Abstract
Amyloses were isolated from diverse botanical sources (apple, mango, maize, and potato), and they were studied in their molecular characteristics (amylose content, molar mass, and molecular weight) using high-performance size-exclusion chromatography as a repetitive and faster protocol. The amylose purity ranged between 85.6–92.6 %, in agreement with the λmax values (601–610 nm), showing that some impurities with molecules of higher molar mass (amylopectin) were present. The standard curve of pullulan showed a high regression coefficient (0.998) inside of the limits of molar mass of amylose. Chromatograms of amylose showed the presence of components of high molar mass with a principal peak that corresponds to amylose. Molar mass of amylose ranged between 1.2 and 8.5 × 105 g/mol with polydispersity values between 1.3–4.1, indicating a narrow range of molar mass distribution of the amyloses analyzed. The high-performance size-exclusion chromatography coupled with a refractive index methodology used in this study may be considered simple and rapid for molecular studies of amylose.
Introduction
Starch is composed of two macromolecules of different structures: Amylose is the mainly linear component and amylopectin is the branched molecule. Starch owes much of its functionality and physical organization into a granular structure to these molecules.[Citation1] It is of great importance to understand the molecular and structural characteristics of starch components so as to suggest possible applications of this polymer in diverse systems. The average molecular weights of amylose and amylopectin influences the functional properties of starch, as demonstrated in gels,[Citation2] extrusion products,[Citation3] and starch pastes.[Citation4] It is, therefore, important to have a reproducible technique for measuring this macromolecular feature. Several studies have been carried out on molecular and structural characteristics of amylopectin using high-performance size-exclusion chromatography (HPSEC),[Citation5–Citation8] HPSEC coupled with multi-angle laser light scattering (MALLS),[Citation9–Citation15] or field flow fractionation (FFF) coupled with MALLS,[Citation15,Citation16] due to this molecule being responsible for diverse physicochemical and functional characteristics of starch, such as viscosity, swelling of the granule, thermal characteristics, crystallinity, and starch digestibility. On the other hand, amylose determines some of the main functional properties of starch including gel formation and starch retrogradation.[Citation17] These properties are strongly dependent on the macromolecular features of amylose. It was reported that a minimal molecular weight of 40,500 g/mol is necessary for amylose gelation.[Citation18] Diverse techniques have been reported for amylose characterization, some give mean–average values, such as classical light scattering, other include intrinsic viscosity measurement;[Citation19–Citation21] however, for a polydisperse in a polymer such as amylose, the average value is not enough to fully predict its physicochemical and functional properties and a technique which a macromolecular distribution is more powerful as HPSEC–MALLS.[Citation22]
A pre-requisite step for determining the molecular weight distribution of amylose and amylopectin and their average molar mass by any of these techniques is the complete dissolution of the starch sample in an appropriate solvent without any degradation of the constituent macromolecules. High temperatures and high pH increase the solubility of starch in aqueous solvents, but may result in molecular size reduction resulting from degradation, depolymerization, or oxidation.[Citation23] The fulfillment of this requirement is necessary to get information representative of the initial starch sample. A solubilization procedure was tested using microwave heating and a high solubilization percentage was obtained for amylase, but when the amount of amylopectin in the starch increased the solubilization decreased.[Citation10] This method has advantages for the sample; it can be solubilized just prior to chromatographic analysis, thus minimizing variation in analysis due to starch aggregation.[Citation11]
Recently, the same procedure was tested in the molecular study of starches isolated from non-conventional source using a MALLS detector.[Citation24] However, the size-exclusion chromatography (SEC) system using a HPLC coupled a refractive index (RI; HPLC–RI) might be feasible for molecular studies of amylose for polymers with low polydispersity. Solvent such as dimethyl sulfoxide (DMSO) with LiBr has been used for analysis of starch and its components (amylose and amylopectin) using SEC–MALLS equipment.[Citation23,Citation25,Citation26] The present work proposed a methodology for molecular studies of amylose using a microwave heating for solubilization of the sample and thereafter, analysis in the HPSEC–RI system.
Materials and Methods
Starch Isolation
Immature apple (Malus domestica cultivar Golden Delicious), determined on the basis of the starch index (the skin color and size were the parameters used for cutting of the fruit) were collected at the local farm “La Campana” in Cuauhtémoc, Chihuahua (México). The methodology reported by Bello-Pérez et al.[Citation27] was used for starch isolation. Green mangos (Mangifera indica L.) variety “Ataulfo” were also purchased in the local market and the starch was isolated with the procedure previously reported by Agustiniano-Osornio et al.[Citation28] Potato starch was carefully isolated using a modification of the procedure of Kim et al.[Citation29] and maize starch was obtained with the method suggested by Adkins and Greenwood.[Citation30] The white-starch sediments were dried in a convection oven (Lab-Line Instruments Inc., Imperial V, Melrose Park, IL) at 40°C for 48 h, carefully ground with a mortar and pestle to pass through a US No. 100 sieve and stored at room temperature (25°C) in a glass container.[Citation31,Citation32] Amylose, from the starches previously mentioned, was isolated by the thymol and n-butanol complexation method.[Citation33] Commercial amylose type III from potato (Sigma Chemical Co., St Louis, MO, USA) was used as a control and subsequently was named as commercial.
Amylose Content
The purity of amylose was determined by the total amylose content test of Hoover and Ratnayake[Citation34] and the wavelength at the maximum absorption (λmax) of the blue iodine–amylose complex.[Citation35] The total amylose contents of starch samples were determined by above procedure, but with prior deffating with hot n-propanol-water (3:1 v/v) for at least 7 h. In order to correct for over estimation of apparent and total amylose content (due to complex formation between I2 and the outer branches of amylopectin), amylose content was calculated from a standard curve prepared using mixtures of pure potato amylose and amylopectin (over the range 0–100% amylose).
SEC-RI Study
Pullulan standards (Fluka Chemie GmbH, Steinhein, Switzerland) of diverse molar mass (1.6 × 106, 3.8 × 105, 1.8 × 105, 1.0 × 105, 4.8 × 104, and 1.2 × 104 g/mol) were used to obtain a calibration line. Pullulans were dissolved in HPLC-grade water at 25 ºC overnight, filtered using 0.2 µm nylon syringe filter (Daigger & Company Inc., Vernon Hills, IL) and injected into the HPSEC–RI system. The solubilization procedure of amyloses was carried out as reported by Millan-Testa et al.[Citation24] using 50 s of microwave heating. The supernatant solution was filtered through a 5 µm nylon syringe filter (Daigger & Company Inc., Vernon Hills, IL). The solution was injected (50 μL) onto the HPLC AT 1100 equipment (Angilent Technology, Deutchland GmbH Waldbronn, Germany) with the gel permeation chromatography-size exclusion chromatography (GPC-SEC) PL aquagel-OH mixed, 8 µm column (7.5 mm ID × 300 mm; Agilent Technologies Deutchland GmbH Waldbronn, Germany). The column and the detector were maintained at 30ºC. The eluant was HPLC-grade water, carefully degassed and filtered before use through Durapore GV (0.2 µm) membranes. The flow rate was 1.0 mL/min. The data analysis was realized using the GPC software of Agilent (Agilent Technologies Deutchland GmbH Waldbronn, Germany). The carbohydrate concentration of the supernatant solution after filtration was measured by the sulphuric acid-phenol colorimetric method.[Citation10] The procedure was carried at least five times for each sample.
Statistical Analysis
Researchers used a completely random design. An analysis of variance (ANOVA) was used at a 5% significance level (α = 0.05). Results were obtained and normality test was verified using a Sigma-Plot statistical program, version 11.0.[Citation36] When significant differences were found, researchers applied the Tukey range test for the comparison of means.[Citation37]
Results and Discussion
Amylose Characteristics
The purity values of the amylose samples evaluated in this work ranged from 85.6 to 92.6% (), which agrees with their λmax values (between 601 and 610 nm). Previous works reported λmax values of pure amylose between 650 and 660 nm.[Citation22,Citation38,Citation39] However, some contrasting data have been reported. Radosta et al.[Citation38] determined in one sample of synthetic amylose an amylose content of 99.0% and λmax of 660 nm, while in a second one they reported 96.3% of purity and λmax of 667 nm. In the same sense, λmax values between 652 and 662 nm were reported for amylose isolated from diverse botanical sources.[Citation22] On further studies,[Citation40] the investigators previously mentioned determined that these samples had a high molar mass population that contaminated amylose solutions, which were removed by ultracentrifugation. Amylose molar mass values after ultracentrifugation were lower than its original value in the amylose solution.
TABLE 1 Amylose content and λmax of amyloses from diverse botanical sources
Standard Curve of Pullulan
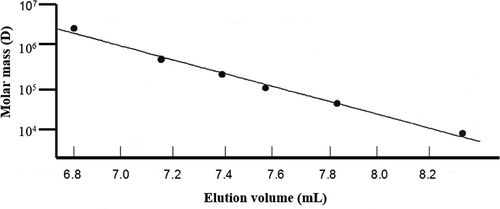
A semi-logarithmic plot of molar mass versus elution volume for pullulan standards is shown in . The regression coefficient of the ratio between molar mass and elution volume was 0.998, showing that the SEC system used for elution of pullulan standards is reproducible because in five standard curves was obtained a standard error of 0.003929 with a p < 0.05. The use of the SEC in studies of molecular structure of polymers with medium molar mass is feasible, because HPSEC alone allows the determination of amylose or amylopectin molar mass if pullulan or dextran standards are available.[Citation22] For polymers with high molar mass (such as amylopectin), HPSEC coupled with MALLS is recommended.[Citation11,Citation23] However, molar mass determination for amylose was realized avoiding the use of a calibration plot.[Citation22,Citation40]
Molecular Characteristics
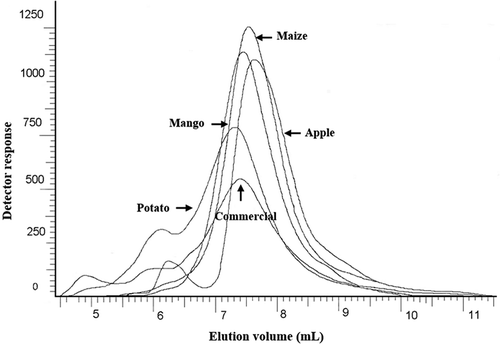
Complete solubilization of starch components without degradation is important in molecular studies. The solubility of amylose samples ranged from 85.0 to 99.7% () and depends on the botanical source as it was in the following descending order commercial > apple > potato > mango > maize. Bello-Pérez et al.[Citation10] reported an amylose solubility value of 100% using microwave heating, similar values were reported when amylose solutions were ultracentrifuged.[Citation40] Chromatograms of amyloses () showed that potato had two small peaks at 4.8 and 6.0 mL as well as commercial sample, whereas apple amylose had only one peak at 6.2 mL. These components of high molar mass contribute to a higher amylose molar mass, because during amylose isolation an amount of amylopectin was not separated. The samples studied presented a main peak at an elution volume between 7.2 and 7.6 mL due to the amylose component. The pattern observed in the chromatograms samples showed an influence in the global molar mass by the chain length distribution of amylose. Components of higher molar mass were found in some amyloses studies, which were separated after ultracentrifugation, contributing to higher molar mass values.[Citation40] However, SEC–MALLS–RI study of some amyloses did not show the presence of a component of higher molar mass, but appreciable differences in the molar masses were observed.[Citation38]
TABLE 2 Number-average (Mn) and weight-average (Mw) molar masses of starches amyloses*
Molar masses of amyloses were between 1.2 and 8.5 × 105 g/mol. In general, the values of this study were lower than those obtained for diverse amyloses (between 4.0 × 105 and 1.2 × 106 g/mol) reported by Roger and Colonna,[Citation22] since potato amyloses of this study presented lower molar mass values (2.0 and 3.0 × 105 g/mol) than that reported for amylose isolated from potato (9.8 × 105 g/mol).[Citation22] Amylose maize presented a similar behavior; the molar mass obtained in this work (1.2 × 105 g/mol) was smaller than the value (8.7 × 105 g/mol) reported by the same authors.[Citation22] Nevertheless, these authors determined molar mass values for the same amylose (between 3.4 × 105 and 1.0 × 106 g/mol), using a modified procedure.[Citation40] Other authors reported molar mass values of potato amylase higher than those obtained here in the same botanical source using HPSEC system (2.1–4.4 × 107 g/mol).[Citation38] The polydispersity assessed by the ratio Mw/Mn is a distribution measurement of the size of the polymers present in the sample. The Mw/Mn values () were in a range from 1.3 to 4.1, showing that the methodology used for determining amylose molar mass can be suggested for the study of its structural characteristics. Similar polydispersity values (1.7–4.4) were reported for amylose of diverse sources.[Citation22] Roger and Colonna[Citation40] reported that values did not decrease when ultracentrifugated and a narrower range value of polydispersity was obtained (2.54–3.81).
The solubilization percentage in the samples analyzed was high, indicating that the determination of molecular characteristic of amylose using HPSEC–RI gives information about the totality of the polymer. Some amyloses presented few amounts of polymers of higher molar mass from amylopectin as it was demonstrated by the amylose content, λmax and the chromatograms. The molar masses of amyloses determined by HPSEC–RI are in the range of values reported for amyloses with low polydispersity, showing a narrow range of molar mass distribution of amylose chains. The use of HPSEC–RI methodology can be feasible for molecular studies of amylose where expensive detectors (such as MALLS, Viscosity, etc.) are not required.
Conclusion
The solubilization percentage in the samples analyzed was high, indicating that the determination of molecular characteristic of amylose using HPSEC–RI gives information about of the totality of the polymer. Some amyloses presented few amounts of polymers of higher molar mass from amylopectin as it was evidenced from the results of amylose content, λmax and the chromatograms. The molar masses of amyloses determined by HPSEC–RI are in the range of those reported for amyloses with low values of polydispersity, showing a narrow range of molar mass distribution of amylose chains. The HPSEC–RI methodology might be feasible for molecular studies of amylose where expensive detectors (such as MALLS, viscosity) are not required.
Acknowledgments
The authors would like to thank Arturo Ramos Martínez and Emilio Ochoa Reyes for their technical assistance.
References
- French, D. Organization of Starch Granules. In Starch: Chemistry and Technology; Paschall, R.L.; Whistlerjames, N.; Bemillereugene, F.; Eds.; Academic Press: San Diego, CA, 1984; 183–247 pp.
- Clark, A.H.; Gidley, M.J.; Richardson, R.K.; Ross-Murphy, S.B. Rheological Studies of Aqueous Amylose Gels: The Effect of Chain Length and Concentration on Gel Modulus. Macromolecules 1989, 22(1), 346–351.
- Della-Valle, G.; Colonna, P.; Patria, A.; Vergnes, B. Influence of Amylose Content on the Viscous Behavior of Low Hydrated Molten Starches. Journal of Rheology (1978–present) 1996, 40(3), 347–362.
- Doublier, J.L.; Colonna, P.; Mercier, C. Extrusion Cooking and Drum Drying of Wheat Starch. II. Rheological Characterization of Starch Pastes. Cereal Chemistry 1986, 63(3), 240–246.
- Hizukuri, S. Polymodal Distribution of the Chain Lengths of Amylopectins, and Its Significance. Carbohydrate Research 1986, 147(2), 342–347.
- Ong, M.H.; Blanshard, J.M.V. Texture Determinants in Cooked, Parboiled Rice. I: Rice Starch Amylose and the Fine Stucture of Amylopectin. Journal of Cereal Science 1995, 21(3), 251–260.
- Striegel, A.M.; Timpa, J.D. Molecular Characterization of Polysaccharides Dissolved in Me2NAc-LiCl by Gel-Permeation Chromatography. Carbohydrate Research 1995, 267(2), 271–290.
- Takeda, C.; Takeda, Y.; Hizukuri, S. Structure of the Amylopectin Fraction of Amylomaize. Carbohydrate Research 1993, 246(1), 273–281.
- Bello-Pérez, L.A.; Colonna, P.; Roger, P.; Paredes-López, O. Macromolecular Features of Amaranth Starch. Cereal Chemistry 1998, 75(4), 395–402.
- Bello-Pérez, L.A.; Roger, P.; Baud, B.; Colonna, P. Macromolecular Features of Starches Determined by Aqueous High-Performance Size Exclusion Chromatography. Journal of Cereal Science 1998, 27(3), 267–278.
- Fishman, M.L.; Rodriguez, L.; Chau, H.K. Molar Masses and Sizes of Starches by High-Performance Size-Exclusion Chromatography with on-Line Multi-Angle Laser Light Scattering Detection. Journal of Agricultural and Food Chemistry 1996, 44(10), 3182–3188.
- Ji, Y.; Wong, K.; Hasjim, J.; Pollak, L.M.; Duvick, S.; Jane, J.; White, P.J. Structure and Function of Starch from Advanced Generations of New Corn Lines. Carbohydrate Polymers 2003, 54(3), 305–319.
- Rolland-Sabaté, A.; Georges Amani, N.G.; Dufour, D.; Guilois, S.; Colonna, P. Macromolecular Characteristics of Ten Yam (Dioscorea spp) Starches. Journal of the Science of Food and Agriculture 2003, 83(9), 927–936.
- Yoo, S.-H.; Jane, J.-l. Molecular Weights and Gyration Radii of Amylopectins Determined by High-Performance Size-Exclusion Chromatography Equipped with Multi-Angle Laser-Light Scattering and Refractive Index Detectors. Carbohydrate Polymers 2002, 49(3), 307–314.
- Yoo, S.-H.; Jane, J.-l. Structural and Physical Characteristics of Waxy and Other Wheat Starches. Carbohydrate Polymers 2002, 49(3), 297–305.
- Roger, P.; Baud, B.; Colonna, P. Characterization of Starch Polysaccharides by Flow Field-Flow Fractionation–Multi-Angle Laser Light Scattering–Differential Refractometer Index. Journal of Chromatography A 2001, 917(1–2), 179–185.
- Biliaderis, C.G. The Structure and Interactions of Starch with Food Constituents. Canadian Journal of Physiology and Pharmacology 1991, 69(1), 60–78.
- Gidley, M.J.; Bulpin, P.V. Aggregation of Amylose in Aqueous Systems: The Effect of Chain Length on Phase Behavior and Aggregation Kinetics. Macromolecules 1989, 22(1), 341–346.
- Banks, W.; Greenwood, C.T. Starch and Its Components; Edinburgh University Press: Edinburgh, 1975; 342 p.
- Kodama, M.; Noda, H.; Kamata, T. Conformation of Amylose in Water. I. Light-Scattering and Sedimentation-Equilibrium Measurements. Biopolymers 1978, 17(4), 985–1002.
- Ring, S.G.; L’Anson, K.; Morris, V.J. Static and Dynamic Light Scattering Studies of Amylose Solutions. Macromolecules 1985, 18(2), 182–188.
- Roger, P.; Colonna, P. Evidence of the Presence of Large Aggregates Contaminating Amylose Solutions. Carbohydrate Polymers 1993, 21(2–3), 83–89.
- Zhong, F.; Yokoyama, W.; Wang, Q.; Shoemaker, C.F. Rice Starch, Amylopectin, and Amylose: Molecular Weight and Solubility in Dimethyl Sulfoxide-Based Solvents. Journal of Agricultural and Food Chemistry 2006, 54(6), 2320–2326.
- Millan-Testa, C.E.; Mendez-Montealvo, M.G.; Ottenhof, M.A.; Farhat, I.A.; Bello-Pérez, L.A. Determination of the Molecular and Structural Characteristics of Okenia, Mango, and Banana Starches. Journal of Agricultural and Food Chemistry 2005, 53(3), 495–501.
- Jackson, D.S. Solubility Behavior of Granular Corn Starches in Methyl Sulfoxide (DMSO) As Measured by High Performance Size Exclusion Chromatography. Starch/Stärke 1991, 43(11), 422–427.
- Yokoyama, W.; Renner-Nantz, J.J.; Shoemaker, C.F. Starch Molecular Mass and Size by Size-Exclusion Chromatography in DMSO-Libr Coupled with Multiple Angle Laser Light Scattering. Cereal Chemistry 1998, 75(4), 530–535.
- Bello-Pérez, L.A.; Agama-Acevedo, E.; Sáyago-Ayerdi, S.G.; Moreno-Damian, E.; Figueroa, J.D.C. Some Structural, Physicochemical, and Functional Studies of Banana Starches Isolated from Two Varieties Growing in Guerrero, México. Starch/Stärke 2000, 52(2–3), 68–73.
- Agustiniano-Osornio, J.C.; González-Soto, R.A.; Flores-Huicochea, E.; Manrique-Quevedo, N.; Sánchez-Hernández, L.; Bello-Pérez, L.A. Resistant Starch Production from Mango Starch Using a Single-Screw Extruder. Journal of the Science of Food and Agriculture 2005, 85(12), 2105–2110.
- Kim, Y.S.; Wiesenborn, D.P.; Orr, P.H.; Grant, L.A. Screening Potato Starch for Novel Properties Using Differential Scanning Calorimetry. Journal of Food Science 1995, 60(5), 1060–1065.
- Adkins, G.K.; Greenwood, C.T. The Isolation of Cereal Starches in the Laboratory. Starch/Stärke 1966, 18(7), 213–218.
- Bello-Pérez, L.A.; Agama-Acevedo, E.; Sánchez-Hernández, L.; Paredes-López, O. Isolation and Partial Characterization of Banana Starches. Journal of Agricultural and Food Chemistry 1999, 47(3), 854–857.
- Bello-Pérez, L.A.; Aparicio-Saguilán, A.; Méndez-Montealvo, G.; Solorza-Feria, J.; Flores-Huicochea, E. Isolation and Partial Characterization of Mango (Magnifera indica L.) Starch: Morphological, Physicochemical, and Functional Studies. Plant Foods for Human Nutrition 2005, 60(1), 7–12.
- Banks, W.; Greenwood, C.T. The Fractionation of Labortory-Isolated Cereal Gereal Starches Using Dimethyl Sulphoxide. Starch/Stärke 1967, 19(12), 394–398.
- Hoover, R.; Ratnayake, W.S. Starch Characteristics of Black Bean, Chick Pea, Lentil, Navy Bean, and Pinto Bean Cultivars Grown in Canada. Food Chemistry 2002, 78(4), 489–498.
- Bello-Pérez, L.A.; Paredes-López, O.; Roger, P.; Colonna, P. Amylopectin—Properties and Fine Structure. Food Chemistry 1996, 56(2), 171–176.
- Fox, E.; Shotton, K.; Ulrich, C. Sigma-Stat User’s Manual; Jandel Scientific Co.: San Rafael, CA, 1995.
- Walpole, R.E.; Myers, R.H.; Myers, S.L. Probabilidad y Estadística para Ingenieros [Probability and Statistics for Engineers], 6th e; Prentice-Hall Hispanoamericana: México, 1999; 739 pp.
- Radosta, S.; Haberer, M.; Vorwerg, W. Molecular Characteristics of Amylose and Starch in Dimethyl Sulfoxide. Biomacromolecules 2001, 2(3), 970–978.
- Wang, L.Z.; White, P.J. Structure and Properties of Amylose, Amylopectin, and Intermediate Materials of Oat Starches. Cereal Chemistry 1994, 71(3), 263–268.
- Roger, P.; Tran, V.; Lesec, J.; Colonna, P. Isolation and Characterisation of Single Chain Amylose. Journal of Cereal Science 1996, 24(3), 247–262.