?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Free amino acid, flavor 5’-nucleotides, organic acid, and the values of equivalent umami concentration of cultivable mushrooms, namely Pleurotus eryngii, Agrocybe aegerita, and Lentinus edodes, were investigated at different cultivation stages, mycelia, primordium, and fruit bodies. Fruit bodies were higher than mycelia in contents of total free amino acid, flavor 5’-nucleotides, and the values of equivalent umami concentration in three cultivated mushrooms, but lower in contents of total organic acid in P. eryngii and L. edodes, respectively. Contents of total organic acid at three cultivation stages of three cultivated mushrooms ranged from 32.65 to 125.50 mg g–1 with the primordium stage of L. edodes being the highest. Overall, the amount of the nutrition and flavor components of mushroom might be related with mushroom species, fermentation way, and cultivated time. Some compounds could be also extracted from mycelia or primordium of mushrooms other than fruit body.
INTRODUCTION
Mushrooms have been used as nutraceuticals and functional foods for centuries, due to their nutritious and subtle flavor.[Citation1,Citation2] The free amino acids, 5’-nucleotides, and organic acids of mushrooms are not only the important nutrient compounds, but the primarily contributor to peculiar umami taste as non-volatile flavor substances.[Citation3] Craske and Reuter[Citation4] separated the amino acids present in an aqueous extract of Boletus edulis, and demonstrated that the most intense mushroom flavor was associated with highly basic amino acids. 5’-nucleotides and organic acids, were also accepted that they were contributions to the flavor of mushrooms.[Citation5,Citation6] The equivalent umami concentration (EUC) of mushrooms had been calculated in order to understand the umami-like taste characteristics.[Citation5,Citation7] Recently, there was an increased interest on the evaluation of nutrient and non-volatile flavor substances in various edible mushroom species.[Citation6] Li et al.[Citation8] showed that different cultivated mushroom species had different concentration of free amino acids, 5’-nucleotides, and organic acids. The different grade of pine-mushrooms had significantly difference in the concentration of non-volatile flavor substances, such as free amino acids, decreased in the order of second grade > third grade > fourth grade > first grade.[Citation9] Moreover, even if the same mushroom, the non-volatile flavor substances of its different parts were also significantly difference.[Citation5] Cho and others[Citation5] showed that free amino acids and 5’-nucleotides were higher in the pileus than in the stipe, irrespective of pine-mushroom grades. However, there were few studies on the difference of nutrient and non-volatile flavor substances in different cultivation stages, especially mycelia and primordium stages of cultivated mushrooms which were always three main cultivation stages, include mycelia, primordium, and fruit bodies.
In this study, selected nutrient and non-volatile flavor substances in three cultivation stages (mycelia, primordium, and fruit body) of Pleurotus eryngii, Agrocybe aegerita, and Lentinus edodes, including their free amino acids, 5’-nucleotides, and organic acids were evaluated. Their EUC were also evaluated as an important index of taste.
Materials and methods
Mushroom Mycelia, Primordium, and Fruit Body
The seed culture of three strains, Pleurotus eryngii, Agrocybe aegerita, and Lentinus edodes, were inoculated onto potato dextrose agar (PDA) at 25°C for 14 days. The product code in China are as follow: Pleurotus eryngii (ID: 2008058), Agrocybe aegirit (ID: 2008033), Lentinus edodes (ID: 2009009). For the production of mycelia, the mycelia from PDA were inoculated into 500 mL flasks containing 250 mL of potato dextrose liquid at 25°C and 25 × g for 14 days in a shaker. After 14 days of incubation, the mycelia were harvested and washed five times with deionized water and then freeze-dried. For the production of primordium and fruit bodies, the mycelia from PDA were inoculated into growth media (Pleurotus eryngii, corn cob 30%, sawdust 30%, wheat bran 20%, corn flour, 18%, lime 2%; Agrocybe aegerita, cotton seed hulls 82%, wheat bran 15%, lime 3%; Lentinus edodes, sawdust 78%, wheat bran 20%, brown sugar 2%). Primordium of Pleurotus eryngii, Agrocybe aegerita, and Lentinus edodes were removed carefully from growth media and freeze-dried after incubated 25, 33, and 54 days, respectively. Fruit bodies were also harvested and freeze-dried after incubated 30, 40, and 60 days, respectively. The freeze-dried mycelia, primordium, and fruit bodies were randomly selected and ground in liquid nitrogen using mortar and pestle.
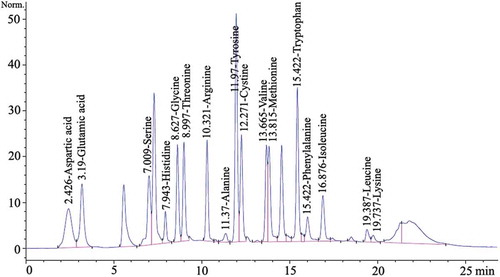
Free Amino Acid Assay
Each powdered mushroom sample (10 mg) was suspended in 2 mL of 0.1 mol L–1 HCl for 45 min in a shaker with speed of 15 × g at ambient temperature. The filtrate was centrifuged at 9000 × g for 5 min and then passed through a 0.22-μm polyvinylidene difluoride (PVDF) filter. The filtrate was mixed with borate buffer and o-phthalaldehyde reagent (Sigma Chemical Co., St. Louis, MO) in the tube, shaken to facilitate derivatization and then immediately injected into a high-performance liquid chromatography (HPLC) system. The HPLC system consisted of a Shimadzu LC-10AT VP pump, a Rheodyne 7725i injector, a 20 mL sample loop, a Hitachi L-7485 fluorescence detector with fluorescence excitation at 340 nm and emission at 450 nm, and a Hypersil AA-ODS column (2.1 × 200 mm, Agilent Technologies, USA). The mobile phases were A: 0.05 mol L–1 sodium acetate (pH 5.7) containing 50 mL L–1 tetrahydofuran; B: deionized water; and C: methanol. The gradient was A:B:C 80:0:20 (v/v/v) for 38 min, 0:33:67 for 2 min, and 0:100:0 for 3 min. The flow rate was 1.2 mL min–1.[Citation10] Each amino acid was identified using the authentic amino acid (Sigma Chemical Co., St. Louis, MO, USA) and quantified by the calibration curve of the authentic compound (), including alanine (r2 = 0.9965), arginine (r2 = 0.9960), isoleucine (r2 = 0.9934), cystine (r2 = 0.9818), glutamic acid (r2 = 0.9976), glycine (r2 = 0.9931), histidine (r2 = 0.9951), aspartic acid (r2 = 0.9949), leucine (r2 = 0.9944), lysine (r2 = 0.9857), methionine (r2 = 0.9944), phenylalanine (r2= 0.9925), serine (r2 = 0.9933), threonine (r2 = 0.9942), tryptophan (r2 = 0.9936), tyrosine (r2 = 0.9936), and valine (r2 = 0.9952).
FIGURE 1 HPLC chromatographic fingerprints of authentic amino acid (0.25 µM mL–1). HPLC peaks: Aspartic acid (2.426 min), Glutamic acid (3.190 min), Serine (7.009 min), Histidine (7.944 min), Glycine (8.627 min), Threonine (8.997 min), Arginine (10.321 min), Alanine (11.370 min), Tyrosine (11.970 min), Cystine (12.271 min), Valine (13.665 min), Methionine (13.815 min), Tryptophan (15.422 min), Phenylalanine (15.422 min), Isoleucine (16.876 min), Leucine (19.387 min), Lysine (19.737 min).
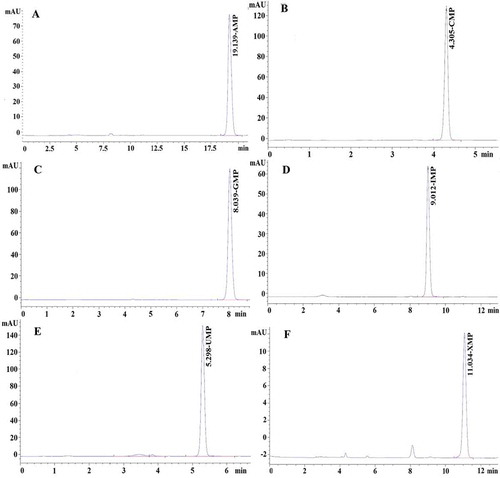
5’-Nucleotide Assay
5’-nucleotides were extracted and analyzed as described by Taylor et al.[Citation11] Each powdered mushroom sample (50 mg) was extracted with 25 mL of deionized water. This suspension was heated to boiling for 1 min, cooled, and then centrifuged at 11,000 × g for 15 min. The extraction was repeated with 20 mL deionized water. The combined supernatant was freeze-dried and resolved in 2 mL deionized water, then filtered (0.22 μm PVDF filter) prior to HPLC injection. HPLC analysis was performed using an Agilent 1200 HPLC system with an Agilent HC-C18 column (4.6 × 250 mm, 5 μm, Agilent, USA). HPLC conditions were as follows: mobile phase, 0.01 M KH2 PO4/H3PO4 (pH 4.68); flow rate 1.0 mL min–1; UV detection wavelength, 259 nm; oven temperature 30°C. Each 5’-nucleotide was identified and quantified using 5’-nucleotide standards (Sigma Chemical Co., St. Louis, MO, USA) as showed in , including 5’-adenosine monophosphate (5’-AMP, r2 = 0.9990), 5’-cytosine monophosphate (5’-CMP, r2 = 0.9985), 5’-guanosine monophosphate (5’-GMP, r2 = 0.9995), 5’-inosine monophosphate (5’-IMP, r2 = 0.9973), 5’-uridine monophosphate (5’-UMP, r2 = 0.9885), 5’-xanthosine monophosphate (5’-XMP, r2 = 0.9975).
FIGURE 2 HPLC chromatographic fingerprints of 5’-nucleotide (0.05 mg L–1). A: 5’-adenosine monophosphate (5’-AMP); B: 5’-cytosine monophosphate (5’-CMP); C: 5’-guanosine monophosphate (5’-GMP); D: 5’-inosine monophosphate (5’-IMP); E: 5’-uridine monophosphate (5’-UMP; F, 5’-xanthosine monophosphate (5’-XMP).
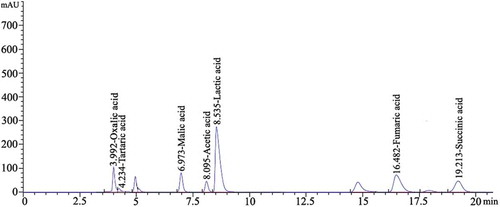
Assay of Organic Acids
Each powdered mushroom sample (10 mg) was suspended in 2 mL deionized water and subjected to ultrasound (400 W, 30 min, ambient temperature) using a high intensity ultrasonic processor. The suspension was centrifuged at 11,000 × g for 15 min and the supernatant was filtered through a 0.22 μm PVDF filter prior to analysis by HPLC. The HPLC system above was fitted with a Venusil MP-C18 column (4.6 × 250 mm, 5 m, Agela Technologies, China). HPLC conditions were as follows: mobile phase, 0.01 M NH4H2PO4 (pH 2.51), flow rate 1.0 mL min–1, UV detection wavelength, 214 nm, oven temperature, 30°C. Each organic acid was identified and quantified using authentic standards (Sigma Chemical Co., St. Louis, MO, USA) as showed in , including tartaric acid (r2 = 0.8918), malic acid (r2 = 0.9999), oxalic acid (r2 = 0.9984), acetic acid (r2 = 0.9999), lactic acid (r2 = 0.9995), fumaric acid (r2 = 0.9999), succinic acid (r2 = 0.9981).
EUC
The EUC (g monosodium glutamate [MSG] per 100 g dried material) is the concentration of MSG equivalent to the umami intensity of that given by the mixture of umami amino acid (aspartic and glutamic) and 5’-nucleotide and is represented by the following addition equation.[Citation8,Citation12]
where Y is the EUC of the mixture in terms of g MSG per 100 g dried material; ai is the concentration (g per 100 g dried material) of each umami amino acid (aspartic or glutamic); aj is the concentration (g per 100 g dried material) of each umami 5’-nucleotide (5’-IMP, 5’-GMP, 5’-XMP, or 5’-AMP); bi is the relative umami concentration (RUC) for each umami amino acid to MSG (Aspartic, 0.077 or Glutamic, 1); bj is the RUC for each umami 5’-nucleotide to 5’-IMP (5’-IMP, 1; 5’-GMP, 2.3; 5’-XMP, 0.61 or 5’-AMP, 0.18); and 121.8 is a synergistic constant based on the concentration of g per 100 g dried material used.
Statistical Analysis
Each mushroom sample was conducted in triplicate. Experimental data was subjected to a one-way analysis of variance (ANOVA) for multiple comparisons. Significant difference among means was at the level of p < 0.05.
Results and Discussion
Free Amino Acid in Three Cultivated Mushrooms at Different Cultivation Stages
Total free amino acid levels in three cultivation stages of Pleurotus eryngii, Agrocybe aegerita, and Lentinus edodes ranged from 36.39 to 72.01 mg g–1 (). Three cultivation stages of Pleurotus eryngii, Agrocybe aegerita, or Lentinus edodes showed different free amino acid profiles as showed in the reported by Lee et al.[Citation13] Contents of alanine, glycine, isoleucine, methionine, phenylalanine, threonine, tryptophan, and tyrosine in Pleurotus eryngii were all significantly higher in mycelia and primordium than those in fruit bodies. The glycine, isoleucine, leucine, threonine, and valine levels of Agrocybe aegerita and the isoleucine, cystine, glycine, histidine, leucine, threonine, and tryptophan of Lentinus edodes were higher in mycelia than that in fruit bodies. Moreover, the total free amino acid in fruit bodies of Pleurotus eryngii and Lentinus edodes were all lower than that in mycelia, while the opposite case was present in Agrocybe aegerita. The reason might be related with the different fermentation ways of mycelia and fruit bodies, or mushrooms species in that free amino acid might be used for the synthesis of other nitrogenous components with increasing cultivated time. Therefore, free amino acid contents in mycelia were comparable to those in fruit bodies, and mycelia production period was short, for these reasons, mycelia might be a good raw material source for free amino acid components extraction. Currently, the mycelia of C. militaris were already available in Taiwan for use in the formulation of nutraceuticals and functional foods. However, consistent with three mushrooms in our study, Lee et al.[Citation13] revealed that the fruit body of Hypsizigus marmoreus was higher than that of mycelia in contents of MSG-like free amino acid which gave the most typical mushroom taste.[Citation13] The fruit bodies and primordium showed more taste characteristics than mycelia. In addition, in comparison with the previous study, the contents of total and MSG-like free amino acid in fruit bodies of three mushrooms were all higher compared to the reported by Li et al.[Citation8] The difference might be due to the different species and detection methods.
TABLE 1 Contents of free amino acid in three cultivated mushrooms (mg g–1)
5’-Nucleotide in Three Edible Mushrooms at Different Cultivation Stages
Total 5’-nucleotide levels in three cultivation stages of Pleurotus eryngii, Agrocybe aegerita, and Lentinus edodes ranged from 0.54 to 3.02 mg g–1 (). The content of total 5’-nucleotide in three mushrooms was all higher in fruit bodies and primordium than that in mycelia, respectively. Furthermore, 5’-nucleotides in fruit body and primordium were all higher than that in mycelia of three mushrooms as reported Hypsizigus marmoreus by Lee et al.[Citation13] Contents of 5’-nucleotides might be related with mushrooms species and the cultivated time. The reason might be that nucleotides were also important components of genetic material in cell which increased with growth of mushroom. Flavor 5’-nucleotides were responsible for the umami or palatable taste, including 5’-guanosine monophosphate (5’-GMP), 5’-inosine monophosphate (5’-IMP), and 5’-xanthosine monophosphate (5’-XMP). Flavor 5’-nucleotide levels were lower compared with previously reported concentrations in A. blazei and C. comatus which were well known for their better taste.[Citation3,Citation8] Yang et al.[Citation9] defined three ranges of flavor 5’-nucleotides, low (<1 mg g–1), medium (1–5 mg g–1) and high (>5 mg g–1), according to which different cultivation stages of three mushroom fall within the low category in our study.
TABLE 2 Levels of 5’-nucleotide levels in three edible mushrooms at different cultivation stages (mg g–1)
Organic Acid Levels in Three Edible Mushrooms at Different Cultivation Stages
Total organic acid levels in three cultivation stages of Pleurotus eryngii, Agrocybe aegerita, and Lentinus edodes ranged from 32.65 to 125.50 mg g−1 (). The results also showed that contents of total organic acid in three cultivated mushrooms were all lower compared to those reported by Li et al.[Citation8] The organic acid profiles in three mushrooms also showed obvious differently. Contents of tartaric acid, acetic acid, lactic acid, and total organic acid in Pleurotus eryngii were all significantly higher in mycelia than those in primordium and fruit bodies. On the contrary, the level of succinic acid in mycelia was lower than that of primordium and fruit bodies. The malic acid, succinic acid, and total organic acid in Agrocybe aegerita were all significantly higher in fruit bodies than those in mycelia. The level of tartaric acid and oxalic acid in primordium was higher than that of mycelia and fruit bodies in Agrocybe aegerita. In Lentinus edodes, the contents of tartaric acid and total organic acid were all significantly higher in mycelia and primordium than those in fruit bodies. The levels of malic acid, acetic acid, fumaric acid, and succinic acid were significantly higher in primordium and fruit bodies than those in mycelia. Moreover, fumaric acid in most samples could not be detected in our study. In contrast, other research showed that fumaric acid were detected in all mushrooms, range from 1.56 to 96.11 mg g–1.[Citation8] Some differences were found between the results reported herein and the ones described by other authors. This might be due to numerous factors such as the different extraction methodology applied and also environmental conditions related to sample collection. However, malic acid, oxalic acid, and succinic acid were found in all the samples and they were also present in many plants and animals.[Citation14] The reason might be that they were all the tricarboxylic acid cycle intermediates. Previous studies showed that organic acids might have a protective role against various diseases due to their antioxidant activity, such as the case of tartaric, malic, citric or succinic acids, and the organic acids were also essential flavor components in alcoholic beverages such as wine and sake.[Citation8,Citation14] Organic acids from mushroom might be served as a more safe food flavoring additive.
TABLE 3 Organic acid levels in three edible mushrooms at different cultivation stages (mg g–1)
EUC in Three Edible Mushrooms at Different Cultivation Stages
EUC values of primordium and fruit bodies in Pleurotus eryngii were approximate 5- and 4-fold higher than those in mycelia, respectively. Contents of EUC in primordium and fruit bodies of Agrocybe aegerita were also significantly higher than that in mycelia, about 3- and 16-fold, respectively. To Lentinus edodes, the EUC values showed the same order as Agrocybe aegerita, ranged from 16.76 to 99.75 (). Although there were significant differences in the contents of free amino acid, flavor 5’-nucleotides, and organic acid among the three cultivated stages of mushrooms, the EUC values were constant higher in primordium and fruit bodies than that in mycelia as Hypsizigus marmoreus reported by Lee et al.[Citation13] The EUC values of fruit bodies in three cultivated mushrooms were all approximate at second level, while the EUC values of mycelia in three cultivated mushrooms were all at third level according to the ratings grouped EUC values into four levels, first level of >1000 g MSG per 100 g dried material, second level of 100–1000 g MSG per 100 g, third level of 10–100 g MSG per 100 g, and fourth level of <10 g MSG per 100 g.[Citation7] EUC value might be also influenced by fermentation ways or correlated positively with cultivated time. Therefore, sensory EUC values of fruit bodies could be beneficial to be used as functional foods with a palatable umami taste or food-flavoring materials.
TABLE 4 EUC value in three edible mushrooms at different cultivation stages
Conclusions
Free amino acid, 5’-nucleotides, and organic acid were obviously different at three cultivation stages of Pleurotus eryngii, Agrocybe aegerita, or Lentinus edodes, namely mycelia, primordium, and fruit bodies. Contents of some components in mushrooms were related with mushroom species and cultivated time. Because of the short production period, primordium, and mycelia might be more suitable for nutraceuticals industry to extract those nutrients. However, the contents of MSG-like free amino acids, flavor 5’-nucleotides, and EUC values were all higher in fruit bodies than that in mycelia of three mushrooms, respectively. The results revealed that Pleurotus eryngii, Agrocybe aegerita, and Lentinus edodes, possess a relatively strong umami taste at fruit bodies cultivation stage indicating that fruit bodies of the three mushrooms could served as well-flavored and functional foods. Moreover, difference of free amino acids, 5’-nucleotides, and organic acid at different cultivation stages might due to the different fermentation ways and the changes in gene expression related to those chemicals. Changes in gene expression of special nutrients and flavor at different cultivation stages of mushroom would be the interesting point of our further research.
FUNDING
This work was financially supported by the program of the National Natural Science Foundation of China (No. 31300112), Major Science and Technology Program for Water Pollution Control and Treatment (No. 2012ZX07204-004-003), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Additional information
Funding
REFERENCES
- Vidovic, S.; Zekovic, Z.; Jokic, S. Clavaria Mushrooms and Extracts: Investigation on Valuable Components and Antioxidant Properties. International Journal of Food Properties 2014, 17, 2072–2081.
- Mariga, A.M.; Yang, W.J.; Mugambi, D.K.; Pei, F.; Zhao, L.Y.; Shao, Y.N.; Hu, Q.H. Antiproliferative and Immunostimulatory Activity of a Protein from Pleurotus Eryngii. Journal of the Science of Food and Agriculture 2014, 94, 3152–3162.
- Tsai, S.Y.; Tsai, H.L.; Mau, J.L. Non-Volatile Taste Components of Agaricus Blazei, Agrocybe Cylindracea, and Boletus Edulis. Food Chemistry 2008, 107, 977–983.
- Craske, J.D.; Reuter, F.H. The Nitrogenous Constituents of the Dehydrated Mushroom, Boletus Edulis and Their Relation to Flavour. Journal of the Science of Food and Agriculture 1965, 16, 243–250.
- Cho, I.H.; Cho, H.K.; Kim, Y.S. Comparison of Umami-Taste Active Components in the Pileus and Stipe of Pine-Mushrooms (Tricholoma Matsutake Sing.) of Different Grades. Food Chemistry 2010, 118, 804–807.
- Saad, W.Z.; Hashim, M.; Ahmad, S.; Abdullah, N. Effects of Heat Treatment on Total Phenolic Contents, Antioxidant, and Anti-Inflammatory Activities of Pleurotus Sajor-Caju Extract. International Journal of Food Properties 2014, 17, 219–225.
- Mau, J.L. The Umami Taste of Edible and Medicinal Mushrooms. International Journal of Medicinal Mushrooms 2005, 7, 119–125.
- Li, W.; Gu, Z.; Yang, Y.; Zhou, S.; Liu, Y.F.; Zhang, J.S. Non-Volatile Taste Components of Several Cultivated Mushrooms. Food Chemistry 2014, 143, 427–431.
- Zhang, Y.; Venkitasamy, C.; Pan, Z.L.; Wang, W. Recent Developments on Umami Ingredients of Edible Mushrooms—A Review. Trends in Food Science & Technology 2013, 33, 78–92.
- Mau, J.L.; Chyau, C.C.; Li, J.Y.; Tseng, Y.H. Flavor Compounds in Straw Mushrooms Volvariella Volvacea Harvested at Different Stages of Maturity. Journal of Agricultural and Food Chemistry 1997, 45, 4726–4729.
- Taylor, M.W.; Hershey, R.A.; Levine, R.A.; Coy, K.; Olivelle, S. Improved Method of Resolving Nucleotides by Reverse-Phase High Performance Liquid Chromatography. Journal of Chromatography A 1981, 219, 133–139.
- Yamaguchi, S.; Yoshikawa, T.; Ikeda, S.; Ninomiya, T. Measurement of the Relative Taste Intensity of Some A-Amino Acid and 5’-Nucleotides. Journal of Food Science 1971, 36, 846–849.
- Lee, Y.L.; Jian, S.Y.; Mau, J.L. Composition and Non-Volatile Taste Components of Hypsizigus Marmoreus. LWT–Food Science and Technology 2009, 42, 594–598.
- Ribeiro, B.; Andrade, P.B.; Baptista, P.; Barros, L.; Ferreira, I.C.F.R.; Seabra, R.M.; Valentao, P. Leucopaxillus Giganteus Mycelium: Effect of Nitrogen Source on Organic Acids and Alkaloids. Journal of Agricultural and Food Chemistry 2008, 56, 4769–4774.