?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The influence of different heat treatments on the protein aggregates and changes in gelation properties of rennet-induced milk gels were investigated. In the heated milk, a visible difference in milk serum proteins was found resulting from the formation of protein aggregates. Meanwhile, the size of protein aggregates increased from 25 to 170 nm with increasing the intensity of heat treatment. Furthermore, the differences in textural variables of rennet gels were found among the heat treatments using the principal component analysis. The water holding capacity and cheese curd yield of rennet gels obtained from the heated milk were significantly greater than those of raw milk (p < 0.05). It was also found heat treatments above 80°C could endow rennet-induced milk gels with novel textural properties.
INTRODUCTION
Whey is produced as byproduct in the cheese industry. It is a complex mixture of different proteins. In general, the main components include β-lactoglobulin (~55%) and α-lactalbumin (~24%).[Citation1] The protein-rich whey was considered a waste product suitable only for livestock feed or dumping into rivers aggravating environmental problems.[Citation2] Whey proteins may be retained by heat treatment and other technologies, such as ultra-filtration and high pressure processing.[Citation3,Citation4] However, the negative effects of integrating whey protein such as on the development of proteolysis in cheddar cheese and on the reduced melting and stretching properties in mozzarella cheese made attempts to commercialize these cheeses between 1980 to 1989 unsuccessful.[Citation5] It was found that heat treatment (at 72°C for 5 min) affected the sensory and chemical properties of urfa cheese.[Citation6] Most studies were also performed to reduce the drawbacks such as by pH control and by optimization for production.[Citation7–Citation11]
On the other hand, the whey protein-enriched cheese manufacture found applications in cheese that are consumed fresh as well as some soft ripened cheese varieties.[Citation12] When whey proteins are integrated into these cheeses, this not only improves the nutrient value and yield, but also causes changes to functional properties.[Citation3] In many cheese plants, the heat treatment has been selected to retain whey proteins for economic reason. Furthermore, the heat treatment of cheese milk above typical pasteurization conditions results in changes in the body, texture, and other properties that are found in traditional cheeses.[Citation13,Citation14]
Textural properties of rennet gels are important for controlling subsequent processing conditions and for determining the consumer acceptability of a final product. Textural properties are influenced not only by the native milk properties, such as proteins, fat, and native pH, but also by the heat processing conditions.[Citation15] Heat treatment may increase the shear storage modulus and the curd firming rates.[Citation16] The denatured whey proteins can be incorporated into milk curd, resulting in a higher yield, and the interactions of whey proteins with casein micelles interfere with the rennet coagulation process, resulting in weak curd structures.[Citation13] Although many investigations have been focused on the effects of heat treatment on the textural properties of traditional cheese products, there is little information on the heat-induced textural changes in rennet gels for the purpose of controlling the process of production and developing different cheese varieties.
The sensory characteristics of cheese seem to be the main obstacle to some customers, especially in East Asia.[Citation17] By improving the taste or expanding the product categories, this problem could be advantageously solved. The present research aims to assess the textural properties of rennet gel for supervising the optimization of pretreatment and predicting the final products as an assisting indicator. Furthermore, the protein aggregates and their sizes were also preliminarily evaluated.
MATERIALS AND METHODS
Heat Treatments
Raw milk (RM) was heated as described by Alloggio et al.[Citation18] with the following modifications; the milk was dispensed into test tubes (Ф10 × 150 mm) in 10-mL aliquots. The tubes were immersed in a boiling water bath until the desired temperature (70, 80, or 90°C) was reached, and then transferred rapidly to a stirred water bath at the desired temperature and held for 2, 5, and 8 min, respectively. Finally, the milk was cooled to an ambient temperature by immersing the test tubes in a stirred water bath at 0–4°C.
Preparation of Serum
This pretreatment was performed using the method established by Guyomarc’h et al.[Citation19] Briefly, the heated milk was centrifuged (4500 × g, 4°C) for 20 min to collect the skimmed milk. A second centrifugation (25,000 × g) at 4°C for 1 h was used to further clarify the sample. The supernatant was filtered through a 0.22 μm membrane filter (Millipore Corporation, USA) for size exclusion chromatography (SEC) and dynamic light scanning (DLS) analysis.
SEC
The SEC was performed according to the method of Sandra and Dalgleish.[Citation20] A chromatography system (ÄKTApurifier, GE, USA) equipped with a 2.0 mL sample loop and an ultra violet detector (280 nm) was used. The column was a Pharmacia XK-16 column packed with Sephacryl S-500 High Resolution gel (GE, USA). The mobile phase was 20 mM Tris-HCl buffer (pH 7.0) containing 0.02% (w/v) NaN3. The flow rate was set at 1.0 mL/min and a 180 min run was performed for each sample.
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
The SDS-PAGE was performed on some selected fractions separated by SEC. The stacking gel (5%) and separating gel (12.5%) were used on a Mini-Protean Tetra Electrophoresis System (Bio-Rad Laboratories, Hercules, CA, USA). The gels were stained with Coomassie blue R250.
DLS
The DLS analysis was performed using a particle size analyzer (ZetaPLAS, Brookhaven Instruments Corporation, USA) according to the method of Beliciu and Moraru[Citation21] with the following modifications. The particle size measurements were conducted at constant temperature of 25°C with a wavelength of 660 nm. No any dilution was used before the DLS analysis. The multimodal size distribution (MSD) was analyzed with the software packages of ZetaPlus Particle Sizing Software Version 3.83.
Preparation of Rennet Gel
The rennet gel of heated milk was performed in a beaker (Ф65 × 100 mm). In details, the heated milk was tempered at 30°C for 2 h using a water bath. Commercial calf rennet (Naturen, Chr. Hansen’s Laboratory, Germany) was added to reach a final rennet concentration of 0.2 g/L. The milk sample (80 mL) was sealed and placed at 30°C for coagulation (4 h). The coagulated sample was stored at 4–6°C until analyzed (~2 h).
Instrumental Textural Profile Analysis (TPA) of Gels
Both the undisturbed gel and the homogeneous stirred gel were analyzed on a texture analyzer (TA.XT.plus, Stable Micro Systems Ltd, UK) with a 5 kg load cell. The stirred gel was prepared by stirring the undisturbed gel with a stirrer at 100 rpm for 15 min, and a 15-min equilibration period was elapsed before measurements. The measurements were conducted at 4–6°C using a 35-mm diameter perspex probe. The test speed was set to 1.0 mm/s for a distance of 10.0 mm. Hardness (Har), adhesiveness (Adh), springiness (Spr), cohesiveness (Coh), chewiness (Che), gumminess (Gum), and resilience (Res) were recorded using the TPA macro in software program called Texture Expert Excede Version 1.0 (Stable Micro Systems Software).
Water Holding Capacity (WHC) and Cheese Curd Yield (CCY)
WHC and CCY of rennet gel were estimated as described by Pandey et al.[Citation22] and by Macheboeuf et al.[Citation23]
Microstructure of Curd
The rennet gel was centrifuged at 13,500 × g for 15 min (4°C). The microstructure of curd was observed by scanning electron microscope (SEM) according to the method of Serrano et al.[Citation24]
Statistical Analysis
All the analysis mentioned above were performed in triplicate. Experimental data were statistically analyzed using PASW Statistics 18.0 Software (SPSS Inc, USA). One-way analysis of variance (ANOVA) using the least significant difference (LSD) test (p < 0.05) was applied to examine the effect of the heat treatments. The principal component analysis (PCA) was applied for dimensionality reduction. The components where the eigenvalue was greater than 1 were used. The regression analysis was carried out for model building.
RESULTS AND DISCUSSION
Evaluation of Milk Protein Aggregates
shows the typical elution profiles of milk serum proteins. Note that the heat treatments were simplified as temperature/time (hereinafter the same). Milk proteins and aggregates were eluted successively from large to small by the size of molecule, i.e., fat or casein residues, protein aggregates, polymers of whey proteins, and whey proteins.[Citation19,Citation25] Moreover, the large and small whey proteins can be distinguished in the profiles of SEC.
FIGURE 1 Effect of heating temperature and time on the protein aggregation in milk serum. The typical elution profiles from 40 to 140 min were used for observation. The heated milk was simplified as temperature/time. Raw milk (RM) in all groups was for comparison. mAU: milli-absorbance units. Upward arrow points to the whey proteins, and downward arrow points to the protein aggregates.
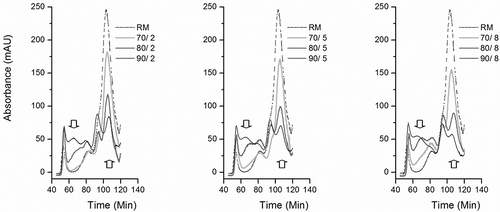
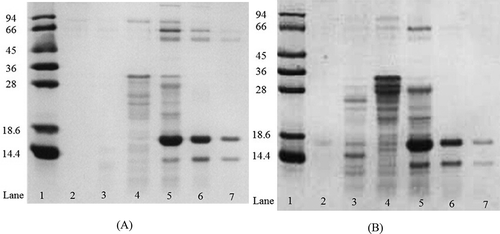
In contrast to that of RM, the profiles of the SEC elution from the heated milk showed large changes. The elution fragment (~105 min), composed of main whey proteins, β-lactoglobulin (18 kDa) and α-lactoalbumin (14 kDa; lanes 6 and 7 in a and b), decreased in absorbance due to the denaturation and aggregation of the whey proteins. Therefore, the elution fragment (from ~65 to ~88 min), used as the indicator of the serum protein aggregates (shown in lanes 3, 4, and 5 of ), increased with the increasing intensity of heat treatment. The finding is in agreement with the studies of Guyomarc’h et al.[Citation19] on heated milk, and Angel and Dalgleish[Citation26] on reconstituted skim milk powder.
FIGURE 2 SDS-PAGE analysis of the SEC fractions from (A) RM, and from (B) the heated milk. Lane 1, the standard proteins (kDa). Lanes 2–7 were from 53–56, 65–68, 77–80, 85–88, 105–108, and 113–116 min of SEC elution, respectively.
For the same heating time, clear differences in protein aggregates and whey proteins could be observed among the obvious heat treatments, i.e., 70, 80, and 90°C (). As we know, the heat treatment of milk above 60°C leads to the denaturation of the whey proteins, resulting in their interaction with each other and with κ-casein to form heat-induced serum and micelle-bound protein aggregates, respectively, in the serum phase of milk, and on the surface of the casein micelles.[Citation27,Citation28] In our study, the level of whey proteins showed a large change between RM and the heated milk, especially in those above 80°C. Meanwhile, the visible difference was also found in the protein aggregates.
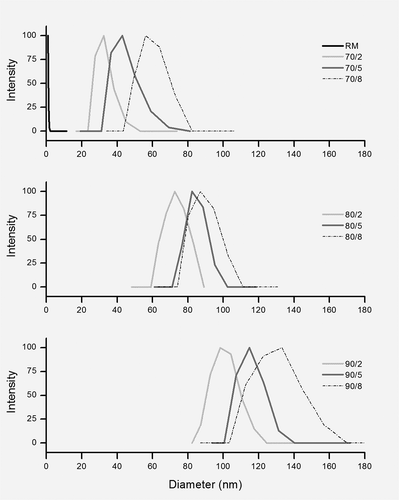
To better observe, at the molecular level, the changes occurring to the hydrodynamic size of the protein aggregates, DLS was also employed. shows the typical profiles of MSD as affected by the heat treatments. In RM, no protein aggregates but whey proteins (<2 nm) were found in MSD. When milk was heated, the protein aggregates were produced in the different sizes. Angel and Dalgleish[Citation26] isolated the whey protein/κ-casein aggregates with SEC and detected their size in elute (50–110 nm). For the first time, in milk serum, we report the sizes distributions of protein aggregates with DLS and observe their differences due to the heat treatments. Altogether, the size of protein aggregates increased from 25 to 170 nm (30–140 nm, mainly) with increasing the intensity of heat treatment.
FIGURE 3 The typical profiles of multimodal size distribution of whey proteins and milk protein aggregates in milk serum.
Casein micelles has been described as an adhesive hard sphere particle.[Citation29,Citation30] The extended brush-like κ-casein layer on the micelles causes colloidal stability mostly due to steric repulsion, and the hydrolysis of the polyelectrolyte brush causes destabilization making the micelles sticky spheres.[Citation30] The denatured whey proteins due to heat treatment can react with κ-casein and then cause the destabilization of protein matrix.
Textural Properties of Gels
The textural variables were acquired to assess the texture of the undisturbed gels () and homogeneously stirred gels (). The undisturbed gel from heated milk had the lower Har, Adh, Spr, Gum, and Che than those of RM. Only the treatments of 80/8 and 90/8 significantly increased Coh of the undisturbed gel (p < 0.05) compared to that of RM. Changes of Res were not clear among the heated and unheated gels. On the other hand, the stirred gel from heated milk had the lower Har, Adh, Gum, and Che, and higher Spr, Coh, and Res than that of RM.
TABLE 1 Effects of heat treatment on the textural variables in the undisturbed gel
TABLE 2 Effects of heat treatment on the textural variables in the stirred gel
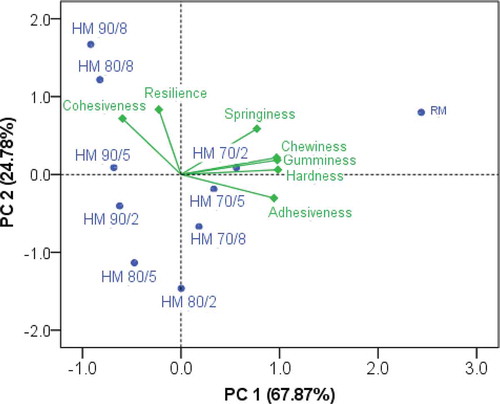
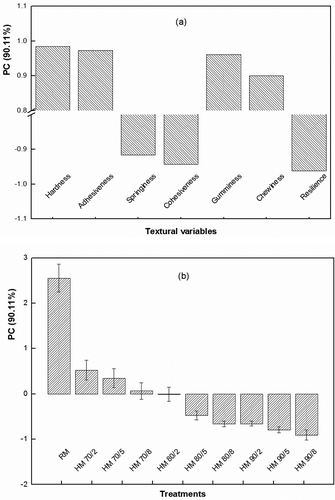
The heating temperature, time, or their combinations significantly influenced the textural variables excluding the effect of temperature on Res of the undisturbed gel ( and ), but the trends of changes in these variables among obvious heat treatments were not clear. In spite of that, the direction of these changes was more clearly seen through PCA () that is explained below. The stirring process changed the texture of rennet gel. When the gel was stirred, Har, Adh, Gum, and Che greatly decreased, and Coh highly increased. Also, the stirring process highly increased Res (from 0.027–0.051 to 0.188–0.395), and slightly increased Spr (from 0.788–0.832 to 0.848–0.890) in the heated samples. In the meantime, the slight decreases of Spr and Res were found in the RM samples.
If a food has lower Adh compared with Coh, then the probe is likely to remain clean,[Citation31] i.e., the food has the ability to hold together. The higher ratios of Adh to Coh were found in the undisturbed gels (263.22–458.04) than the stirred gels (36.49–81.87) as well as the RM samples (566.81 versus 150.32). The result suggested that the stirring process strengthened the capability of curd to hold together, which might lead to the textural changes of final products. This finding increased the positive effects of the stirring process.
Comparison of these heat treatments is illustrated in the PCA diagram (cumulative variance explained = 92.65%) for the undisturbed gels (). This diagram showed the distinctive position of the RM sample because of its relatively high Har, Adh, Spr, Gum, and Che compared to the heated samples. The samples of 90/8, 80/8, 90/5, and 90/2 showed clear difference in Coh compared with the rest of the samples in which much similar Coh was found. Likewise, the samples of 90/8, 80/8, and 90/5 showed clear difference in Res compared to the rest of the samples. shows PCA on the textural variables of the stirred gels. Only one principal component (PC; the variance explained = 90.11%) was produced to distinguish the heat treatments. A distinctive difference between RM and the heated milk was found. Totally, the clear trends were observed with the increasing intensity of heat treatments up to the treatment of 80/2 in the both gels.
WHC and CCY
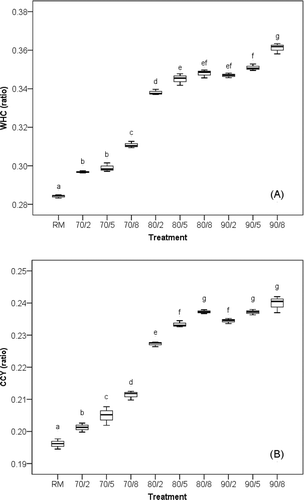
shows the effects of heat on WHC and CCY of rennet gel. WHC and CCY obtained from the heated milk were significantly greater than those of RM. Increasing the intensity of heat treatment from 70/2 to 90/8 increased WHC by 4.4 to 27.1% (a) compared with RM, and increased CCY by 2.6 to 22.3% (b).
WHC of rennet gel is important parameters in the cheese-making process and affects parameters such as yield, moisture content, and textural attributes.[Citation20] WHC and CCY had a close relationship (r = 0.991). The latter was always used for the preliminary evaluation on the yield of cheese (CY).[Citation4,Citation32] The great increase of CCY was found in the treatments of 80°C. Increasing the temperature of heat treatment from 70 to 80°C increased CCY by 2.6–7.8 to 15.9–21.0% compared with RM.
To access the effects of heat, the CCY equations of rennet gel were estimated using stepwise regression analysis with the parameters of heat treatment (temperature, Kelvin; time, min). The following equations were derived from individual components or a combination of two components.
The temperature of heat treatment showed a relatively strong correlation with CCY. When the temperature and time were incorporated into Eq. (3), the correlation between the heat treatment and CCY increased (from 0.794 to 0.853). Therefore, CCY depends on the combination of temperature and time of heat treatment.
The regression analysis of CCY with the parameters of the PCs from textural variables was carried out to build a statistical model between CCY of rennet gels and their textural property. In the undisturbed gel, the following correlations were built with the PCs (PC1 and PC2, standardized values).
When PC2 was account into Eq. (6), the correlation between the textural property of gels and their CCY increased slightly. In the stirred gel, the only PC (standardized values) was used to build the following correlation.
Thus, the Eq. (6) and Eq. (7) for the undisturbed and stirred gel, respectively, could predict CCY with relatively high accuracy. Usually, the predictive equations for CCY have evolved from compositions of milk, moisture, and salt in cheese and whey solids as separate factors.[Citation33] Application of the attributes of cheese would delay these predictions. Also, heat treatment has a positive effect on CCY due to increased moisture content and more effective recovery of whey proteins.[Citation34] Although the use of heat (or texture) parameters alone is hard to predict CCY, it would be a significant attempt for substituting the moisture content in cheese with these parameters under a given production process.
Microstructure of Curd
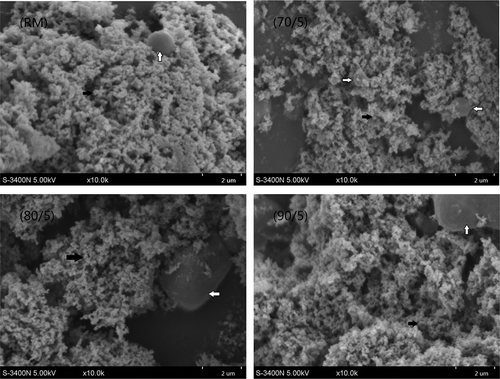
Even though changes in milk proteins and the texture of gels were observed as a result of heat treatments, the results of SEM did not show obvious differences in the microstructure of cheese curd among the heat treatments. As shown in , fat globules were largely spherical in shape with the diameters close to 1.5 μm, and were present in the matrix of casein micelle. Therefore, they can function as breakers of casein network.[Citation35] In contrast to RM, although there were similar proteins network in the microstructure of the heated samples, numerous smaller protein complexes appeared in the relatively regular spheres, especially in the treatments of 90°C (). This phenomenon might due to the reaction of denatured whey proteins and formation of complexes, which could hinder the micelles to further expand.
CONCLUSIONS
Sizes distributions of the protein aggregates in heated milk changed regularly with the increasing temperature and time of heat treatment from ~25 to ~170 nm in diameter. The attributes of rennet-induced milk gels were assessed as affected by the different heat treatments. The textural variables, WHC, and CCY gels were closely correlated with the heat treatments. The correlation coefficient between WHC and CCY was 0.991. The correlation between the heat treatment and CCY increased from 0.794 to 0.853. Heat treatments above 80°C could endow rennet gel with novel textural properties. The statistical models of CCY were preliminarily built for predicting the effects of heat treatments and subsequent textural properties, which achieved the high correlations with heat treatments and with texture indicating that these variables could be used as the assisting factors for predicting cheese yield in the future studies.
FUNDING
The authors acknowledge financial support from the National Natural Science Foundation of the People’s Republic of China (Project Number 31401557) and Food Science and Engineering—the most important discipline of Zhejiang Province (Project Number JYTsp20141091).
Additional information
Funding
REFERENCES
- Swaisgood, H.E. Characteristics of Milk. In Food Chemistry; Fennema, O.R.; Ed.; Marcel Dekker: New York, NY, 1996; 841–878.
- Laleye, L.C.; Jobe, B.; Wasesa, A.A.H. Comparative Study on Heat Stability and Functionality of Camel and Bovine Milk Whey Proteins. Journal of Dairy Science 2008, 91, 4527–4534.
- Hinrichs, J. Incorporation of Whey Proteins in Cheese. International Dairy Journal 2001, 11, 495–503.
- Huppertz, T.; Fox, P.F.; Kelly, A.L. Effects of High Pressure Treatment on the Yield of Cheese Curd from Bovine Milk. Innovative Food Science & Emerging Technologies 2004, 5, 1–8.
- Horton, B.S. Whatever Happened to the Ultrafiltration of Milk? Australian Journal of Dairy Technology 1997, 52, 47–49.
- Atasoy, A.F.; Yetismeyen, A.; Turkoglu, H.; Ozer, B. Effects of Heat Treatment and Starter Culture on the Properties of Traditional Urfa Cheeses (a White-Brined Turkish Cheese) Produced from Bovine Milk. Food Control 2008, 19, 278–285.
- Brandsma, R.L.; Rizvi, S.S.H. Depletion of Whey Proteins and Calcium by Microfiltration of Acidified Skim Milk for Cheesemaking. Journal of Dairy Science 1999, 82, 2063–2069.
- Brandsma, R.L.; Rizvi, S.S.H. Effect of Manufacturing Treatments on the Rheological Character of Mozzarella Cheese Made from Microfiltration Retentate Depleted of Whey Proteins. International Journal of Food Science and Technology 2001, 36, 601–610.
- Neocleous, M.; Barbano, D.M.; Rudan, M.A. Impact of Low Concentration Factor Microfiltration on the Composition and Aging of Cheddar Cheese. Journal of Dairy Science 2002, 85, 2425–2437.
- Lluis-Arroyo, D.; Flores-Najera, A.; Cruz-Guerrero, A.; Gallardo-Escamilla, F.; Lobato-Calleros, C.; Jimenez-Guzman, J.; Garcia-Garibay, M. Effect of An Exopolysaccharide-Producing Strain of Streptococcus Thermophilus on the Yield and Texture of Mexican Manchego-Type Cheese. International Journal of Food Properties 2014, 17, 1680–1693.
- Hiroyuki, S.; Morimasa, T. Quantifying Thermally Induced Flowability of Rennet Cheese Curds. International Journal of Food Properties 2015, 18, 2277–2283.
- Ardisson-Korat, A.V.; Rizvi, S.S.H. Vatless Manufacturing of Low-Moisture Part-Skim Mozzarella Cheese from Highly Concentrated Skim Milk. Journal of Dairy Science 2004, 87, 3601–3613.
- Singh, H.; Waungana, A. Influence of Heat Treatment of Milk on Cheese Making Properties. International Dairy Journal 2001, 11, 543–551.
- Kethireddipalli, P.; Hill, A.R.; Dalgleish, D.G. Protein Interactions in Heat-Treated Milk and Effect on Rennet Coagulation. International Dairy Journal 2010, 20, 838–843.
- Lucey, J.A.; Johnson, M.E.; Horne, D.S. Invited Review: Perspectives on the Basis of the Rheology and Texture Properties of Cheese. Journal of Dairy Science 2003, 9, 2725–2743.
- Guinee, T.P.; O’Callaghan, D.J.; Pudja, P.D.; O’Brien, N. Rennet Coagulation Properties of Retentates Obtained by Ultrafiltration of Skim Milks Heated to Different Temperatures. International Dairy Journal 1996, 6, 581–596.
- Zhang, X.Y.; Guo, H.Y.; Zhao, L.; Sun, W.F.; Zeng, S.S.; Lu, X.M.; Cao, X.; Ren, Z.F.Sensory Profile and Beijing Youth Preference of Seven Cheese Varieties. Food Quality and Preference 2011, 22, 101–109.
- Alloggio, V.; Caponio, F.; Pasqualone, A.; Gomes, T. Effect of Heat Treatment on the Rennet Clotting Time of Goat and Cow Milk. Food Chemistry 2000, 70, 51–55.
- Guyomarc’h, F.; Law, A.J.R.; Dalgleish, D.G. Formation of Soluble and Micelle-Bound Aggregates in Heated Milk. Journal of Agricultural and Food Chemistry 2003, 51, 4652–4660.
- Sandra, S.; Dalgleish, D.G. Effects of Ultra-High-Pressure Homogenization and Heating on Structural Properties of Casein Micelles in Reconstituted Skim Milk Powder. International Dairy Journal 2005, 15, 1095–1104.
- Beliciu, C.M.; Moraru, C.I. Effect of Solvent and Temperature on the Size Distribution of Casein Micelles Measured by Dynamic Light Scattering. Journal of Dairy Science 2009, 92, 1829–1839.
- Pandey, P.K.; Ramaswamy, H.S.; St-Gelais, D. Water-Holding Capacity and Gel Strength of Rennet Curd As Affected by High-Pressure Treatment of Milk. Food Research International 2000, 33, 655–663.
- Macheboeuf, D.; Coulon, J.D.; D’hour, P. Effect of Breed, Protein Genetic Variants, and Feeding on Cows’ Milk Coagulation Properties. Journal of Dairy Research 1993, 60, 43–54.
- Serrano, J.; Velazquez, G.; Lopetcharat, K.; Ramirez, J.A; Torres, J.A. Effect of Moderate Pressure Treatments on Microstructure, Texture, and Sensory Properties of Stirred-Curd Cheddar Shreds. Journal of Dairy Science 2004, 87, 3172–3182.
- Wang, W.J.; Zhang, L.W.; Li, Y.H.; Feng, Z. Heat-Induced Protein Aggregates and Difference in the Textural Properties of Whole Milk Gel. Journal of Food Quality 2012, 35, 247–254.
- Angel, C.R.; Dalgleish, D.G. Structures and Some Properties of Soluble Protein Complexes Formed by the Heating of Reconstituted Skim Milk Powder. Food Research International 2006, 39, 472–479.
- Jean, K.; Renan, M.; Famelart, M.H.; Guyomarc’H, F. Structure and Surface Properties of the Serum Heat-Induced Protein Aggregates Isolated from Heated Skim Milk. International Dairy Journal 2006, 16, 303–315.
- Lee, S.K.; Huss, M.; Klostermeyer, H.; Anema, S.G. The Effect of Pre-Denatured Whey Proteins on the Textural and Micro-Structural Properties of Model Processed Cheese Spreads. International Dairy Journal 2013, 32, 79–88.
- De Kruif, C.G. Supra-Aggregates of Casein Micelles As a Prelude to Coagulation. Journal of Dairy Science 1998, 81, 3019–3028.
- De Kruif, C.G.; Zhulina, E.B. κ-Casein As a Polyelectrolyte Brush on the Surface of Casein Micelles. Colloids and Surfaces A—Physicochemical and Engineering Aspects 1996, 117, 151–159.
- Liu, H.; Xu, X.M.; Guo, S.D. Comparison of Full-Fat and Low-Fat Cheese Analogues with Or Without Pectin Gel Through Microstructure, Texture, Rheology, Thermal, and Sensory Analysis. International Journal of Food Science and Technology 2008, 43, 1581–1592.
- Zamora, A.; Ferragut, V.; Jaramillo, P.D.; Guamis, B.; Trujillo, A.J. Effects of Ultra-High Pressure Homogenization on the Cheese-Making Properties of Milk. Journal of Dairy Science 2007, 90, 13–23.
- Emmons, D.B.; Modler, H.W. Invited Review: A Commentary on Predictive Cheese Yield Formulas. Journal of Dairy Science 2010, 93, 5517–5537.
- Rynne, N.M.; Beresford, T.P.; Kelly, A.L; Guinee, T.P. Effect of Milk Pasteurization Temperature and in Situ Whey Protein Denaturation on the Composition, Texture, and Heat-Induced Functionality of Half-Fat Cheddar Cheese. International Dairy Journal 2004, 14, 989–1001.
- Lopez, C.; Camiera, B.; Gassia, J.Y. Development of the Milk Fat Microstructure During the Manufacture and Ripening of Emmental Cheese Observed by Confocal Laser Scanning Microscopy. International Dairy Journal 2007, 17, 235–247.