Abstract
We assessed extracts from Psidium brownianum for antifungal activity and identified the phenolic phytocompounds. Minimum inhibitory concentration was determined by microdilution and IC50 was calculated. The minimun fungicidal concentration and the morphology of Candida were evaluated. Extracts analyzed by high-performance liquid chromatography demonstrated flavonoids and phenolic acids. The minimum inhibitory concentration was 8192 µg/mL and the IC50 varied between 1056 and 5128 µg/mL. Extracts showed fungistatic effect and altered the dimorphism of the strains, being the better result observed using the decoction, that affected the fungal dimorphism of the strain CA ATCC40006 at 4096 µg/mL.
INTRODUCTION
Infections caused by Candida species have been considered to be one of the biggest problems related to human fungal pathogens. The great and severe impact of the pathogenesis is related to the impairment of the immune system and the use of certain drugs that allow the emergence of invasive candidiasis, as for example, antibiotics against anaerobic microorganisms.[Citation1] Candida albicans is a commensal polymorphic fungus and part of the human microbiota[Citation2] with the highest prevalence of isolation either in healthy or diseased organisms.[Citation3] Regarded as a harmless commensal microbe, it may change status, becoming, in favorable conditions, an opportunistic pathogen capable of causing common clinical situations, such as thrush and vaginitis, to severe life-threatening infections, such as systemic infection known as candidemia.[Citation4]
A similar behavior is found in the fungus Candida tropicalis, which belongs to the same family (Saccharomycetaceae—Ascomycete) and same clade (CUG) as C. albicans.[Citation5] This fungus is responsible for high mortality rates ranging from 40 to 70%,[Citation6] where there are reports stating that in the intestine of cancer patients, it is much more invasive than C. albicans.[Citation7] Drug resistance of these microorganisms has been determined and often reported to the point that many classes of antifungals have already been produced. The arsenal of drugs is designed to neutralize the virulence factors expressed by fungi as well as resistance mechanisms additivity or even multiple mechanisms.[Citation8–Citation10]
Some studies have sought alternative sources of compounds able to neutralize virulence factors and mechanisms of fungal resistance, such as those investigating natural products (whether from plants or animals or other organisms) and their bioactive potential against fungi.[Citation11Citation12] However, many of them have been unable to explain the mechanisms by which these products act. Plants of the genus Psidium have been studied with regard to antifungal activity, in particular the species Psidium guajava.[Citation13] Besides them, Psidium sartorianum,[Citation14] Psidium acutangulum,[Citation15] and Psidium guineense Sw[Citation16] have also been evaluated. Psidium brownianum Mart. ex DC. Occurs as a shrub or tree, reaching up to 0.5 to 8 m. It is a glabrous plant with leathery leaves and usually short petiole. Its flowers are white, and the fruit can vary between elliptical and striated and globose and grooved.[Citation17Citation18] It can often be found in regenerating areas, and its presence has been recorded in Northeast and Southeast Brazil, inhabiting different geographic areas, such as Caatinga, Cerrado, and Atlantic Forest.[Citation18] The ethnobotanical use of P. brownianum has been observed in some places as food (fruit) or medicinal purposes (sprouts), for example, for the treatment of flu.[Citation19] To date, there has been no report on biological activity of this species. The aim of this study was to evaluate the antifungal activity of extracts of P. brownianum, comparing the kinds of extracts by their methods of preparation (decoction, infusin, and hydroethanolic), efficacy and effect against the fungal morphology.
MATERIAL AND METHODS
Collection Area
The area is characterized as an enclave of Cerrado sensu stricto[Citation20] it is located in northeast Chapada do Araripe (7° 21.685’ S and 39° 28.605’ W, at 907 m asl; 7° 21.793’ S and 39° 28.605’ W, at 902 m asl; 7° 21.787’ S and 39° 28.558’ W, at 906 m asl) municipality of Crato, Ceara, in Northeast Brazil (). Chapada do Araripe is situated at the border of the states of Ceará, Piauí, and Pernambuco. It is a plateau, with a maximum altitude of 1000 m and minimum of 700 m. The predominant soil type is dystrophic red latosol.[Citation21] The average annual precipitation is about 760 mm, concentrated between the months of January and April (66.3%), and the average annual temperature is 24.1°C.[Citation22]
Plant Material
The study was conducted using young, healthy leaves of a Psidium species locally known as araçá de veado, which were collected and transported to the Laboratory of Microbiology and Molecular Biology at the Regional University of Cariri—URCA. Twigs with flowers of the species were also collected and vouchers were produced and deposited in the Herbarium Dárdano de Andrade Lima at the university under No. 10161, where the species was identified as Psidium brownianum DC. The collection period included January, February, March, and April, known as the “wintry block of the Cariri Ceara region.” Collections were made between 8:30 and 10:30 am, and the plant material was taken to the laboratory. Leaves uncontaminated by parasites were washed and dried before being weighted and stored under refrigeration. Altogether, there were 2866 kg of leaves in perfect condition, and this quantity was divided for preparation of three types of extracts: hydroethanolic extract (70%)—EHPB, aqueous extract by decoction—AEPBD and water extract by infusion—AEPBI.
Preparation of Extracts
Aqueous extracts
Two types of aqueous extracts with natural tap water were prepared, each using 399.9 g of leaves mixed with 6 L of water (based on a proportion of 10 g/150 mL, equivalent to one cup of tea—150 mL). The decoction was made by mixing roughly cut leaves in cold water and then boiling for 15 min. Afterward, the tea was allowed to cool (4 h and 45 min), filtered and then stored under refrigeration. As for the infusion, the water was boiled without leaves, which were placed in the water after turning off the heat. The pot was covered with a lid and allowed to stand for 4 h 45 min until the tea cooled down,[Citation23] and the preparation was then filtered and stored under refrigeration. Infusion and decoction were frozen (–60°C) and lyophilized to dryness. The powdered extracts were stored under refrigeration for testing, using 24.9 and 23.3 g extract powder from the decoction and infusion, respectively.
EHPB
The EHPB (70%) was prepared by trituration with cold extraction, using a total of 2 kg leaves in a proportion of 5 g/mL of hydroethanol solution.[Citation23] The leaves were cut to increase contact surface with the solvent, and the mixture was left at room temperature protected from air and light, for a period of 96 h to for maximum extraction efficiency. The mixture was then filtered and placed in a rotary evaporator (Q-344B—Quimis—Brazil) at 40 rpm and 60°C to concentrate the extract. Finally, the crude extract was frozen, lyophilized (568 g) and then stored under refrigeration.
Chemical Analysis
Chemical, apparatus, and general procedures
All chemicals were of analytical grade. Methanol, acetic acid, gallic acid, caffeic acid, ellagic acid, and chlorogenic acid were purchased from Merck (Darmstadt, Germany). Quercetin, quercitrin, rutin, kaempferol, luteolin, catechin, and coumarin were acquired from Sigma Chemical Co. (St. Louis, MO, USA). High-performance liquid chromatography–diode array detector (HPLC-DAD) was performed with a Shimadzu Prominence Auto Sampler (SIL-20A) HPLC system (Shimadzu, Kyoto, Japan), equipped with Shimadzu LC-20AT reciprocating pumps connected to a DGU 20A5 degasser with a CBM 20A integrator, SPD-M20A DAD, and LC solution 1.22 SP1 software.
HPLC-DAD
Reverse-phase chromatographic analyses were carried out under gradient conditions using C18 column (4.6 mm × 250 mm) packed with 5 μm diameter particles; the mobile phase was water containing 2% formic acid (A) and acetonitrile (B), and the composition gradient was: 17% of B until 10 min and changed to obtain 20, 30, 50, 60, 70, 20, and 10% B at 20, 30, 40, 50, 60, 70, and 80 min, respectively, following the method described[Citation24] with slight modifications. The extracts solutions of P. brownianum (hidroethanolic—EHPB, infusion—AEPBI and decoction—AEPBD) were prepared at 20 mg/mL and the mobile phase were filtered through 0.45 μm membrane filter (Millipore) and then degassed by ultrasonic bath prior to use. The flow rate was 0.7 mL/min and the injection volume was 50 μL. Stock solutions of standards references were prepared in water: acetonitrile (1:1; v/v) at a concentration range of 0.025–0.250 mg/mL catechin, coumarin, quercetin, quercitrin, kaempferol, luteolin, and rutin, and 0.035–0.350 mg/mL for gallic, caffeic, ellagic, and chlorogenic acids. Quantification was carried out by integration of the peaks using the external standard method, at 270 nm for gallic acid and coumarin, 281 nm for catechin, 327 nm for chlorogenic, ellagic, and caffeic acids, and 366 for quercetin, quercitrin, luteolin, kaempferol, and rutin. The chromatography peaks were confirmed by comparing its retention time with those of reference standards and by DAD spectra (200 to 600 nm). Calibration curve for gallic acid: Y = 13682x + 1284.5 (r = 0.9999); catechin: Y = 11956x + 1260.3 (r = 0.9995); caffeic acid: Y = 11943x + 1198.7 (r = 0.9998); chlorogenic acid: Y = 12601x + 1327.1 (r = 0.9994); ellagic acid: Y = 13075x + 1283.9 (r = 0.9997); rutin: Y = 12853x + 1186.7 (r = 0.9996); quercetin: Y = 11968x + 1273.9 (r = 0.9998); quercitrin: Y = 12658x + 1249.7 (r = 0.9995), coumarin: Y = 13159x + 1358.2 (r = 0.9990), kaempferol: Y = 11983x + 1275.8 (r = 0.9999), and luteolin: Y = 12496x + 1195.4 (r = 0.9997). All chromatography operations were carried out at ambient temperature and in triplicate.
Limit of Detection (LOD) and Limit of Quantification (LOQ)
LOD and LOQ were calculated based on the standard deviation of the responses and the slope using three independent analytical curves.[Citation25] LOD and LOQ were calculated as 3.3 and 10 σ/S, respectively, where σ is the standard deviation of the response and S is the slope of the calibration curve.
Antifungal Assay
Strains and culture media used
Standard types of strains were obtained from the Culture Collection of Oswaldo Cruz of the Brazilian Institute of Quality Control in Health (INCQS) and clinical isolates of the yeasts Candida albicans and Candida tropicalis were provided by Dr. Edeltrudes Oliveira Lima (Mycology Laboratory of Paraíba Federal University), namely CA INCQS 40006, CA LM 62, CA LM 77, CA LM 109, CA LM 111, CA LM 122, CT INCQS 40042, CT LM 18, CT LM 20, and CT LM 23. These strains were inoculated into Sabouraud dextrose agar (SDA, KASVI) and incubated for 24 h at 37°C. Afterward, small aliquots of yeast were transferred to test tubes each containing 3 mL of sterile saline (0.9%). The concentration of the inoculum was standardized by 0.5 McFarland, giving a standard yeast suspension of 1 × 105 cells/mL.[Citation26] The inocula thus prepared were used to determine the minimum inhibitory concentration (MIC) in Sabouraud dextrose broth (SDB, HIMEDIA), double concentrated. Another culture medium was used for analysis of yeast micromorphology. The potato dextrose agar (PDA, DIFCO) was prepared by diluting it more than that recommended by the manufacturer to make it a depleted medium capable of stimulating yeast to produce hyphae. Agar was added to this diluted medium to obtain a solid medium.
Drugs, reagents, and preparation of solutions
Dimethyl sulfoxide (DMSO, Merck, Darmstadt, Germany) was used for dilution of the extracts, and the antifungal fluconazole (Capsule—FLUCOMED), diluted in water, was used as the reference drug. The matrix solutions of the extracts were prepared by weighing 0.3 g of each extract and then diluting in 1 mL of DMSO. To obtain the desired concentration for testing, the extracts were further diluted in sterile distilled water so that the concentration of DMSO in the natural product did not exert any activity in the test cells.[Citation27]
Microbiological Screening
Microbiological screening was performed to select the yeasts to be used in microbiological testing. The microdilution broth was chosen to perform this procedure, and this was done by determining the MIC.[Citation28] The plates prepared to carry out this test would be used later in tests to find the minimum fungicidal concentration (MFC), besides facilitating the demonstration of cell viability curve and calculating the IC50 of the test products.
Determination of MIC
This test was performed by the broth microdilution method in 96-well plates. Each well was filled with 100 µL of SDB containing 10% fungal inoculum, and then, 100 µL of the natural product (16384 µg/mL) or fluconazole (antifungal reference) at the same concentration, were added to the first well, followed by twofold serial dilution. The concentrations in the wells ranged from 64 to 8192 µg/mL. The last well contained no extract or drug and served as the normal growth control.[Citation28] Controls for diluent of the products (using saline instead of inoculum) and the sterile medium were also prepared. All tests were performed in triplicate. The plates were incubated at 37°C for 24 h and afterward read in an enzyme linked immuno sorbent assay (ELISA) spectrophotometer (Thermoplate®) at a wavelength of 630 nm. The MIC was defined as “the lowest concentration of an antimicrobial agent that inhibit the visible growth of a microorganism in dilution assays.”[Citation26] The results obtained in the ELISA readout were used to construct the cell viability curve and the IC50 of the extracts of P. brownianum.
Determination of MFC
For this test, a small sterile rod was placed in each well of the MIC test plate (except for sterility control). After mixing the medium in each well, the rod was taken to a large petri dish containing SDA, streaking its surface and transferring the solution (medium + inoculum + natural product) for subculture of yeast and checking cell viability. After 24 h incubation, the plates were inspected for any formation of colonies of Candida[Citation29] (with modifications). The concentration at which there was no growth of fungal colonies was considered the MFC of the natural product.
Effect of Natural Products on Fungal Morphology
To determine if the natural product caused any change in fungal morphology, by inhibiting the development of hyphae, sterile micromorphological chamber slides were prepared for observation of yeasts. Three milliliters of PDA medium depleted by dilution were added to chambers, containing the natural product concentrations MIC/2, MIC and MIC × 2. Aliquots of the inoculi were taken from the petri dishes to make two parallel streaks on the solid medium, which were then covered with a sterile coverslip. The chambers were placed in the incubator for 24 h (37°C) and inspected under a light microscope using a 40× objective. A camera was attached to the microscope to capture images randomly at 5× zoom. A control for yeast growth (hyphae stimulated by depleting medium) was performed, as well as a control with the conventional antifungal fluconazole for comparative purposes and a control with DMSO at 100 and 0.5% (the concentration in the natural products used in the tests with some modifications).[Citation30Citation31]
Statistical Analysis
The results of the tests were done in triplicate. Data obtained for each sample and concentration were checked for their normal distribution and then analyzed by one-way ANOVA by post-hoc Tukey test. EC50 values were obtained by non-linear regression for the purpose of interpolating values from standard curves (using the software Graphpad Prism, v. 5.0) of the percentage growth values plotted against concentration and EC50 values are expressed as μg/mL.
RESULTS AND DISCUSSION
HPLC was used to analyze the chemical composition of the P. brownianum extracts, which detected and quantified the phenolic compounds present. However, as it can be seen in , the HPLC profile, the HPLC profile showed other minor compounds in addition to gallic acid (retention time-tR 9.98 min, peak 1), catechin (tR = 14.35 min, peak 2), chlorogenic acid (tR = 22.03 min, peak 3), caffeic acid (tR = 25.11 min, peak 4), ellagic (tR = 33.07 min, peak 5), rutin (tR = 38.49 min, peak 6), quercitrin (tR = 47.68 min, peak 7), quercetin (tR = 49.73 min, peak 8), coumarin (tR = 53.81 min, peak 9), kaempferol (tR = 55.78 min, peak 10), and luteolin (tR = 61.94 min, peak 11). The findings for each extract are shown in , with the peaks confirmed by chromatography, and the amounts for each chemical constituent are given in , demonstrating the presence of flavonoids and phenolic acids.
TABLE 1 Phenolics and flavonoids composition of Psidium brownianum
FIGURE 2 High-performance liquid chromatography phenolics and flavonoids profile of Psidium brownianum.
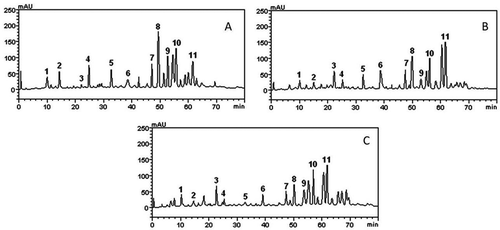
The different times of exposure to temperature of the aqueous (decoction and infusion) extracts resulted in the extraction of compounds in different amounts, where the decoction showed slightly higher amounts than the infusion with little significant difference. However, the decreasing order of the major constituents also varied: decoction: luteolin > quercetin > kaempferol > quercitrin > rutin; and infusion: luteolin > kaempferol > quercetin > chlorogenic acid > quercitrin acid. The results for the EHPB of the species showed the five major ones in the following order: quercetin > kaempferol > coumarin > quercitrin > luteolin. In terms of the amount of phenolic compounds extracted from 20 mg of each extract, the order was EHPB > AEPBD > AEPBI, showing that the EHPB was richer in these phytochemicals ().
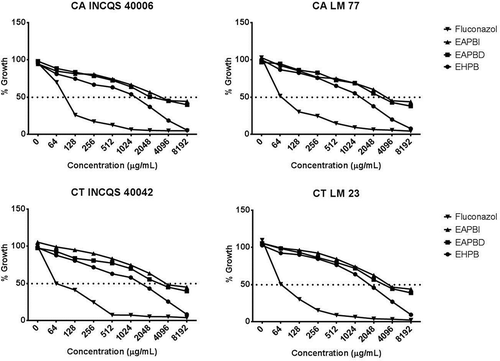
The antimicrobial screening revealed that a minimum concentration of the extract able to inhibit the growth of the strains was the same (8192 µg/mL), and thus, the selection of the strains was performed randomly. For continuity of the work, we selected the yeast strains INCQS CA 40006, CA LM 77, INCQS 400042 CT, and CT LM 23. Therefore, for comparative purposes, a standard strain and a clinical strain isolated from each species were used. Fluconazole was used as the control, which, as expected, had a lower IC50 (58.63 to 76.72 µg/mL) compared to the extracts (1056.82 to 5128.61 µg/mL; ). In this context, extracts of P. brownianum demonstrated antifungal potential due the fact of inhibit the virulence mechanism of morphogenesis. So, these extracts can be used against the candidiasis, but not against systemic infections due the high doses required for this activity.
TABLE 2 IC50 of all products assayed (µg/mL)
The IC50 was calculated for each test product using the ELISA readings, where the IC50 values differed but were generally high, ranging from 1056.82 to 5128.61 µg/mL (). A graph demonstrating the effect on cell viability of the selected yeasts is presented in . It is important to note that the type of solvent and extraction method did not influence the results obtained from the MIC and MFC tests. Also, the determination of the potential to inhibit the transition of yeasts was not affected, since aqueous extracts (obtained using different times of exposure to heat) and EHPBs showed the same effect at the same concentrations tested.
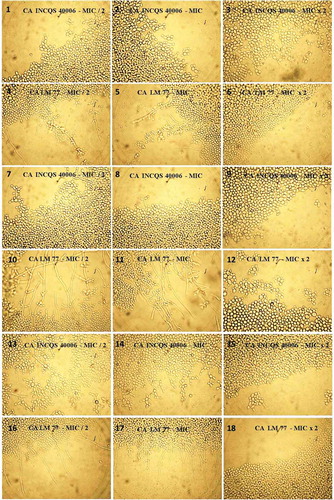
To determine the concentration of the test products necessary to exert a fungicidal effect, tested concentrations ranged from 64 to 8192 µg/mL and after 24 h incubation, fungal growth was observed in all cases, showing that the minimum fungicidal concentration was higher than 8192 µg/mL. DMSO (100%) was found to have an effect on the morphology of Candida yeasts (data not shown), but at the concentration at which the products were diluted for testing, this activity was absent, as can be seen in .
FIGURE 4 Controls used in micromorphologic assay of Candida yeast under the effect of the products of Psidium brownianum.

Fluconazole, a drug used as a control in testing, is a drug that acts on the fungal membrane, causing loss of integrity.[Citation32] It showed an inhibitory effect on hyphae at a lower concentration compared to the products tested, as can be seen in . This effect was expected, since the MIC of this antifungal was well below the values expressed by the products tested. The extracts tested, at the highest concentration (16384 µg/mL), prevented the morphological transition of yeasts, with the exception of strain CA 40006, which was affected by MIC/2 (4096 µg/mL) of decoctio, with progressive inhibition at higher concentrations (8192 and 16384 µg/mL; and ). The extracts probably influenced the genetic and biochemical processes occurring in the cell wall of yeast, disrupting the formation of pseudohyphae and hyphae and thereby affecting one of the factors responsible for the virulence of the species, the ability to invade substrates. The activity of phenolic compounds against Candida has been reported in some studies,[Citation33–Citation36] also demonstrating a concentration-dependent effect.[Citation37] The extracts evaluated here just started to show some antifungal activity when the concentration of phenolic compounds in the extract gradually increased as demonstrated in the cell viability curves.
Micromorphological analysis could indicate a possible mechanism of action of the tested products, because it allows the observation of changes in morphology of the strains. The formation of hyphae and pseudohyphae, observable in a micromorphology assay are important virulence factors in the development of candidiasis.[Citation38] The morphological transition between the yeast and hyphal forms of Candida, that is, dimorphism, is one of the pathogenicity mechanisms appearing as a fitness attribute in superficial and systemic infections, where yeast and hyphae have spreading and invasive potential, respectively.[Citation2] Morphological changes in Candida are stimulated by various environmental factors, and thus, changes in pH, CO2, temperature, depletion of nutrients, serum or presence of N-acetylglucosamine, as well as quorum sensing mechanisms, can promote the formation of hyphae.[Citation2] This hyphal growth depends on the expression of various genes of cell wall proteins, transduction pathways, transcription factors, down regulation, activation of cyclic nucleotide-dependent protein kinase, phosphorylation reactions, and some ribosomal proteins.[Citation39] The adhesion of the hyphae to the surface at the time of invasion, is favored by a set of proteins, adhesins.[Citation2]
In our assays, nutrient depletion was the factor that triggered the development of hyphae. In the presence of depleted medium, the fungi responded to the stress condition by activating the genetic and biochemical processes necessary for the formation of pseudohyphae and hyphae. shows the growth of strains in depleted medium. A previous study found that the flavonoid extract from multifloral honey containing luteolin was able to inhibit the dimorphic conversion of C. albicans and that this effect could be related to the inhibition of the production of reactive oxygen species and changes in cellular levels of intracellular glutathione, both factors that significantly influence the conversion of yeasts to hyphae.[Citation37] Also, aimed at elucidating the probable mechanism of action of the effect of this extract on yeast transition, another study found that the inhibitory activity of the flavonoid extract of honey could be attributed to changes in cell cycle progression, membrane integrity, mitochondrial function, and also biogenesis.[Citation40]
Methanolic extract of Leiothrix spiralis and luteolin were effective against Candida species; however, only the extract interfered with formation of hyphae, indicating that effect on inhibition of hyphae cannot be attributed to flavonoid luteolin alone, but it was probably due to phenolic compounds presents in methanolic extract.[Citation41] Therefore, considering the potential of phenolic compounds for inhibiting the dimorphic transition process in yeast and their existence in extracts of P. brownianum, we can assume that the effect exhibited by extracts was due to these phytochemicals through a single contribution (except for luteolin) or in a synergistic fashion. Luteolin is the major compound in the aqueous extracts, and the fact that its presence did not interfere in the extracts’ effect on yeast morphology suggests that the inhibitory effect was due to the activity of other compounds or to their synergistic action. The possibility of luteolin being involved in this possible synergistic process cannot be ruled out.
In the ether extract of Italian multifloral honey, flavonoids including luteolin, quercetin, apigenin, kaempferol and isorhamnetin were found. The extract presented a better effect than components separately assayed, suggesting that synergism between phenolic compounds is an important factor in natural products exerting an antimicrobial effect.[Citation42] Finally, given the above, this study showed low antimicrobial activity of P. brownianum against Candida. However, in relation to mucocutaneous fungal infections caused by Candida,[Citation43] the topical use of this natural product can be demonstrated as a local inhibitor of fungal virulence, hindering morphogenesis and the invasion of tissues. Our results did not focus on a direct clinical indication, as many complementary tests are needed, such as toxicity tests.
CONCLUSION
In this study, it was found that the mechanism of action by which the extracts of P. brownianum act is by altering capacity for morphological transition in yeasts, preventing the conversion from yeast to hyphae and pseudohyphae. The complete elucidation of this mechanism needs further study. The antifungal potential observed is presumably attributed to phenolic compounds found in the extracts (flavonoids and phenolic acids), whose levels did not significantly differ between the extracts, and their possible synergistic effect. However, more specific studies are needed to reach a definitive conclusion. This is the first report of biological activity for this species, and the results reveal that P. brownianum is a source of phytochemicals able to inhibit the morphological transition of strains of C. albicans and C. tropicalis, neutralizing one of their major virulence factors, the ability to invade substrates and consequently tissues. This effect is potentially promising, but more studies are needed for the possible development of drugs for topical use.
FUNDING
The authors are grateful to the Brazilian Research agencies: CNPq, CAPES, and FUNCAP for the financial and grant support.
REFERENCES
- Kriengkauykiat, J.; Ito, J.I.; Dadwal, S.S. Epidemiology and Treatment Approaches in Management of Invasive Fungal Infections. Clinical Epidemiology 2011, 3, 175–191.
- Mayer, F.L.; Wilson, D.; Hube, B. Candida Albicans Pathogenicity Mechanisms. Virulence 2013, 4, 119–128.
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida Glabrata, Candida Parapsilosis, and Candida Tropicalis: Biology, Epidemiology, Pathogenicity, and Antifungal Resistance. FEMS Microbiology Reviews 2012, 36, 288–305.
- Fazly, A.; Jain, C.; Dehner, A.C.; Issi, L.; Lilly, E.A.; Ali, A.; Cao, H.; Fidel Jr., P.L.; Rao, R.P.; Kaufman, P.D. Chemical Screening Identifies Filastatin, a Small Molecule Inhibitor of Candida Albicans Adhesion, Morphogenesis, and Pathogenesis, Proceedings of the National Academy of Sciences 2013, 110, 13594–13599.
- Kim, J.; Sudbery, P. Candida Albicans, a Major Human Fungal Pathogen. The Journal of Microbiology 2011, 49, 171–177.
- Chen, Y.L.; Yu, S.J.; Huang, H.Y.; Chang, Y.L.; Lehman, V.N.; Silao, F.G.S.; Bigol, U.G.; Bungay, A.A.C.; Averette, A.; Heitman, J. Calcineurin Controls Hyphal Growth, Virulence, and Drug Tolerance of Candida Tropicalis. Eukaryotic Cell 2014, 13, 844–854.
- Kothavade, R.J.; Kura, M.M.; Valand, A.G.; Panthaki, M.H. Candida Tropicalis: Its Prevalence, Pathogenicity, and Increasing Resistance to Fluconazole. Journal of Medical Microbiology 2010, 59, 873–880.
- Jiang, C.; Dong, D.; Yu, B.; Cai, G.; Wang, X.; Ji, Y.; Peng, Y. Mechanisms of Azole Resistance in 52 Clinical Isolates of Candida Tropicalis in China. Journal of Antimicrobial Chemotherapy 2013, 68, 778–785.
- Pfaller, M.A. Antifungal Drug Resistance: Mechanisms, Epidemiology, and Consequences for Treatment. The American Journal of Medicine 2012, 125, S3–S13.
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Giannini, M.J.S. Candida Species: Current Epidemiology, Pathogenicity, Biofilm Formation, Natural Antifungal Products, and New Therapeutic Options. Journal of Medical Microbiology 2013, 62, 10–24.
- Giordani, C.; Santin, R.; Cleff, M. Survey of Plant Extracts with Anti-Candida Activity in the 2005–2013 Period. Revista Brasileira de Plantas Medicinais 2015, 17, 175–185.
- Chen, M.; Zhai, L.; Arendrup, M.C. In Vitro Activity of 23 Tea Extractions and Epigallocatechin Gallate Against Candida Species. Medical Mycology 2015, 53, 194–198.
- Suwanmanee, S.; Kitisin, T.; Luplertlop, N. In Vitro Screening of 10 Edible Thai Plants for Potential Antifungal Properties. Evidence-Based Complementary and Alternative Medicine 2014, 1–7.
- Camacho-Hernández, I.L.; Cisneros-Rodríguez, C.; Uribe-Beltrán, M.J.; Ríos-Morgan, A.; Delgado-Vargas, F. Antifungal Activity of Fruit Pulp Extract from Psidium Sartorianum. Fitoterapia 2004, 75, 401–404.
- Wen, L.; Haddad, M.; Fernández, I.; Espinoza, G.; Ruiz, C.; Neyra, E.; Bustamante B.; Rojas, R. Antifungal activity of four plants used in traditional Peruvian medicine. Isolation of 3′-formyl - 2′, 4′, 6′- trihidroxidihidrochalcona, Psidium acutangulum active ingrediente (in Spanish). Revista da Sociedade Chemistry Peru 2011, 77, 199–204.
- Lapenna, E.A.M.; Ramírez, G.E.M.; Díaz, L.; Aguillón, K.; Marín, H. Bactericidal and fungicidal activity of some Venezuelan plants used in traditional medicine (in Spanish). Journal of the National Institute of Hygiene Rafael Rangel 2003, 34, 6–9.
- Oliveira, A.G. de. Myrtaceae diversity of salt marshes of the Conceição da Barra and São Mateus, the Conceição da Barra and São Mateus, Espirito Santo, Brazil (in Portuguese). Masters dissertation. Post-graduate program in Botany. National School of Tropical Botany. Institute of Research Botanical Garden of Rio de Janeiro. 2013.
- Sobral, M.; Proença, C.; Souza, M.; Mazine, F.; Lucas, E. Myrtaceae in Species List flora of Brazil (in Portuguese). Botanical Garden of Rio de Janeiro. Retrieved from http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB171
- De Jesus, M.C.F. Survey of the species of “restinga” used in Pontal do Ipiranga communities and Degredo, Linhares, ES (in Portuguese). Dissertation in Tropical Biodiversity. Department of Agricultural and Biological Sciences, Federal University of Espirito Santo, São Mateus. 2012.
- Ribeiro, J.F.; Walter, B.M.T. Fitofisionomias do Bioma Cerrado. In The Cerrado vegetation types. In Cerrado: Environment and Flora (in Portuguese); Sano, S.M.; Almeida, S.P.; Eds.; EMBRAPA-CPAC: Brasília, 1998; 89–166 pp.
- Brasil. Exploratory-map Recognition Solos: State of Ceara, scale 1: 600,000 (in Portuguese). Superintendência de Desenvolvimento do Nordeste (SUDENE), 1972.
- Costa, I.R.; Araújo, F.S.; Lima-Verde, L.W. Flora and autoecological aspects of an enclave of Cerrado in the Araripe, Northeast Brazil (in Portuguese). Acta Botanica Brasilica 2004, 18, 759–770.
- Matos, F.J.A. Live pharmacies, 4th Ed; Publisher UFC: Fortaleza (in Portuguese), 2002; 36–40 pp.
- Kamdem, J.P.; Olalekan, E.O.; Hassan, W.; Kade, J.; Yetunde, O.; Boligon, A.A. Trichilia Catigua (Catuaba) Bark Extract Exerts Neuroprotection Against Oxidative Stress Induced by Different Neurotoxic Agents in Rathippocampal Slices. Industrial Crops and Products 2013, 50, 625–632.
- Silva, A.R.H.; Moreira, L.R.; Brum, E.S.; Freitas, M.L.; Boligon, A.A.; Margareth, L.A.; Roman, S.S.; Mazzanti, C.M.; Brandão, R. Biochemical and Hematological Effects of Acute and Sub-Acute Administration to Ethyl Acetate Fraction from the Stem Bark Scutia Buxifolia Reissek in Mice. Journal of Ethnopharmacology 2014, 153, 908–916.
- NCCLS Norma M27-A2. Método de Referência para Testes de Diluição em Caldo para Determinação da Sensibilidade à Terapia Antifúngica das leveduras; Norma Aprovada—Segunda Edição. Norma M27-A2 do NCCLS (ISBN 1-56238-469-4). NCCLS, Wayne, Pennsylvania, 2002.
- Stoppa, M.A.; Casemiro, L.A.; Vinholis, A.H.C.; Cunha, W.R.; Silva, M.L.A.; Martins, C.H.G.; Furtado, N.A.J.C. Comparative study between the recommended methodologies at CLSI and EUCAST on the evaluation of the antifungal activity (in Portuguese). New Chemical 2009, 32, 498–502.
- Javadpour, M.M.; Juban, M.M.; Lo, W.C.; Bishop, S.M.; Alberty, J.B.; Cowell, S.M.; Becker, C.L.; Mclaughlin, M.L. De Novo Antimicrobial Peptides with Low Mammalian Cell Toxicity. Journal of Medicinal Chemistry 1996, 39, 107–3113.
- Ernst, E.J.; Klepser, M.E.; Ernst, M.E.; Messer, S.A.; Pfaller, M.A. In Vitro Pharmacodynamic Properties of MK-0991 Determined by Time-Kill Methods. Diagnostic Microbiology and Infectious Disease 1999, 33, 75–80.
- Sidrin, J.J.C.; Rocha, M.F.G. Medical mycology under the light of contemporary authors. Rio de Janeiro: Guanabara Koogan, 2010; 388 p.
- Mendes, J.M. Antifungal activity of research Eugenia caryophyllata Thunb. essential oil against strains of Candida tropicalis (in Portuguese). Dissertation in Natural Products and Synthetic Bioactive. Federal University of Paraíba-UFPB, João Pessoa - PB, 2011.
- Costa, L.C.G.; Alves, S.F.; Nogueira, S.A.; Carvalho, G.K. Determination of fluconazole content of magistral and industrial capsules (in Portuguese). Faculty magazine Montes Belos 2014, 7, 47–56.
- Alves, C.T.; Ferreira, I.C.; Barros, L.; Silva, S.; Azeredo, J.; Henriques, M. Antifungal Activity of Phenolic Compounds Identified in Flowers from North Eastern Portugal Against Candida Species. Future Microbiology 2014, 9, 139–146.
- Barros, L.; Dueñas, M.; Alves, C.T.; Silva, S.; Henriques, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antifungal Activity and Detailed Chemical Characterization of Cistus Ladanifer Phenolic Extracts. Industrial Crops and Products 2013, 41, 41–45.
- Tempesti, T.C.; Alvarez, M.G.; de Araújo, M.F.; Júnior, F.E.A.C.; de Carvalho, M.G.; Durantini, E.N. Antifungal Activity of a Novel Quercetin Derivative Bearing a Trifluoromethyl Group on Candida Albicans. Medicinal Chemistry Research 2012, 21, 2217–2222.
- Vashisth, P.; Nikhil, K.; Pemmaraju, S.C.; Pruthi, P.A.; Mallick, V.; Singh, H.; Patel A.; Mishra, N.C.; Singh, R.P.; Pruthi, V. Antibiofilm Activity Of Quercetin-Encapsulated Cytocompatible Nanofibers Against Candida Albicans. Journal of Bioactive and Compatible Polymers 2013, 28, 652–665.
- Candiracci, M.; Citterio, B.; Piatti, E. Antifungal Activity of the Honey Flavonoid Extract Against Candida Albicans. Food Chemistry 2012, 131, 493–499.
- Köhler, J.R.; Arturo, C.; John, P. The Spectrum of Fungi That Infects Humans. Cold Spring Harbor Perspectives in Medicine 2015, 5, a019273.
- Kim, J.; Sudbery, P: Candida Albicans, a Major Human Fungal Pathogen. The Journal of Microbiology 2011, 49, 171–172.
- Canonico, B.; Candiracci, M.; Citterio, B.; Curci, R.; Squarzoni, S.; Mazzoni, A.; Papa, S.; Piatti, E. Honey Flavonoids Inhibit Candida Albicans Morphogenesis by Affecting DNA Behavior and Mitochondrial Function. Future Microbiology 2014, 9, 445–456.
- Araújo, M.G.F.; Hilário, F.; Vilegas, W.; Santos, L.C.; Brunetti, I.L.; Sotomayor, C.E.l Bauab, T.M. Correlation Among Antioxidant, Antimicrobial, Hemolytic, and Antiproliferative Properties of Leiothrix Spiralis Leaves Extract. International Journal of Molecular Sciences 2012, 13, 9260–9277.
- Candiracci, M.; Citterio, B.; Diamantini, G.; Blasa, M.; Accorsi, A.; Piatti, E. Honey Flavonoids, Natural Antifungal Agents Against Candida Albicans. International Journal of Food Properties 2011, 14, 799–808.
- Reyes, J.V.; Arenas, R. Mucocutaneous candidiasis. A review (in Spanish). Mexican Journal of Mycology 2007, 25, 91–104.