?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The chemical composition and antioxidant activity of juice extracted from seven samples of bergamot (Citrus bergamia Risso) collected in different areas of Reggio Calabria Province were investigated. The ascorbic acid, total polyphenol, and flavonoid contents were determined. Total flavonoids and polyphenols were analyzed by ultraviolet spectra, while flavanone content was analyzed by high-performance liquid chromatography. The antioxidant activity of the fractions was assessed using three representative assays: 2,2′-azinobis(3-ethylbenzothiazoline 6-sulfonic acid), 1,1-Diphenyl-2-picryl-hydrazyl radical quenching and β-carotene bleaching test. The main flavanones were naringin, neohesperedin, and neoeriocitrin, and their average content 242.4 ± 1.8, 183.0 ± 0.6, and 247.0 ± 1.4 mg mL–1, respectively. The results showed that bergamot juice possessed a good quality and a valuable source of health promoting constituents. In fact it contained eriocitrin, naringin, neoeriocitrin, and neohesperedin, which may contribute differentially to the antioxidant capacity.
INTRODUCTION
Bergamot (Citrus bergamia Risso) is thought to be a hybrid of the sour orange and citron or lemon. The fruit is spherical like an orange but yellow like a lemon. It has been known in the Mediterranean for several centuries and was described as early as 1708. Production is mostly limited to the Ionian coastal areas of the province of Reggio di Calabria (Italy). Bergamot is a symbol of the entire province, which grows about 90% of world production of this citrus fruit. This fruit is also cultivated in Côte d’Ivoire. However, the quality of the obtained essence is not comparable due to the argillite, limestone, and alluvial deposits found there.[Citation1] There are three bergamot cultivars: Fantastico, Castagnaro, and Femminello. The Fantastico cultivar is the most representative (90%).[Citation2]
Citrus derivatives are important in the field of food technology. However, even today, the technological processes often provide only partial extraction of all possible derivatives. In regards to, in particular, bergamot, the aim of the producer is limited almost exclusively to the extraction of the essential oil; the juice and peel are used only occasionally, although they could be a source of profit for growers.
The juice is generally considered a waste product, which represents a serious environmental and economic problem for the industries. Most studies have focused on its essential oil as this constitutes a raw material for the perfume and food industries.[Citation3,Citation4] However, the presence of neoriesperidin, naringin, and neoeriocitrin in its juice has also been reported.[Citation5–Citation7]
Bergamot juice and albedo are rich in neoeriocitrin, neohesperidin, naringin, rutin, neodesmin, and rhoifolin. The profile of the flavonoids in fruits of bergamot differs from other citrus not only in terms of quality but also quantity.[Citation8,Citation9] Flavonoids are specially known for their antioxidant activities,[Citation10–Citation12] which play a significant role in cardiovascular health and in prevention of cancer.[Citation13–Citation15] Other than fighting free radicals, they are also known for their antihistamine, antimicrobial, memory enhancing, and even mood-boosting properties. In recent years, the beneficial properties of bergamot juice have been generating interest, and have been the subject of several studies.[Citation16–Citation20] Some researchers have focused on the molecular mechanisms of the bioactive compounds contained in the bergamot juice. The obtained results suggest that the flavonoids of bergamot juice may be useful for the development of alternative pharmacological strategies aimed at reducing the inflammatory process.[Citation21–Citation23]
Several studies have shown that flavonoids present in bergamot juice, lower cholesterol levels by modulating hepatic HMG-CoA levels.[Citation24–Citation26] Recently a research group of University of Catanzaro (Italy) has shown that administration of bergamot juice leads to a significant reduction in serum cholesterol and triglycerides. Moreover, the flavonoids present in the juice of this citrus fruit have a lipid-lowering action. Its effectiveness was tested on patients with hypercholesterolemia with and without type 2 diabetes, and on rats who also had an altered lipid profile. Intake of bergamot juice 20 min before meals helped reduce both cholesterol and triglycerides in patients. Moreover, none of the participants in the trial showed intolerance or side effects after taking the bergamot juice.[Citation27] An analytical differentiation of citrus juices, based on the differing concentrations of certain minor components, including flavonoids, plays an important role in determining chemotaxonomic markers to ascertain the authenticity of commercial products.[Citation28] The aim of the present article was to evaluate the chemical composition and antioxidant activity in juice obtained from bergamot fruits collected Catona and Africo in a 90 km coastal stretch of the province of Reggio Calabria, Southern Italy.
MATERIALS AND METHODS
Plant Material and Juice Extraction
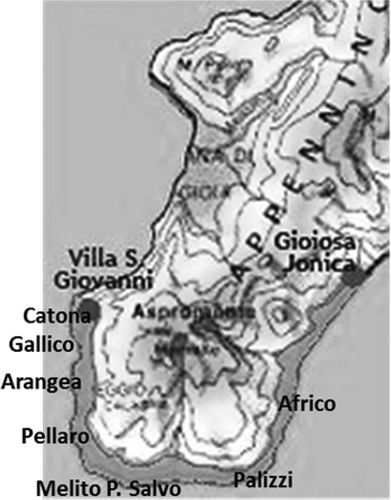
Citrus bergamia Risso fruits cv Fantastico were collected in February 2014 from plantations located between Catona and Africo (Reggio Calabria, Italy). Plantations were chosen in seven areas of the coast were bergamot is cultivated: Catona, Gallico, Arangea, Pellaro, Africo, Melito Porto Salvo, and Palizzi (). All plantations were made up of plants aged 15–20 years and irrigated by sprinkler. Fruits were harvested at maturity stage and at full fruit size from a random sample of 15 plants in order to obtain a set of fruits that are representative. Plants received similar water and fertilizer treatments. Fruits were examined for integrity and absence of dust and insect contamination. Immediately after harvesting, the juice was extracted by mechanical pressure and frozen at –80°C until analyzed.
Reagent
Hesperidin, narirutin, naringin, neohesperidin, neoeriocitrin, linoleic acid, and β-carotene were purchased from Sigma Chemical Company (Milan, Italy). Ascorbic acid, Folin-Ciocalteu (FC) reagent and 2,2-diphenil-1-picrylhydrazyl radical (DPPH) and 2,2′-azinobis (3-ethyl-benzothiazoline-6-sulphonate; ABTS) were supplied by Carlo Erba (Milan, Italy). Solvents and reagents not expressly specified had a high degree of purity and were supplied by Carlo Erba (Milan, Italy).
Physicochemical Analysis
The bergamot were squeezed and the juice was centrifuged and filtered to determine the following analyses: The color of fresh juice was measured at 25°C using a Konica Minolta CM-700/600d spectrophotometer (Konica Minolta Sensing, Japan). Data were expressed as L* (lightness/darkness in a range 0–100), a* (greenness/redness in a range between –60 and +60), and b* (blueness/yellowness in a range between –60 and +60). The total soluble solids (TSS) were determined using a digital refractometer PR-201α (Atago, Tokyo, Japan), previously calibrated at 20°C and the results expressed as degrees Brix; The pH was measured at ambient temperature with a pH meter (Model Basic 20, Crison) previously calibrated with standard solutions pH 4 and pH 7; The total acidity (TA) was determined using the International Federation of Fruit Juice producers test:[Citation29] A potentiometric titration of the acidity of the juice, with a solution of 0.25 N NaOH up to pH 8.1. The results were expressed as g L–1 of anhydrous and hydrate citric acid. Ascorbic acid was determined using the International Federation of Fruit Juice producers test:[Citation29] A potentiometric titration of the acidity of the juice, with a solution of 2,6-dichloroindophenol. Total flavanones were determined with a colorimetric method using alkaline diethylene glycol for determination of the bitter rhamnoglycoside naringin and other flavanones that may be present in citrus fruits.[Citation30] The bergamot juice was analyzed for total phenolics by the FC colorimetric method.[Citation31] All determinations above described were made in triplicate.
Flavanone Analysis
Flavanone glycosides, expressed as hesperidin equivalents (mg L–1), were determined by liquid chromatograph according to the official methodologies of the International Federation of Fruit Juice producers.[Citation29] Fresh bergamot juice previously centrifuged at 4000 rpm for 20 min, was filtered through a 0.45 μm membrane filter. Separation of flavanones was performed by high-performance liquid chromatography (HPLC) using a Phenomenex C18 column (150 mm × 3 mm). The solvent system was buffer solution A: ACN/H2O/H2PO4 (70:26:4) and buffer solution B: KH2PO4 pH 3.5, with flow-rate 500 μL/min in gradient conditions. The analysis was monitored at 287 nm. The gradient program was as follows: starting condition, 85% A, 15% B; 5 min, 70% A, 30% B; 20 min, 50% A, 50% B; 30 min, 25% A, 75% B; 35 min, 5% A, 95% B; 40 min, 85% A, 15% B. The column was operated at 25°C and flow rate was 1 mL min–1. Identification of compounds was performed by comparing their retention time with those of standards and confirmed with characteristic spectra using the photodiode array detector.
Flavanone glycoside evaluation was calculated according to the external standard method by integration of the peak areas of individual compounds to that of standard curve prepared using hesperidin standard.
Antioxidant Activity
DPPH assay
This experimental procedure was described by Loizzo et al.[Citation32] In an ethanol solution of DPPH radical (final concentration was 1.0 × 10–4 M), samples at different concentrations were added. The reaction mixtures were shaken and kept in the dark for 30 min. The absorbance of the resulting solutions was measured in 1 cm cuvettes using a Perkin Elmer Lambda 40 ultraviolet/visible (UV/Vis) spectrophotometer at λ = 517 nm against blank without DPPH. A decrease of DPPH solution absorbance indicates an increase of DPPH radical scavenging activity. This activity is given as % DPPH radical-scavenging that is calculated in the equation:
ABTS assay
ABTS assay was based on the method previously described by Loizzo et al.[Citation32] with slight modifications. ABTS radical cation (ABTS+) was produced by the reaction of a 7 mM ABTS solution with 2.45 mM potassium persulphate. The mixture was stored in the dark at room temperature for 12 h before use. The ABTS+ solution was diluted with ethanol to an absorbance of 0.70 ± 0.05 at λ = 734 nm. After addition of 25 μL of sample or Trolox standard to 2 mL of diluted ABTS+ solution, absorbance was measured at exactly 6 min after mixing. Appropriate solvent blanks were run in each assay. The scavenging ability of the sample was calculated according to the following equation:
where A0 is the absorbance of the control reaction and A is the absorbance in the presence of samples.
ß-carotene bleaching test
Antioxidant activity was determined using β-carotene bleaching as previously described by Menichini et al.[Citation33] Briefly, β-carotene solution was added to linoleic acid and 100% Tween 20. The emulsion was mixed with of samples at different concentrations and tubes were placed at 45°C in a water bath for 60 min. Propyl gallate was used as standard. The absorbance of the samples, standard and control was measured at 470 nm against a blank at t = 0 and successively at 30 and 60 min.
Statistical Analysis
All experiments were carried out in triplicate. Data were expressed as means ± standard deviation (SD). Analysis of variance (one-way ANOVA) was conducted using SPSS version 17.0 for Windows (SPSS Inc., Chicago, IL) and the Tukey’s test was used to determine any significant difference among all treatments at p < 0.05. The concentration giving 50% inhibition (IC50) was calculated by non-linear regression with the use of Prism GraphPad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA, USA). The dose-response curve was obtained by plotting the percentage inhibition versus concentration. Differences within and between groups were evaluated by one-way ANOVA test followed by a multicomparison Dunnett’s test compared with the positive controls.
RESULTS AND DISCUSSION
The bergamot juice extracted from fruits collected in seven different areas of the coast of the province of Reggio Calabria (Italy), were examined in order to determine their antioxidant activity as related to their phenolic composition. shows the areas where the fruits were collected with their latitude and longitude. There were significant differences (p < 0.05) in all color attributes among samples studied (). With respect to lightness (L*), the lowest value corresponded to the Melito, Catona, and Palizzi samples, while the highest values were those samples from Gallico, Arangea, and Pellaro.
TABLE 1 Sites of collection of Citrus bergamia in province of Reggio Calabria (Italy)
TABLE 2 Average colorimetric values of bergamot juice
shows the values of the chemical-physical characteristics (pH, titratable acidity, soluble solid, and formol index) of the bergamot juice. The pH values of the juices analyzed are within normal range (2.54–2.84) and the differences between them are significant (p < 0.05). The lowest value was in the Africo sample and the highest (pH 2.84) in the Catona sample. Titratable acidity was significantly different: values were in the range 11.38–14.83. Soluble solid content measured for bergamot juice was in the range 8.11–11.61 Brix. The formol index was used to estimate the total content of amino acids in a juice, and partly to estimate its purity. In the seven juices analyzed, the values obtained show that there are significant differences.
TABLE 3 Physicochemical data of bergamot juice
shows that the ascorbic acid, total flavonoid and total polyphenol content in all treated samples was significantly different (p < 0.05). Vitamin C (ascorbic acid), flavonoid, and phenolic compounds are essential components for human health, and have a high antioxidant activity, providing protection against free radicals and consequently participating in the prevention of many degenerative diseases. Ascorbic acid content was in the range 89.40–285.35 mg 100 mL–1. The Gallico sample was significantly lower (89.4 ± 2.1 mg 100 mL–1) than that of other samples. Similarly, the total flavonoid content showed the lowest value for the Gallico and Palizzi samples (51.1 ± 2.2 mg 100 mL–1 and 74.0 ± 2.1 mg 100 mL–1, respectively). The highest content of total flavonoids was found in the samples from Arangea and Pellaro (148.2 ± 3.4 mg 100 mL–1 and 147.8 ± 4.6 mg 100 mL–1, respectively). With regard to total polyphenols, significant differences in the various samples analyzed were observed, their content being between 180.5 ± 2.9 mg 100 mL–1 and 233.4 ± 0.5 mg 100 mL–1. The Catona sample was significantly lower (180.5 ± 2.9 mg 100 mL–1) compared to the other samples.
TABLE 4 Values of ascorbic acid, total flavonoid and total phenolic of Citrus bergamia (juice)
shows the data collected for the flavonoid content of bergamot juice. The most abundant components in bergamot juices are naringin, neoeriocitrin, and neohesperedin, as reported by Gionfriddo et al.,[Citation5] Kawaii et al.,[Citation6] Calabrò et al.,[Citation34] Dugo et al.,[Citation8] Gattuso et al.,[Citation35] and Nogata.[Citation9]
TABLE 5 Flavanone content (mg L–1) in bergamot juice
The lowest values of flavonoids identified by HPLC on the basis of retention times and quantified from the peak areas compared to standards, are those collected from the areas of Gallico and Palizzi.
Reactive oxygen species (ROS) are closely related to many pathological conditions, such as inflammation, tumors, cardiovascular disease, cerebral ischemia, and diabetes. DPPH· and ABTS· are stable free radicals, which have been widely accepted as a tool for estimating free radical scavenging activities of antioxidants.[Citation36] All samples exhibited a radical scavenging activity against both radicals in a concentration-dependent manner (). The area of collection influenced the DPPH radical scavenging activity with a range of IC50 values from 19.6 ± 2.0 to 31.4 ± 1.1 µg mL–1 (Melito and Palizzi samples, respectively). The sample was also Melito the most effective also in the ABTS test with IC50 value of 17.4 ± 1.6 µg mL–1 followed by the Africo sample (IC50 value of 18.9 ± 2.4 µg mL–1). Correlation analysis revealed that the DPPH assay is positively correlated with total phenol and flavonoid content. However, total phenols also positively correlated with ABTS data. The potential of bergamot juice to inhibit lipid peroxidation was evaluated using the β-carotene/linoleic acid bleaching test, which measures the capacity for inhibiting conjugated diene hydroperoxide formation during linoleic acid oxidation. Even in this case the Melito was sample the most active (IC50 values of 25.7 ± 1.2 and 24.9 ± 3.0 µg mL–1 at 30 and 60 min of incubation). The correlation analysis revealed that data obtained from this assay are positively correlated with vitamin C and total flavonoids, which are mainly responsible for antioxidant activity. A significant positive correlation was also observed with eriocitrin, naringin, neoeriocitrin, and neohesperidin content. Previously, Gardner et al.[Citation37] reported that vitamin C was found to account for 65–100% of the antioxidant potential of citrus juice.
TABLE 6 Antioxidant activity of Citrus bergamia Risso
The antioxidant potential of C. bergamia juice has been previously investigated by Trovato et al.[Citation38] that found a noticeable effect on scavenging DPPH radicals with IC50 value of 25.01 µL. Xu et al.[Citation39] studied the total phenolic content and antioxidant activities of 15 citrus variety juices. The total phenolic content ranged from 751.82 to 1555.49 mgL–1 for C. limon and hybrid 439 (C. eticulate × C. sinensis), respectively. It was interesting that Hybrid 439 achieved the highest DPPH inhibitory activity with a percentage of inhibition of 61.62% followed by C. sinensis Osbeck cv Hamlin and cv Liubencheng that exhibited a DPPH· scavenging ability of 60.24 and 60.13%. Interestingly in Citrus investigated by Xu et al.,[Citation39] neohesperidin was found only in two varieties.
CONCLUSIONS
For all the studied characteristics there were statistically significant differences (p < 0.05) between the juices analyzed. The purpose of this thesis was to see how the microclimate of a given area of cultivation may influence the quality and, therefore, the chemical composition of the juice itself. Results obtained in this study are a contribution to the characterization of bergamot fruits (Citrus bergamia Risso) cultivated in different areas of the province of Reggio Calabria (Italy).
REFERENCES
- Mandalari, G.; Bennett, R.B.; Bisignano, G.; Saija, A.; Dugo, G.; Lo Curto, R.B.; Faulds, C.B.; Waldron, K.W. Characterization of Bergamot (Citrus Bergamia Risso) Pulp, a Major Co-Product of Essential Oil Extraction. Journal of Agricultural and Food Chemistry 2006, 54, 197–203.
- Dugo, G.; Lamonica, G.; Cotroneo, A.; Trozzi, A.; Crispo, F.; Licandro, G.; Gioffrè, D. La Composizione Della Frazione Volatile Dell’essenza Di Bergamotto [Composition of volatile fraction of bergamot essential oil]. Stazione Sperimentale Per L’industria Delle Essenze E Dei Derivati Agrumari Reggio Calabria (1987). Collana Di Monografie Sugli Oli Essenziali E Sui Derivati Agrumari.
- Ricci, H.; Rovesti, P. Impieghi Cosmetologici Dell’olio Essenziale Di Bergamotto [Cosmetic uses of bergamot essential oil]. EPPOS, 1979, 61, 18.
- Poiana, M.; Reverchon, E.; Sicari, V.; Mincione, B.; Crispo, F. Supercritical Carbon Dioxide Extraction of Bergamot Oil. Bergapten Content in the Extracts. Italian Journal of Food Science 1994, 4, 59–66.
- Gionfriddo, F.; Postorino, E.; Bovalo, F. I Flavanoni Glucosidici Nel Succo Di Bergamoto. Essenza e Derivati Agrumari [Flavonoid glycoside in bergamot juice]. 1996, 4, 404–416.
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. Quantitation of Flavonoid Constituents in Citrus Fruits. Journal of Agricultural and Food Chemistry 1999, 47, 3565–3571.
- Pernice, R.; Borriello, G.; Ferracane, R.; Borrelli, RC.; Cennamo, F.; Ritieni, A. Bergamot: A Source of Natural Antioxidants for Functionalized Fruit Juices. Food Chemistry 2009, 112, 545–550.
- Dugo, P.; Lo Presti, M.; Ohman, M.; Fazio, A.; Dugo, G.; Mondello, L. Determination of Flavonoids in Citrus Juices by Micro-HPLC-ESI-MS. Journal of Separation Science 2005, 28, 1149–1156.
- Nogata, Y.; Sakamoto, K.; Schiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid Composition of Fruit Tissues of Citrus Species. Bioscience Biotechnology and Biochemistry 2006, 70, 178–192.
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Scientific World Journal 2013. http://dx.doi.org/10.1155/2013/162750.
- Wong, J.Y.; Matanjun, P.; Ooi, Y.B.H.; Chia, K.F. Evaluation of Antioxidant Activities in Relation to Total Phenolics and Flavonoids Content of Selected Malaysian Wild Edible Plants by Multivariate Analysis. International Journal of Food Properties 2014, 17, 1763–1778.
- Li, L.; Wang, S.; Chen, J.; Xie, J.; Wu, H.; Zhan, R.; Li, W. Major Antioxidants and in Vitro Antioxidant Capacity of Eleven Mango (Mangifera Indica L.) Cultivars. International Journal of Food Properties 2014, 17, 1872–1887.
- Hollman, P.C.; Katan, M.B. Dietary Flavonoids: Intake, Health Effects, and Bioavailability. Food Chemistry Toxicology 1999, 37, 937–942.
- Navarra, M.R.; Ursino, N.; Ferlazzo, M.; Schumacher, U.; Valentiner, U. Effect of Citrus Bergamia Juice on Human Neuroblastoma Cells in Vitro and in Metastatic Xenograft Models. Fitoterapia 2014, 95, 83–92.
- Delle Monache, S.; Sanità, P.; Trapasso, E.; Ursino, M.R.; Dugo, P.; Russo, M.; Ferlazzo, N.; Calapai, G.; Angelucci, G.; Navarra, M. Mechanisms Underlying the Anti-Tumoral Effects of Bergamia Juice. PLoS ONE 2013, 8(4), 8–16.
- Barbera, N.; Pendino, F. Bergamot Symposium (Reggio Calabria, November 30–December 2) 1998.
- Matsumoto, H.; Ikoma, Y.; Sugiura, M.; Yano, M.; Hasegawa, Y. Identification and Quantification of the Conjugated Metabolites Derived from Orally Administered Hesperidin in Rat Plasma. Journal of Agricultural and Food Chemistry 2004, 52, 6653–6659.
- Picerno, P.; Sansone, F.; Mencherini, T.; Prota, L.; Aquino, R.P.; Rastrelli, L.; Lauro, M.R. Citrus Bergamia Juice: Phytochemical and Technological Studies. Natural Product Communications 2011, 6, 951–955.
- Ferlazzo, N.; Visalli, G.; Smeriglio, A.; Cirmi, S.; Lombardo, G.E.; Campiglia, P.; Di Pietro, A.; Navarra, M. Flavonoid Fraction of Orange and Bergamot Juices Protect Human Lung Epithelial Cells from Hydrogen Peroxide-Induced Oxidative Stress. Evidence-Based Complementary and Alternative Medicine. 2015, Published online 2015 June 21. DOI:10.1155/2015/957031
- Ursino, M.; Mondello, M.R.; Sdrafkakis. V.; Campolo, L., Taviano, M.F.; Miceli, N. Attività Citoprotettiva Del Succo Di Citrus Bergamia Risso & Poiteau Sull’Apparato Gastrointestinale [Cytoprotective activities of juice “Citrus bergamia Risso & Poiteau” on gastroenterological tract]. Fitomed 2006 (Taormina, July 6–8).
- Impellizzeri, D.; Bruschetta, G.; Di Paola, R.; Ahmad, A.; Campolo, M.; Cuzzocrea, S.; Esposito, E.; Navarra, M. The Anti-Inflammatory and Antioxidant Effects of Bergamot Juice Extract (Bje) in An Experimental Model of Inflammatory Bowel Disease. Clinical Nutrition 2014, 34, 1146–1154.
- Sommella, E.; Pepe, G.; Pagano, F.; Tenore, G.; Marzocco, S.; Manfra, M.; Calabrese, G.; Aquino, R.P.; Campiglia, P. UHPLC Profiling and Effects on LPS-Stimulated J774A.1 Macrophages of Flavonoids from Bergamot (Citrus Bergamia) Juice, An Underestimated Waste Product with High Anti-Inflammatory Potential. Journal of Functional Foods 2014, 7, 641–649.
- Risitano, R.; Currò, M.; Cirmi, S.; Ferlazzo, N.; Campiglia, P.; Caccamo, D.; Ientile, R.; Navarra, M. Flavonoid Fraction of Bergamot Juice Reduces LPS-Induced Inflammatory Response Through SIRT1-Mediated NF-kappab Inhibition in THP-1 Monocytes. PLoS ONE 2014, 9(9). DOI: 10.1371/journal.pone.0107431
- Terpstra, A.H.; Lapre, J.A.; De Vries, H.T.; Beynen, A.C. Dietary Pectin with High Viscosity Lowers Plasma and Liver Cholesterol Concentration and Plasma Cholesteryl Ester Transfer Protein Activity in Hamsters. Journal of Nutrition 1998, 128, 1944–1949.
- Kim, H.K.; Jeong, T.S.; Lee, M.K.; Park, Y.B.; Choi, M.S. Lipid-Lowering Efficacy of Hesperetin Metabolites in High-Cholesterol Fed Rats. Clinical Chimica Acta 2000, 3327, 129–137.
- Marounek, M.; Volek, Z.; Synytsya, A.; Copikova, J. Effect of Pectin and Amidated Pectin on Cholesterol Homeostasis and Cecal Metabolism in Rats Fed a High-Cholesterol Diet. Physiological Research 2007, 56, 433–442.
- Mollace, V.; Sacco, I.; Janda, E.; Malara, C.; Ventrice, D.; Colica, C.; Visalli, V.; Muscoli, S.; Ragusa, S.; Muscoli, C.; Rotiroti, D.; Romeo, F. Hypolipemic and Hypoglycaemic Activity of Bergamot Polyphenols: From Animal Models to Human Studies. Fitoterapia 2011, 82, 309–316.
- Hammond, D.A. Authenticity of Fruit Juices, Jams, and Preserves. In Food Authentication; Ashurst, P.R.; Dennis M.J.; Eds.; Chapman & Hall: London, UK, 1996; 32–36.
- IFU (3-17-58) International Federation of Fruit Juice Producers. Methods of Analysis and Microbiological Methods. Retrieved from http://www.ifu-fruitjuice.com/ifu-methods
- Davis, W.B. Determination of Flavanones in Citrus Fruits. Journal of Industrial and Engineering Chemistry 1947, 19, 476.
- Singleton, V.L.; Orthofer, R.; Lamanuela-Raventòs, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods in Enzymology 1999, 299C, 152–178.
- Loizzo, M.R.; Tundis, R.; Bonesi, M.; Menichini, F.; Mastellone, V.; Avallone, L.; Menichini, F. Radical Scavenging, Antioxidant, and Metal Chelating Activities of Annona Cherimola Mill. (Cherimoya) Peel and Pulp in Relation to Their Total Phenolic and Total Flavonoid Contents. Journal of Food Composition and Analysis 2012, 25, 179–184.
- Menichini, F.; Tundis, R.; Bonesi, M.; Loizzo, M.R.; Conforti, F.; Statti, G.; De Cindio, B.; Houghton, P.J.; Menichini, F. The Influence of Fruit Ripening on the Phytochemical Content and Biological Activity of Capsicum Chinense Jacq. Cultivar Habanero. Food Chemistry 2009, 114, 553–560.
- Calabro, M.L.; Galtieri, V.; Cutroneo, P.; Tommasini, S.; Ficarra, P.; Ficarra, R. Study of the Extraction Procedure by Experimental Design and Validation of a LC Method for Determination of Flavonoids in Citrus Bergamia Juice. Journal of Pharmacological and Biomedical Analysis 2004, 35, 349–363.
- Gattuso, G.; Caristi, C.; Gargiulli, C.; Bellocco, E.; Toscano, G.; Leuzzi, U. Flavonoid Glycosides in Bergamot Juice (Citrus Bergamia Risso). Journal of Agricultural and Food Chemistry 2006, 54, 3929–3935.
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A Review of the Antioxidant Potential of Medicinal Plant Species. Food and Bioproducts Processing 2010, 89, 217–233.
- Gardner, P.T.; White, T.A.C.; McPhail, D.B.; Duthie, G.G. The Relative Contributions of Vitamin C, Carotenoids, and Phenolics to the Antioxidant Potential of Fruit Juices. Food Chemistry 2000, 68, 471–474.
- Trovato, A.; Taviano, M.F.; Pergolizzi, S.; Campolo, L.; De Pasquale, R.; Miceli, N. Citrus Bergamia Risso & Poiteau Juice Protects Against Renal Injury of Diet-Induced Hypercholesterolemia in Rats. Phytotherapy Reserarch 2010, 24, 514–519.
- Xu, G.; Liu, D.; Chen, J.; Ye, X.; Ma, Y.; Shi, J. Juice Components and Antioxidant Capacity of Citrus Varieties Cultivated in China. Food Chemistry 2008, 106, 545–551.