrastructure and Texture of Prawn Muscle (Macrobrachuim Rosenbergii) During Cold Storage. LWT–Food Science and Technology 2007, 40(10), 1747–1754.
- Li, K.; Luo, Y.; Shen, H. Postmortem Changes of Crucian Carp (Carassius Auratus) During Storage in Ice. International Journal of Food Properties 2015, 18(1), 205–212.
- Sáez, M.I.; Suárez, M.D.; Cárdenas, S.; Martínez, T.F. Freezing and Freezing-Thawing Cycles on Textural and Biochemical Changes of Meagre (Argyrosomus Regius, L) Fillets During Further Cold Storage. International Journal of Food Properties 2015, 18(8), 1635–1647.
- Lodish, H.; Berk, A.; Kaiser, C.A. Molecular Cell Biology; W. H. Freeman and Company: New York, NY, 2012.
- Nagase, H.; Woessner, J.F. Matrix Metalloproteinases. Journal of Biological Chemistry 1999, 274(31), 21491–21494.
- Visse, R.; Nagase, H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases Structure, Function, and Biochemistry. Circulation Research 2003, 92(8), 827–839.
- Wu, J.; Lu, B.; Du, M.; Liu, G.; Hara, K.; Su, W.; Cao, M. Purification and Characterization of Gelatinase-Like Proteinases from the Dark Muscle of Common Carp (Cyprinus Carpio). Journal of Agricultural and Food Chemistry 2008, 56(6), 2216–2222.
- Wu, G.; Cao, M.; Chen, S.; Weng, W.; Cai, Q.; Su, W. Purification and Characterization of a Gelatinolytic Metalloproteinase from the Skeletal Muscle of Red Sea Bream (Pagrus Major). Journal of Agricultural and Food Chemistry 2010, 58(9), 5730–5736.
- Robinson, J.J. Characterization of a Metalloproteinase: A Late Stage Specific Gelatinase Activity in the Sea Urchin Embryo. Journal of Cellular Biochemistry 1997, 66(3), 337–345.